Indirect Myocardial Injury in Polytrauma: Mechanistic Pathways and the Clinical Utility of Immunological Markers
Abstract
1. Introduction
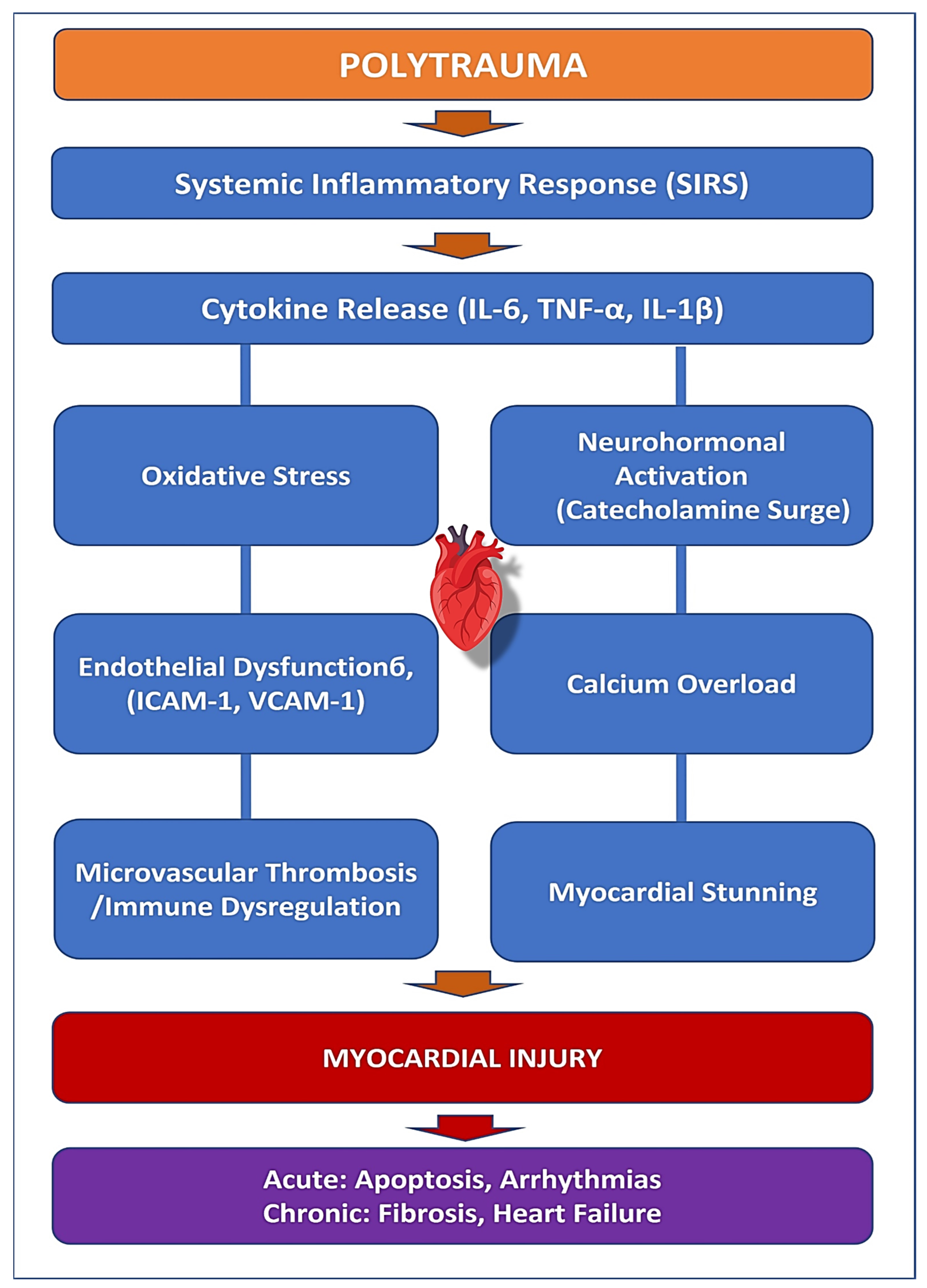
2. Pathophysiology of Myocardial Injury in Polytrauma
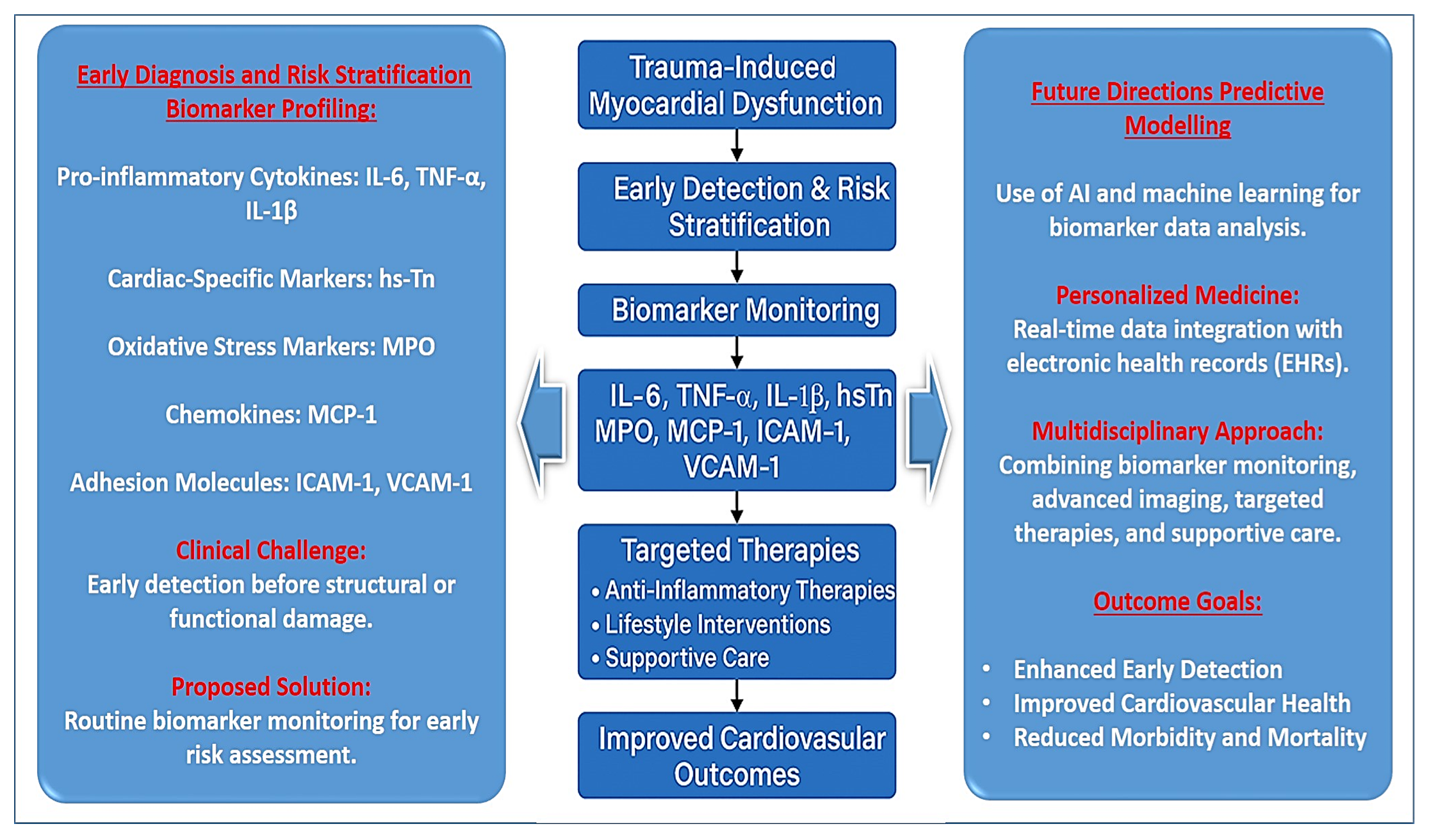
3. Key Immunological Markers in Myocardial Injury Following Polytrauma
4. Clinical Relevance and Future Directions
5. Perspectives and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sztulman, L.; Ritter, A.; de Rosa, R.; Pfeiffer, V.; Leppik, L.; Busse, L.C.; Kontaxi, E.; Störmann, P.; Verboket, R.; Adam, E.; et al. Cardiac damage after polytrauma: The role of systematic transthoracic echocardiography—A pilot study. World J. Emerg. Surg. 2025, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Maayah, M.; Grubman, S.; Allen, S.; Ye, Z.; Park, D.Y.; Vemmou, E.; Gokhan, I.; Sun, W.W.; Possick, S.; Kwan, J.M.; et al. Clinical Interpretation of Serum Troponin in the Era of High-Sensitivity Testing. Diagnostics 2024, 14, 503. [Google Scholar] [CrossRef]
- Van Lieshout, E.M.M.; Verhofstad, M.H.J.; Van Silfhout, D.J.T.; Dubois, E.A. Diagnostic approach for myocardial contusion: A retrospective evaluation of patient data and review of the literature. Eur. J. Trauma Emerg. Surg. 2020, 47, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Kyriazidis, I.P.; Jakob, D.A.; Vargas, J.A.H.; Franco, O.H.; Degiannis, E.; Dorn, P.; Pouwels, S.; Patel, B.; Johnson, I.; Houdlen, C.J.; et al. Accuracy of diagnostic tests in cardiac injury after blunt chest trauma: A systematic review and meta-analysis. World J. Emerg. Surg. 2023, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Ting, R.S.; Weaver, N.A.; King, K.L.; Way, T.L.; Sarrami, P.; Daniel, L.; Dinh, M.; Nair, P.; Hsu, J.; D’Amours, S.K.; et al. Epidemiology of postinjury multiple organ failure: A prospective multicenter observational study. Eur. J. Trauma Emerg. Surg. 2024, 50, 3223–3231. [Google Scholar] [CrossRef]
- Zhou, P.; Ling, L.J.; Xia, X.H.; Yuan, H.; Guo, Z.Q.; Feng, Q.P.; Ma, J. Independent predictors of mortality for critically ill patients with polytrauma: A single center, retrospective study. Heliyon 2024, 10, e25163. [Google Scholar] [CrossRef]
- Meyer, H.J.; Dermendzhiev, T.; Hetz, M.; Osterhoff, G.; Kleber, C.; Denecke, T.; Henkelmann, J.; Metze, M.; Werdehausen, R.; Hempel, G.; et al. Coronary artery calcification detected by initial polytrauma CT in severely injured patients: Retrospective single-center cohort study. Eur. J. Trauma Emerg. Surg. 2024, 50, 1527–1536. [Google Scholar] [CrossRef]
- Becker, N.; Franz, N.; Eguchi, A.; Wagner, A.; Sturm, R.; Rinderknecht, H.; Kobayashi, Y.; Iwasa, M.; Weber, B.; Marzi, I.; et al. Elevated extracellular particle concentration in plasma predicts in-hospital mortality after severe trauma. Front. Immunol. 2024, 15, 1390380. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Huo, T.T.; Zhu, B.L.; Jin, X.; Zhao, Z.H.; Lin, M.H.; Liu, J.X.; Guo, Z.Y.; Xu, W.H.; et al. Peripheral microvessel area better predicts the severity of coronary stenosis of acute myocardial infarction patients over pulse wave velocity. Sci. Rep. 2024, 14, 28584. [Google Scholar] [CrossRef]
- Dhaliwal, K.B.S.; Johal, P.S.; Kumar, N.; Ahmed, R.; Mactaggart, S.; Sakthivel, H.; Verma, R.; Ravalani, A.; Ramphul, K. Noninfectious Systemic Inflammatory Response Syndrome with Organ Damage in Patients Admitted for Acute Myocardial Infarction: A Perspective from the United States. Chest 2024, 166, A474–A475. [Google Scholar] [CrossRef]
- Al-Awar, A.; Hussain, S. Interplay of Reactive Oxygen Species (ROS) and Epigenetic Remodelling in Cardiovascular Diseases Pathogenesis: A Contemporary Perspective. Front. Biosci.-Landmark 2024, 29, 398. [Google Scholar] [CrossRef] [PubMed]
- Börzsei, D.; Kiss, V.; Nagy, A.; Hoffmann, A.; Török, S.; Almasi, N.; Veszelka, M.; Varga, C.; Szabó, R. Moderate-Intensity Swimming Alleviates Oxidative Injury in Ischemic Heart. Appl. Sci. 2024, 14, 2073. [Google Scholar] [CrossRef]
- Divya, K.P.; Kanwar, N.; Anuranjana, P.V.; Kumar, G.; Beegum, F.; George, K.T.; Kumar, N.; Nandakumar, K.; Kanwal, A. SIRT6 in Regulation of Mitochondrial Damage and Associated Cardiac Dysfunctions: A Possible Therapeutic Target for CVDs. Cardiovasc. Toxicol. 2024, 24, 598–621. [Google Scholar] [CrossRef]
- Yan, T.T.; Yu, H.L.; Li, T.; Dong, Y.H. Mechanisms of Cardiovascular Toxicities Induced by Cancer Therapies and Promising Biomarkers for Their Prediction: A Scoping Review. Heart Lung Circ. 2024, 33, 605–638. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Cheng, R.K.; Heckbert, S.R.; Thompson, H.; Zaslavksy, O.; Chlebowski, R.T.; Shadyab, A.H.; Johnson, L.; Wactawski-Wende, J.; Wells, G.; et al. Chronic Oxidative Stress as a Marker of Long-term Radiation-Induced Cardiovascular Outcomes in Breast Cancer. J. Cardiovasc. Transl. 2023, 16, 403–413. [Google Scholar] [CrossRef]
- Aghajani, M.; Aghajani, M.; Moghaddam, E.K.; Faghihi, M.; Imani, A. Acute sleep deprivation (ASD) and cardioprotection: Impact of ASD on oxytocin-mediated sympathetic nervous activation preceding myocardial infarction. Neuropeptides 2024, 107, 102453. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Lötterle, L.; Han, J.Y.; Kalbitz, M.; Henrich, D.; Marzi, I.; Leppik, L.; Weber, B. Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes. J. Clin. Med. 2024, 13, 961. [Google Scholar] [CrossRef]
- Ghanta, S.N.; Kattamuri, L.P.V.; Odueke, A.; Mehta, J.L. Molecular Insights into Ischemia-Reperfusion Injury in Coronary Artery Disease: Mechanisms and Therapeutic Implications: A Comprehensive Review. Antioxidants 2025, 14, 213. [Google Scholar] [CrossRef]
- Chaudhary, M.; Sharma, V.; Bedi, O.; Kaur, A.; Singh, T.G. SGK-1 Signalling Pathway is a Key Factor in Cell Survival in Ischemic Injury. Curr. Drug Targets 2023, 24, 1117–1126. [Google Scholar] [CrossRef]
- Sakaguchi, A.; Nishiyama, C.; Kimura, W. Cardiac regeneration as an environmental adaptation. BBA-Mol. Cell Res. 2020, 1867, 118623. [Google Scholar] [CrossRef]
- Mikolka, P.; Kosutova, P.; Balentova, S.; Cierny, D.; Kopincova, J.; Kolomaznik, M.; Adamkov, M.; Calkovska, A.; Mokra, D. Early Cardiac Injury in Acute Respiratory Distress Syndrome: Comparison of Two Experimental Models. Physiol. Res. 2020, 69, S421–S432. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, J.L.; Fang, Y.; Du, H.L.; Chen, Y.L.; Zhao, S.Q. Molecular biomarkers of blunt cardiac injury: Recent advances and future perspectives. Expert. Rev. Mol. Diagn. 2024, 24, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Dergilev, K.; Zubkova, E.; Guseva, A.; Tsokolaeva, Z.; Goltseva, Y.; Beloglazova, I.; Ratner, E.; Andreev, A.; Partigulov, S.; Lepilin, M.; et al. Tumor Necrosis Factor-Alpha Induces Proangiogenic Profiling of Cardiosphere-Derived Cell Secretome and Increases Its Ability to Stimulate Angiogenic Properties of Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 16575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Xu, T.; Luo, Z.H.; Zhou, H.Y.; Duan, Z.G.; Xiong, X.L.; Huang, M.J.; Li, W. Effect of inflammatory factors on myocardial infarction. BMC Cardiovasc. Disor 2024, 24, 538. [Google Scholar] [CrossRef]
- Peng, Y.J.; Tao, Y.C.; Liu, L.X.; Zhang, J.; Wei, B. Crosstalk among Reactive Oxygen Species, Autophagy and Metabolism in Myocardial Ischemia and Reperfusion Stages. Aging Dis. 2024, 15, 1075–1107. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.N.; Cui, X.R.; Yan, Q.; Xue, T.T.; Jing, X. Insight into myocardial ischemia-reperfusion injury from the perspective of ferroptosis. Perfusion 2024, 40, 1088–1102. [Google Scholar] [CrossRef]
- Hu, D.X.; Prabhakaran, H.S.; Zhang, Y.Y.; Luo, G.X.; He, W.F.; Liou, Y.C. Mitochondrial dysfunction in sepsis: Mechanisms and therapeutic perspectives. Crit. Care 2024, 28, 292. [Google Scholar] [CrossRef]
- Quinn, M.; Zhang, R.Y.K.; Bello, I.; Rye, K.A.; Thomas, S.R. Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction. Antioxidants 2024, 13, 788. [Google Scholar] [CrossRef]
- Dong, Y.L.; Kang, Z.Y.; Zhang, Z.L.; Zhang, Y.Q.; Zhou, H.F.; Liu, Y.F.; Shuai, X.X.; Li, J.Y.; Yin, L.Q.Q.; Wang, X.X.; et al. Single-cell profile reveals the landscape of cardiac immunity and identifies a cardio-protective Ym-1hi neutrophil in myocardial ischemia-reperfusion injury. Sci. Bull. 2024, 69, 949–967. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Klósek, M.; Pietsz, G.; Balwierz, R.; Olczyk, P.; Czuba, Z.P. Ethanolic Extract of Propolis and CAPE as Cardioprotective Agents against LPS and IFN-α Stressed Cardiovascular Injury. Nutrients 2024, 16, 627. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Orekhov, N.A.; Churov, A.V.; Starodubtseva, I.A.; Beloyartsev, D.F.; Kovyanova, T.I.; Sukhorukov, V.N.; Orekhov, A.N. Role of Cathelicidins in Atherosclerosis and Associated Cardiovascular Diseases. J. Mol. Pathol. 2024, 5, 319–334. [Google Scholar] [CrossRef]
- Mitsis, A.; Avraamides, P.; Lakoumentas, J.; Kyriakou, M.; Sokratous, S.; Karmioti, G.; Drakomathioulakis, M.; Theodoropoulos, K.C.; Nasoufidou, A.; Evangeliou, A.; et al. Role of inflammation following an acute myocardial infarction: Design of INFINITY. Biomark. Med. 2023, 17, 971–981. [Google Scholar] [CrossRef]
- Lange, T.H.; Eijken, M.; Baan, C.; Petersen, M.S.; Bibby, B.M.; Jespersen, B.; Moller, B.K. Early Immunological Effects of Ischemia-Reperfusion Injury: No Modulation by Ischemic Preconditioning in a Randomised Crossover Trial in Healthy Humans. Int. J. Mol. Sci. 2019, 20, 2877. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, J.P.G.; Günthel, M.; van den Bosch, E.; Kamphuis, V.P.; Blom, N.A.; Breur, J.; Berger, R.M.F.; Bogers, A.J.J.C.; Koopman, L.; Ten Harkel, A.D.J.; et al. Ventricular function and biomarkers in relation to repair and pulmonary valve replacement for tetralogy of Fallot. Open Heart 2023, 10, e002238. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Quan, L.P.; Cai, C.Y.; Yu, D.Y.; Yan, W.; Wei, Q.J.; Zhang, Z.; Huang, X.N.; Liu, L. Dysregulation and imbalance of innate and adaptive immunity are involved in the cardiomyopathy progression. Front. Cardiovasc. Med. 2022, 9, 973279. [Google Scholar] [CrossRef]
- Yoon, J.A.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Ahn, H.J.; Jeon, S.Y.; Kim, D.; Kang, C. Checkpoint for Considering Interleukin-6 as a Potential Target to Mitigate Secondary Brain Injury after Cardiac Arrest. Brain Sci. 2024, 14, 779. [Google Scholar] [CrossRef]
- Li, X.; Zhou, W.H.; Guo, D.X.; Hu, Y.D.; Zhou, H.L.; Chen, Y. Roles of MDA-LDL/OX-LDL/LOX-1 and TNF-α/TLR4/NF-κB Signaling Pathways in Myocardial Damage by Implantations of Cardiac Pacemakers in Elderly Patients. Curr. Vasc. Pharmacol. 2024, 22, 251–265. [Google Scholar] [CrossRef]
- Garon, E.B.; Lu, S.; Goto, Y.; De Marchi, P.; Paz-Ares, L.; Spigel, D.R.; Thomas, M.; Yang, J.C.H.; Ardizzoni, A.; Barlesi, F.; et al. Canakinumab as Adjuvant Therapy in Patients With Completely Resected Non-Small-Cell Lung Cancer: Results From the CANOPY-A Double-Blind, Randomized Clinical Trial. J. Clin. Oncol. 2024, 42, 180–191. [Google Scholar] [CrossRef]
- Cortes-Ibáñez, F.O.; Johnson, T.; Mascalchi, M.; Katzke, V.; Delorme, S.; Kaaks, R. Cardiac troponin I as predictor for cardiac and other mortality in the German randomized lung cancer screening trial (LUSI). Sci. Rep. 2024, 14, 7197. [Google Scholar] [CrossRef]
- Knochenhauer, T.; Sachse, M.; Bazhanov, I.; Macius, E.; Massoudy, N.; Conradi, L.; Reichenspurner, H.; Blankenberg, S.; Neumann, J.T.; Twerenbold, R.; et al. Troponin dynamics after cardiac surgery interventions B-ACS pilot study-Biomarkers after cardiac surgery. Z. Herz Thorax Gefass 2024, 38, 212–219. [Google Scholar] [CrossRef]
- Wong, C.N.; Gui, X.Y.; Rabkin, S.W. Myeloperoxidase, carnitine, and derivatives of reactive oxidative metabolites in heart failure with preserved versus reduced ejection fraction: A meta-analysis. Int. J. Cardiol. 2024, 399, 131657. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Lund, L.H. Inflammation and myeloperoxidase—The next treatment targets in heart failure? Int. J. Cardiol. 2024, 401, 131834. [Google Scholar] [CrossRef] [PubMed]
- Vicenzetto, C.; Giordani, A.S.; Menghi, C.; Baritussio, A.; Cattini, M.G.P.; Pontara, E.; Bison, E.; Rizzo, S.; De Gaspari, M.; Basso, C.; et al. The Role of the Immune System in Pathobiology and Therapy of Myocarditis: A Review. Biomedicines 2024, 12, 1156. [Google Scholar] [CrossRef]
- Cederstrom, S.; Jernberg, T.; Samnegard, A.; Johansson, F.; Silveira, A.; Tornvall, P.; Lundman, P. Inflammatory biomarkers and long-term outcome in young patients three months after a first myocardial infarction. Cytokine 2024, 182, 156696. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Jiménez, C.M.; Romo, A.N.; Aguilera, J.L.; Crespin, M.C.; Baena, B.T.; Casado-Díaz, A.; Moreno, M.A.G.; Puerta, M.J.M.; Roger, A.J. Nutritional Support Reduces Circulating Cytokines in Patients with Heart Failure. Nutrients 2024, 16, 1637. [Google Scholar] [CrossRef]
- Ponikowska, B.; Iwanek, G.; Zdanowicz, A.; Urban, S.; Zymlinski, R.; Ponikowski, P.; Biegus, J. Biomarkers of Myocardial Injury and Remodeling in Heart Failure. J. Pers. Med. 2022, 12, 799. [Google Scholar] [CrossRef]
- Jaiswal, A.; Vamne, A.; Verma, M.K.; Doctor, B. H-FABP as a diagnostic marker for early detection of young myocardial infarction among Indians. Bioinformation 2022, 18, 506–512. [Google Scholar] [CrossRef]
- Uztimür, M.; Ünal, C.N.; Sagiroglu, M. Evaluation of the Use of Different Sensitive Cardiac Biomarkers in Determining Myocardial Damage in Cows With Subclinical and Clinical Ketoses. Vet. Med. Sci. 2025, 11, e70390. [Google Scholar] [CrossRef]
- Wimalanathan, T.; Paus, M.F.; Skranes, J.B.; Berge, T.; Tveit, A.; Rosjo, H.; Omland, T.; Lyngbakken, M.N.; Heck, S.L. Associations between Growth Differentiation Factor 15, Cardiac Troponin T, and N-terminal pro-B-type Natriuretic Peptide, and Future Myocardial Fibrosis Assessed by Cardiac Magnetic Resonance Imaging: Data from the Akershus Cardiac Examination 1950 Study. J. Appl. Lab. Med. 2024, 10, 392–405. [Google Scholar] [CrossRef]
- Dogon, G.; Rigal, E.; Potel, E.; Josse, M.; Rochette, L.; Bejot, Y.; Vergely, C. Growth/differentiation factor 15 (GDF15) expression in the heart after myocardial infarction and cardioprotective effect of pre-ischemic rGDF15 administration. Sci. Rep. 2024, 14, 12949. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (uPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef]
- Montecillo, J.; Pirker, T.; Pemberton, C.; Chew-Harris, J. suPAR in cardiovascular disease. Adv. Clin. Chem. 2024, 121, 89–131. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, J.; Zhou, J.; Ren, Y.; Gul, N.; Chen, L.; Lu, Y. Soluble suppression of tumorigenicity 2 associated with microvascular obstruction in patients with ST-segment elevation myocardial infarction. BMC Cardiovasc. Disor. 2024, 24, 691. [Google Scholar] [CrossRef] [PubMed]
- Dina, C.; Nyulas, V.A.; Radu, A.; Bungau, S.G. The Impact of Nutritional Status and Nutritional Intervention Strategies on Acute Coronary Syndrome. J. Cardiovasc. Emerg. 2024, 10, 124–132. [Google Scholar] [CrossRef]
- Jovanovic, N.; Zach, V.; Crocini, C.; Bahr, L.S.; Forslund-Startceva, S.K.; Franz, K. A gender perspective on diet, microbiome, and sex hormone interplay in cardiovascular disease. Acta Physiol. 2024, 240, e14228. [Google Scholar] [CrossRef]
- Yao, F.; Liu, C.; Luo, D.; Zhou, Y.L.; Li, Q.Q.; Huang, H.L.; Xu, H.M. Metabolites of Microbiota: A Novel Therapy for Heart Disease. Food Rev. Int. 2024, 41, 1099–1115. [Google Scholar] [CrossRef]
- Laws, J.L.; Maya, T.R.; Gupta, D.K. Stress Echocardiography for Assessment of Diastolic Function. Curr. Cardiol. Rep. 2024, 26, 1461–1469. [Google Scholar] [CrossRef]
- Kottam, A.; Hanneman, K.; Schenone, A.; Daubert, M.A.; Sidhu, G.D.; Gropler, R.J.; Garcia, M.J.; Cardi, A.H.A.C. State-of-the-Art Imaging of Infiltrative Cardiomyopathies: A Scientific Statement From the American Heart Association. Circ.-Cardiovasc. Imag. 2023, 16, e000081. [Google Scholar] [CrossRef]
- Al-Mohaissen, M.A. Echocardiographic assessment of primary microvascular angina and primary coronary microvascular dysfunction. Trends Cardiovas. Med. 2023, 33, 369–383. [Google Scholar] [CrossRef]
- Parsa, S.; Shah, P.Y.; Doijad, R.; Rodriguez, F. Artificial Intelligence in Ischemic Heart Disease Prevention. Curr. Cardiol. Rep. 2025, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Hassan, A.; Khan, D.A.; Khan, S.A.; Bakhshi, A.D.; Akram, M.U.; Babar, M.; Hussain, F.; Abdul, W. AI-CADR: Artificial Intelligence Based Risk Stratification of Coronary Artery Disease Using Novel Non-Invasive Biomarkers. IEEE J. Biomed. Health Inform. 2024, 28, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Yoon, M.; Kim, J.; Lee, J.H.; Oh, I.; Lee, C.J.; Kang, S.M.; Choi, D.J. Artificial Intelligence-Based Electrocardiographic Biomarker for Outcome Prediction in Patients With Acute Heart Failure: Prospective Cohort Study. J. Med. Internet Res. 2024, 26, e52139. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.H.; Lee, H.S.; Kang, S.; Jang, J.H.; Jo, Y.Y.; Son, J.M.; Lee, M.S.; Kwon, J.M.; Kwun, J.S.; Cho, H.W.; et al. AI-enabled ECG index for predicting left ventricular dysfunction in patients with ST-segment elevation myocardial infarction. Sci. Rep. 2024, 14, 16575. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, M.J.; Cho, Y.J.; Kim, J.; Lee, E.; Son, D.; Kim, S.Y.; Soh, M.S. Screening for RV Dysfunction Using Smartphone ECG Analysis App: Validation Study with Acute Pulmonary Embolism Patients. J. Clin. Med. 2024, 13, 4792. [Google Scholar] [CrossRef]
- DeGroat, W.; Abdelhalim, H.; Peker, E.; Sheth, N.; Narayanan, R.; Zeeshan, S.; Liang, B.T.; Ahmed, Z. Multimodal AI/ML for discovering novel biomarkers and predicting disease using multi-omics profiles of patients with cardiovascular diseases. Sci. Rep. 2024, 14, 26503. [Google Scholar] [CrossRef]
- Wright, K. Artificial Intelligence and Digital Biomarkers: A Revolution in Cardiovascular Diagnostics. EMJ Cardiol. 2024, 12, 31–35. [Google Scholar] [CrossRef]
- Mehta, N.N.; deGoma, E.; Shapiro, M.D. IL-6 and Cardiovascular Risk: A Narrative Review. Curr. Atheroscler. Rep. 2024, 27, 12. [Google Scholar] [CrossRef]
- Katkenov, N.; Mukhatayev, Z.; Kozhakhmetov, S.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. Systematic Review on the Role of IL-6 and IL-1β in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2024, 11, 206. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, N.; Zhang, Y.; Liu, Y.; Pei, M.; Liu, G.; Jia, X.; Guo, X. Early detection of chemotherapy-induced cardiotoxicity in breast cancer patients: A comprehensive analysis using speckle tracking echocardiography. Front. Cardiovasc. Med. 2024, 11, 1413827. [Google Scholar] [CrossRef]
- Maturi, B.; Dulal, S.; Sayana, S.B.; Ibrahim, A.; Ramakrishna, M.; Chinta, V.; Sharma, A.; Ravipati, H. Revolutionizing Cardiology: The Role of Artificial Intelligence in Echocardiography. J. Clin. Med. 2025, 14, 625. [Google Scholar] [CrossRef] [PubMed]
| Key Mechanism | Description | Clinical Implications | Early Detection & Diagnostics |
|---|---|---|---|
| Systemic Inflammation (SIRS) | Trauma triggers a massive inflammatory response with increased TNF-α, IL-6, and IL-1β, promoting endothelial dysfunction and myocardial apoptosis. | Increased risk of heart failure, prolonged inflammation, myocardial fibrosis, and remodeling. | Measurement of inflammatory biomarkers (IL-6, TNF-α, IL-1β), CRP levels, and echocardiography for early detection of myocardial dysfunction. |
| Oxidative Stress | Hypoxia, ischemia-reperfusion injury, and immune activation lead to excessive ROS production, mitochondrial dysfunction, and cell death. | Cardiac remodeling, endothelial dysfunction, increased long-term cardiovascular risk. | Detection of oxidative stress markers (MPO, ROS levels), lipid peroxidation assays, and mitochondrial function tests. |
| Neurohormonal Activation | Elevated catecholamines (epinephrine/norepinephrine) increase myocardial oxygen demand, causing contractile dysfunction, myocardial stunning, and arrhythmias. | Myocardial stunning, calcium overload, risk of Takotsubo cardiomyopathy in trauma patients. | Plasma catecholamine levels, HRV (heart rate variability) monitoring, echocardiography for myocardial function assessment. |
| Endothelial Dysfunction | Upregulation of adhesion molecules (ICAM-1, VCAM-1) increases leukocyte infiltration, leading to microvascular thrombosis and myocardial inflammation. | Increased risk of myocardial infarction, thrombosis, organ failure, even without coronary artery disease. | Endothelial function testing (flow-mediated dilation), measurement of ICAM-1 and VCAM-1 levels, and D-dimer tests for thrombotic risk. |
| Immune Dysregulation | Hyperinflammatory response (SIRS) is followed by compensatory immune suppression (CARS), delaying myocardial healing and increasing susceptibility to infections. | Persistent inflammation, fibrotic remodeling, risk of long-term cardiac complications. | Immune profiling (T-cell activation markers), cytokine level assays, and CRP monitoring for prolonged inflammation. |
| Biomarkers & Diagnostics | IL-6, TNF-α, IL-1β, MPO, and adhesion molecules can help in early risk stratification. Advanced imaging (e.g., speckle-tracking echocardiography) can enhance early detection. | Early intervention, personalized treatment strategies, improved diagnostic accuracy. | High-sensitivity cardiac troponin, echocardiographic strain imaging, and biomarker-based risk assessment tools. |
| Therapeutic Considerations | Anti-inflammatory therapies targeting IL-1β and TNF-α, oxidative stress inhibitors, endothelial stabilizers, and neurohormonal modulation are potential treatment options. | Reduction in cardiovascular complications, improved trauma-related cardiac outcomes, enhanced patient survival. | Serial biomarker tracking, cardiac imaging follow-ups, and therapeutic response monitoring using advanced analytics. |
| Biomarker | Role in Myocardial Injury | Clinical Implications | Problems and Issues | Diagnosis and Treatment |
| Interleukin-6 (IL-6) | Central mediator of systemic inflammation, promotes endothelial activation, increases vascular permeability, and facilitates immune cell infiltration into the myocardium. | Correlates with severity of systemic inflammatory response syndrome (SIRS), myocardial dysfunction, and multi-organ failure. | Lacks specificity for myocardial injury; elevated in various inflammatory conditions. | Can serve as an early indicator of myocardial stress, guiding risk stratification and potential anti-inflammatory interventions. |
| Tumor Necrosis Factor-α (TNF-α) | Impairs cardiac contractility, increases endothelial permeability, induces apoptosis, and mitochondrial dysfunction. Upregulates ICAM-1 and VCAM-1, promoting leukocyte adhesion. | Contributes to myocardial fibrosis, endothelial dysfunction, and progression of heart failure. | Anti-TNF therapies have systemic effects and may suppress immune responses, increasing infection risk. | Targeting TNF-α with inhibitors may help reduce inflammation-driven myocardial damage. |
| Interleukin-1 Beta (IL-1β) | Triggers cardiomyocyte hypertrophy, matrix remodeling, and fibrosis. Amplifies inflammatory signalling in myocardial injury. | Linked to prolonged hospital stays and increased mortality in trauma patients. | IL-1β inhibitors are costly and may not be widely available for trauma patients. | IL-1β inhibitors (e.g., Canakinumab) have shown promise in reducing cardiovascular complications. |
| High-Sensitivity Troponin (hs-Tn) | Biomarker of myocardial stress; elevated in trauma patients due to immune-mediated myocardial injury rather than coronary occlusion. | Strong correlation with IL-6 and IL-1β levels; serves as a predictor of myocardial dysfunction. | May be elevated due to non-cardiac causes, including renal dysfunction and muscle damage. | Useful in differentiating trauma-induced cardiac stress from ischemic myocardial infarction. |
| Myeloperoxidase (MPO) | Released by activated neutrophils, contributes to endothelial dysfunction, oxidative stress, and microvascular thrombosis. | Increases risk of long-term cardiac remodeling and heart failure. | Difficult to measure in routine clinical practice; oxidative stress is influenced by multiple factors. | MPO-targeting therapies may help mitigate oxidative damage and improve myocardial recovery. |
| Suppression of Tumorigenicity 2 (sST2) | An indicator of myocardial strain and fibrosis, associated with poor cardiac recovery. | Predicts adverse cardiac outcomes in trauma patients. | Not yet widely adopted in clinical settings; limited availability of standardized testing. | Potential biomarker for early identification of high-risk trauma patients. |
| Monocyte Chemoattractant Protein-1 (MCP-1) | Recruits monocytes and macrophages to injured myocardial tissue, exacerbating inflammation and fibrosis. | Linked to increased mortality in critically ill patients. | High variability in MCP-1 expression; influenced by multiple inflammatory pathways. | Could be a therapeutic target for reducing myocardial inflammation and fibrosis. |
| Intercellular Adhesion Molecule-1 (ICAM-1) | Facilitates leukocyte adhesion and infiltration into the myocardium, worsening local inflammation. | Associated with persistent inflammation and prolonged myocardial dysfunction. | Lacks specificity; elevated in various conditions, including infections and autoimmune diseases. | Monitoring ICAM-1 levels may help assess the severity of inflammatory myocardial injury. |
| Vascular Cell Adhesion Molecule-1 (VCAM-1) | Upregulated in response to TNF-α and IL-6, promoting leukocyte recruitment and endothelial dysfunction. | Contributes to chronic myocardial inflammation and heart failure progression. | Measurement of VCAM-1 levels is not routine; requires specialized laboratory testing. | May serve as a biomarker for assessing vascular inflammation in trauma patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekbossynova, M.; Saliev, T.; Mukarov, M.; Sugralimova, M.; Batpen, A.; Kozhakhmetova, A.; Zhanbolat, A. Indirect Myocardial Injury in Polytrauma: Mechanistic Pathways and the Clinical Utility of Immunological Markers. J. Cardiovasc. Dev. Dis. 2025, 12, 268. https://doi.org/10.3390/jcdd12070268
Bekbossynova M, Saliev T, Mukarov M, Sugralimova M, Batpen A, Kozhakhmetova A, Zhanbolat A. Indirect Myocardial Injury in Polytrauma: Mechanistic Pathways and the Clinical Utility of Immunological Markers. Journal of Cardiovascular Development and Disease. 2025; 12(7):268. https://doi.org/10.3390/jcdd12070268
Chicago/Turabian StyleBekbossynova, Makhabbat, Timur Saliev, Murat Mukarov, Madina Sugralimova, Arman Batpen, Anar Kozhakhmetova, and Aknur Zhanbolat. 2025. "Indirect Myocardial Injury in Polytrauma: Mechanistic Pathways and the Clinical Utility of Immunological Markers" Journal of Cardiovascular Development and Disease 12, no. 7: 268. https://doi.org/10.3390/jcdd12070268
APA StyleBekbossynova, M., Saliev, T., Mukarov, M., Sugralimova, M., Batpen, A., Kozhakhmetova, A., & Zhanbolat, A. (2025). Indirect Myocardial Injury in Polytrauma: Mechanistic Pathways and the Clinical Utility of Immunological Markers. Journal of Cardiovascular Development and Disease, 12(7), 268. https://doi.org/10.3390/jcdd12070268







