Iatrogenic Pneumopericardium After Pericardiocentesis: A Systematic Review and Case Report
Abstract
1. Introduction
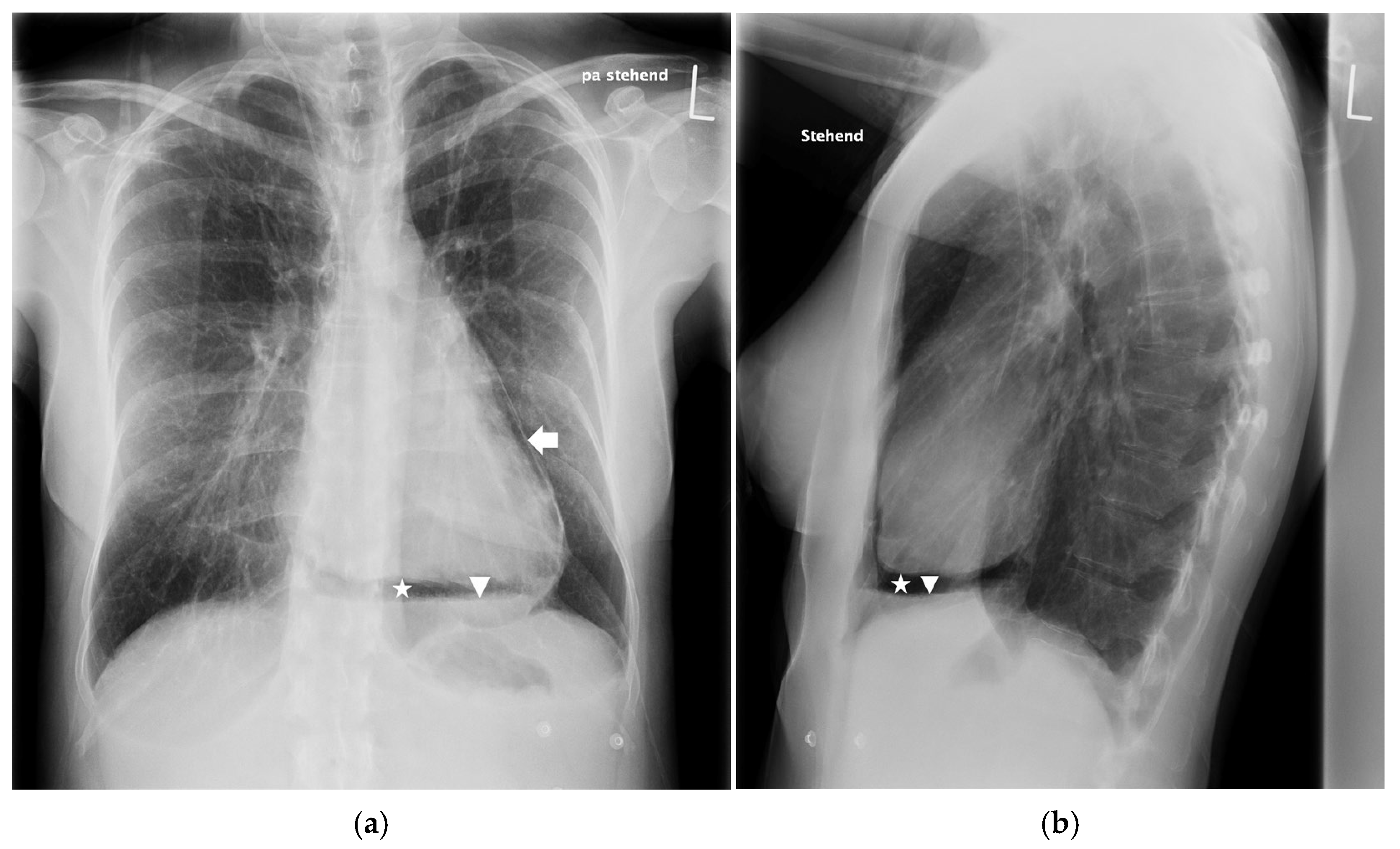
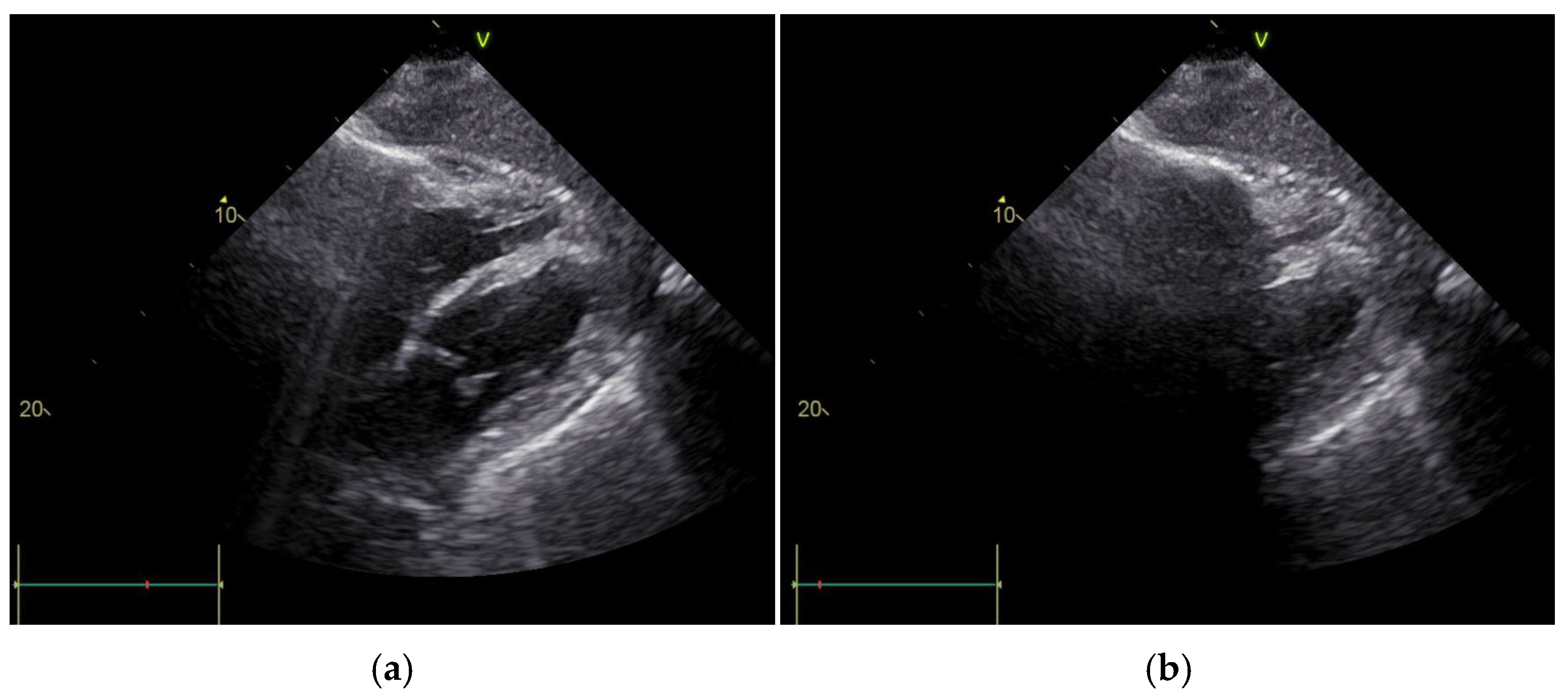
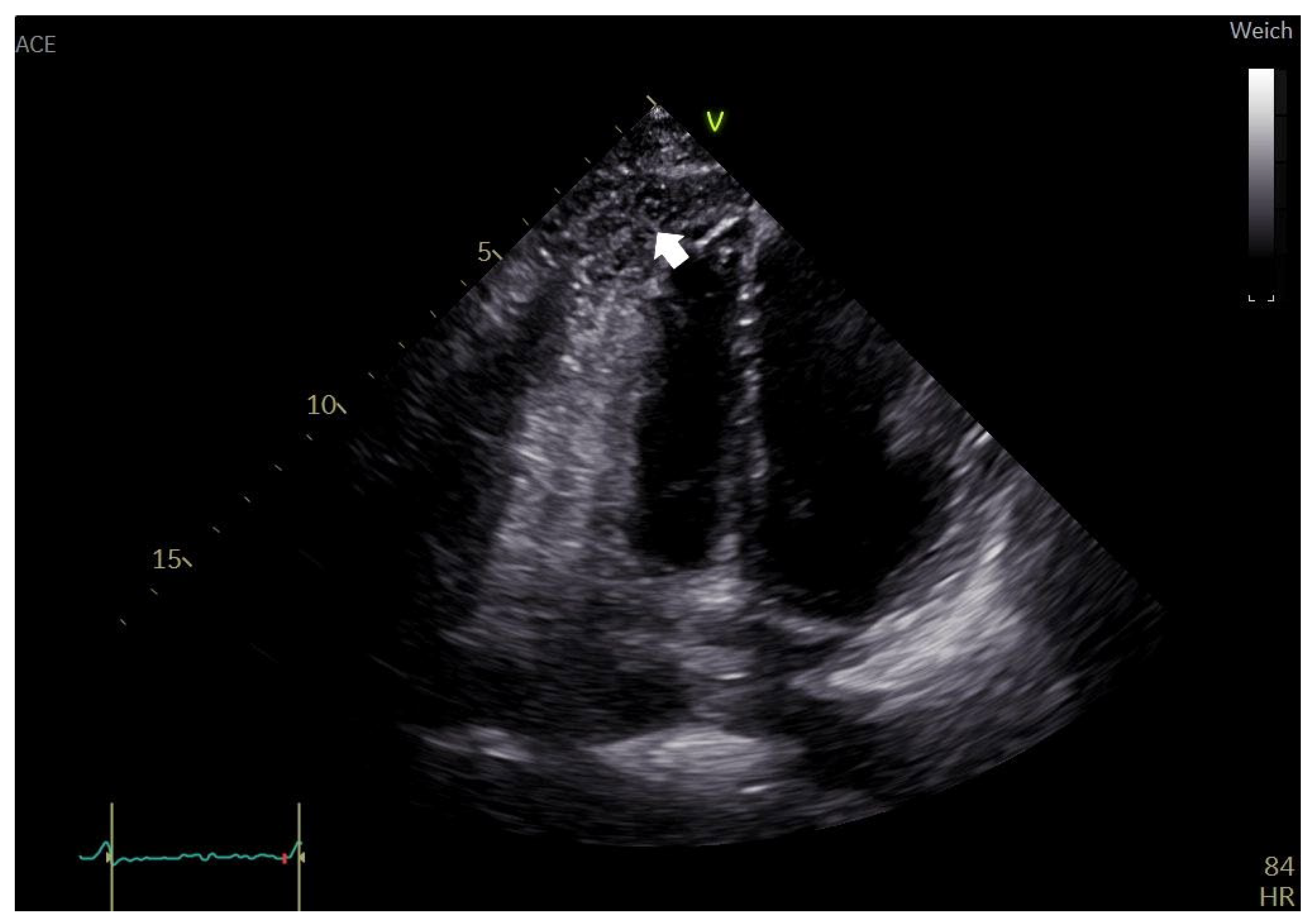
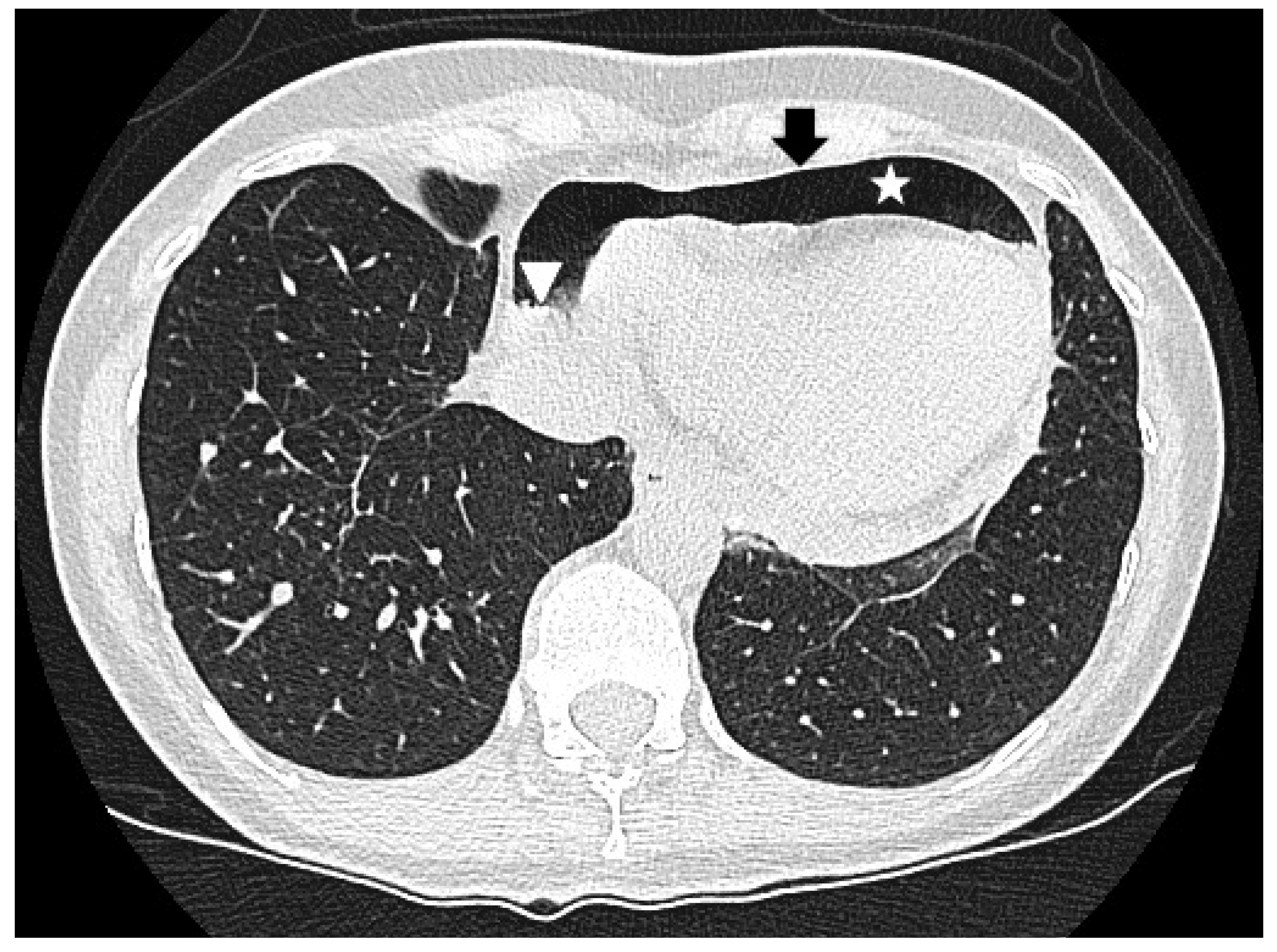
2. Case Report
3. Materials and Methods
3.1. Search Strategy
3.2. Selection Process
3.3. Data Collection, Extraction, and Analysis
3.4. Risk of Bias Assessment
4. Results
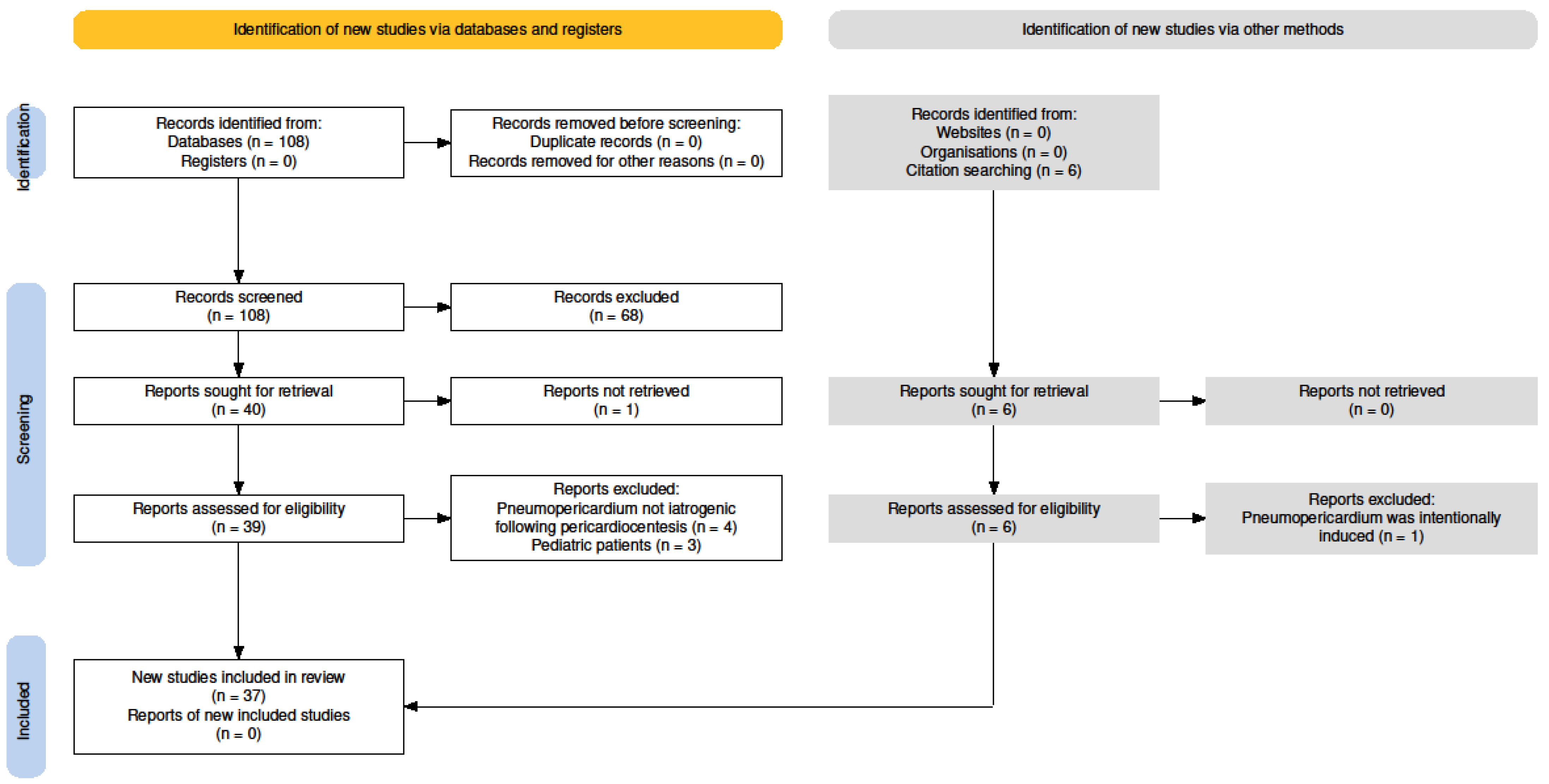
4.1. Study Selection
4.2. Study Characteristics
| Article | Imaging | Air Gap Sign | Swirling Bubbles Sign | Air–Fluid Level | |||||
|---|---|---|---|---|---|---|---|---|---|
| X-Ray | CT | TTE | Identified | Described | Identified | Described | Identified | Visible | |
| Triantafyllis et al. [16] | Yes | No | Yes | No | No | No | Yes | No | No |
| Yilmaz et al. [14] | Yes | Yes | Yes | Yes | Yes | No | No | No | No |
| Mandal [34] | Yes | Yes | No | No | No | No | No | No | No |
| Iskander et al. [19] | No | Yes | Yes | Yes | Yes | Yes | Yes | No | On CT |
| Lee et al. [35] | No | Yes | No | No | No | No | No | No | No |
| Narins et al. [28] | Yes | No | Yes | No | No | No | No | No | No |
| Choi et al. [26] | Yes | No | Yes | No | No | No | Yes | On X-ray | No |
| Satyavolu et al. [24] | Yes | Yes | No | No | No | No | No | No | No |
| Zhu et al. [20] | No | Yes | Yes | No | No | Yes | Yes | No | No |
| Kenzaka et al. [32] | Yes | Yes | No | No | No | No | No | On CT | On X-ray |
| Özkartal et al. [22] | Yes | No | Yes | No | No | No | Yes | No | No |
| Pandey et al. [36] | Yes | Yes | Yes | No | No | No | No | On X-ray | On CT |
| Yuce et al. [23] | Yes | Yes | Yes | No | No | No | Yes | On X-ray | On CT |
| Bedotto et al. [37] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No |
| Delgado-Montero et al. [38] | Yes | Yes | No | No | No | No | No | On X-ray | On CT |
| Mullens et al. [39] | Yes | No | No | No | No | No | No | No | No |
| Methachittiphan et al. [40] | Yes | No | Yes | No | No | No | Yes | No | No |
| Alonso-Ventura et al. [41] | Yes | Yes | Yes | No | No | No | No | No | On X-ray, CT |
| Wakabayashi et al. [27] | No | Yes | Yes | No | No | No | No | No | No |
| Jansen et al. [42] | No | Yes | No | No | No | No | No | No | On CT |
| Bharucha et al. [17] | Yes | No | No | No | No | No | No | No | No |
| Kawanami et al. [43] | Yes | Yes | No | No | No | No | No | No | No |
| Tanabe et al. [21] | Yes | Yes | Yes | No | No | No | No | On X-ray | No |
| Abrahan IV et al. [44] | Yes | Yes | Yes | No | No | No | Yes | On CT | No |
| Adrover Lopez et al. [30] | Yes | Yes | Yes | No | No | No | No | No | No |
| Vijay and Joshi [45] | Yes | Yes | Yes | No | No | No | Yes | On X-ray | On CT |
| Ramírez Martínez et al. [18] | Yes | Yes | Yes | No | No | No | No | On X-ray | No |
| Shah et al. [46] | Yes | Yes | Yes | No | No | No | No | No | No |
| Garcia-Izquierdo et al. [47] | Yes | No | Yes | No | Yes | No | No | No | No |
| Lee et al. [48] | Yes | No | Yes | No | No | Yes | Yes | On X-ray | No |
| Peters et al. [49] | Yes | Yes | Yes | No | No | No | Yes | No | On X-ray |
| Planchat et al. [50] | Yes | Yes | No | No | No | No | No | On X-ray | No |
| Agstam et al. [29] | Yes | No | No | No | No | No | No | No | No |
| Mohanan Nair et al. [33] | Yes | No | Yes | No | No | No | No | No | No |
| Vohra et al. [51] | Yes | Yes | Yes | No | No | Yes | Yes | On X-ray | No |
| Varol et al. [25] | Yes | No | No | No | No | No | No | No | No |
| Kim et al. [31] | Yes | Yes | No | No | No | No | No | No | No |
4.3. Risk of Bias
| Article | Item Number and Corresponding Score | Yes | No | Unclear | N.A. | Score | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||||
| Triantafyllis et al. [16] | Y | N | Y | Y | Y | U | Y | Y | 6 | 1 | 1 | 0 | 6 | Fair |
| Yilmaz et al. [14] | Y | U | Y | Y | U | U | Y | Y | 5 | 0 | 3 | 0 | 5 | Fair |
| Mandal [34] | Y | Y | U | Y | U | Y | Y | Y | 6 | 0 | 2 | 0 | 6 | Fair |
| Iskander et al. [19] | Y | U | Y | Y | Y | Y | Y | Y | 7 | 0 | 1 | 0 | 7 | Good |
| Lee et al. [35] | Y | U | Y | Y | U | U | Y | Y | 5 | 0 | 3 | 0 | 5 | Fair |
| Narins et al. [28] | Y | U | U | Y | U | U | Y | Y | 4 | 0 | 4 | 0 | 4 | Poor |
| Choi et al. [26] | Y | Y | Y | Y | U | Y | Y | Y | 7 | 0 | 1 | 0 | 7 | Good |
| Satyavolu et al. [24] | Y | Y | Y | Y | Y | N | Y | Y | 7 | 1 | 0 | 0 | 7 | Good |
| Zhu et al. [20] | Y | U | Y | Y | U | Y | Y | Y | 6 | 0 | 2 | 0 | 6 | Fair |
| Kenzaka et al. [32] | Y | Y | Y | Y | N | U | Y | Y | 6 | 1 | 1 | 0 | 6 | Fair |
| Özkartal et al. [22] | Y | U | Y | Y | N | U | Y | U | 4 | 1 | 3 | 0 | 4 | Poor |
| Pandey et al. [36] | Y | N | N | Y | N | Y | Y | Y | 5 | 3 | 0 | 0 | 5 | Fair |
| Yuce et al. [23] | Y | N | N | Y | U | U | Y | Y | 4 | 2 | 2 | 0 | 4 | Poor |
| Bedotto et al. [37] | Y | Y | U | Y | N | U | Y | Y | 5 | 1 | 2 | 0 | 5 | Fair |
| Delgado-Montero et al. [38] | Y | N | U | Y | U | N | Y | U | 3 | 2 | 3 | 0 | 3 | Poor |
| Mullens et al. [39] | Y | N | Y | Y | N | N | Y | N | 4 | 4 | 0 | 0 | 4 | Poor |
| Methachittiphan et al. [40] | Y | N | N | Y | N | Y | Y | N | 4 | 4 | 0 | 0 | 4 | Poor |
| Alonso-Ventura et al. [41] | Y | Y | Y | Y | U | U | Y | Y | 6 | 0 | 2 | 0 | 6 | Fair |
| Wakabayashi et al. [27] | Y | N | N | Y | N | Y | Y | Y | 5 | 3 | 0 | 0 | 5 | Fair |
| Jansen et al. [42] | Y | N | N | Y | U | U | Y | U | 3 | 2 | 3 | 0 | 3 | Poor |
| Bharucha et al. [17] | Y | U | Y | Y | U | U | Y | Y | 5 | 0 | 3 | 0 | 5 | Fair |
| Kawanami et al. [43] | Y | Y | U | Y | Y | U | Y | U | 5 | 0 | 3 | 0 | 5 | Fair |
| Tanabe et al. [21] | Y | N | U | Y | U | U | Y | N | 3 | 2 | 3 | 0 | 3 | Poor |
| Abrahan IV et al. [44] | Y | Y | Y | Y | Y | U | Y | Y | 7 | 0 | 1 | 0 | 7 | Good |
| Adrover Lopez et al. [30] | Y | Y | Y | Y | N | U | Y | Y | 6 | 1 | 1 | 0 | 6 | Fair |
| Vijay and Joshi [45] | Y | U | Y | Y | N | Y | Y | Y | 6 | 1 | 1 | 0 | 6 | Fair |
| Ramírez Martínez et al. [18] | Y | N | U | Y | U | Y | Y | Y | 5 | 1 | 2 | 0 | 5 | Fair |
| Shah et al. [46] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | 0 | 0 | 0 | 8 | Good |
| Garcia-Izquierdo et al. [47] | Y | N | U | Y | N | U | Y | N | 3 | 3 | 2 | 0 | 3 | Poor |
| Lee et al. [48] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | 0 | 0 | 0 | 8 | Good |
| Peters et al. [49] | Y | U | U | Y | Y | U | Y | Y | 5 | 0 | 3 | 0 | 5 | Fair |
| Planchat et al. [50] | Y | U | N | Y | U | N | Y | Y | 4 | 2 | 2 | 0 | 4 | Poor |
| Agstam et al. [29] | Y | U | Y | Y | U | N | Y | U | 4 | 1 | 3 | 0 | 4 | Poor |
| Mohanan Nair et al. [33] | Y | U | Y | Y | Y | Y | Y | U | 6 | 0 | 2 | 0 | 6 | Fair |
| Vohra et al. [51] | Y | N | Y | Y | Y | Y | Y | Y | 7 | 1 | 0 | 0 | 7 | Good |
| Varol et al. [25] | Y | N | Y | Y | N | N | Y | N | 4 | 4 | 0 | 0 | 4 | Poor |
| Kim et al. [31] | Y | U | U | Y | N | Y | Y | Y | 5 | 1 | 2 | 0 | 5 | Fair |
| Number of articles applying to this item | 37 | 11 | 21 | 37 | 10 | 14 | 37 | 26 | ||||||
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| JBI | Joanna Briggs Institute |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RV | Right ventricle |
| TTE | Transthoracic echocardiography |
References
- Cummings, R.G.; Wesly, R.L.R.; Adams, D.H.; Lowe, J.E. Pneumopericardium Resulting in Cardiac Tamponade. Ann. Thorac. Surg. 1984, 37, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Gołota, J.J.; Orłowski, T.; Iwanowicz, K.; Snarska, J. Air tamponade of the heart. Pol. J. Cardio.-Thorac. Surg. 2016, 2, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.I.; Visser, F.; Mattheyse, F.; Coetzee, A. Tension Pneumopericardium During Positive-Pressure Ventilation Leading to Cardiac Arrest. J. Cardiothorac. Vasc. Anesth. 2008, 22, 879–882. [Google Scholar] [CrossRef]
- Cohen, D.J.; Baumgart, S.; Stephenson, L.W. Pneumopericardium in Neonates: Is It PEEP or Is It PIP? Ann. Thorac. Surg. 1983, 35, 179–183. [Google Scholar] [CrossRef]
- Maisch, B. Interventional Pericardiology: Pericardiocentesis, Pericardioscopy, Pericardial Biopsy, Balloon Pericardiotomy, and Intrapericardial Therapy; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Aryana, A.; Tung, R.; d’Avila, A. Percutaneous Epicardial Approach to Catheter Ablation of Cardiac Arrhythmias. Clin. Electrophysiol. 2020, 6, 1–20. [Google Scholar] [CrossRef]
- Ackermann, W. The treatment of tuberculous pericarditis with effusion by injection of air and lipiodol into the pericardial sac: Preliminary Report of a Case. Am. Heart J. 1929, 5, 126. [Google Scholar] [CrossRef]
- Imazio, M.; De Ferrari, G.M. Cardiac tamponade: An educational review. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 102–109. [Google Scholar] [CrossRef]
- Sinnaeve, P.R.; Adriaenssens, T. A contemporary look at pericardiocentesis. Trends. Cardiovasc. Med. 2019, 29, 375–383. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lioni, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, n71. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: North Adelaide, Australia, 2024. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Gurkan, O.; Arslanboga, E.N.; Karaca Ozer, P. A rare complication of pericardiocentesis: Pneumopericardium. Echocardiography 2023, 40, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Bricheteau. Observat d’hydropneumopéricarde accompagné d’un bruit de fluctuation perceptible à l’orielle. Arch. Gen. Med. 1844, 4, 334. [Google Scholar]
- Triantafyllis, A.S.; Zamfir, T.; Kontogiannis, N. Pneumopericardium as a complication of pericardiocentesis. Can. Med. Assoc. J. 2023, 195, E193–E194. [Google Scholar] [CrossRef]
- Bharucha, A.H.; Kanyal, R.; Aylward, J.W.; Sivakumar, P.; Webb, I. Pneumopericardium in a patient with trisomy 21 and COVID-19 following emergency pericardiocentesis. Br. J. Cardiol. 2021, 28, 3. [Google Scholar]
- Ramírez Martínez, T.; Blanco Ponce, E.; García-Guimarães, M.; Torres, G.; Gayán Ordás, J. Iatrogenic pneumopericardium following pericardiocentesis. Eur. Heart J. Cardiovasc. Imaging 2024, 25, e251. [Google Scholar] [CrossRef]
- Iskander, S.; Amar, H.; Audrey, B.; Fabien, D. Pneumopericardium: A Rare Complication of Pericardiocentesis. J. Cardiovasc. Ultrasound. 2016, 24, 55. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wang, T.T.; Xu, X.N.; Xu, J.Y.; Fu, H.Y.; Li, G.P.; Liu, T.; Liu, C.L. Iatrogenic pneumopericardium after therapeutic pericardiocentesis for pericardial effusion: A case report. J. Geriatr. Cardiol. 2023, 20, 479–482. [Google Scholar] [CrossRef]
- Tanabe, J.; Murakami, H.; Akazawa, Y.; Nakamura, D.; Sera, F.; Oka, T.; Fukushima, K.; Ohtani, T.; Hosen, N.; Sakata, Y. Pneumopericardium After the Removal of a Pericardiocentesis Drain. Circ. Rep. 2023, 5, 313–314. [Google Scholar] [CrossRef]
- Özkartal, T.; Schlossbauer, S.A.; Faletra, F.; Pedrazzini, G. Pericardiocentesis Complicated by Pneumopericardium. JACC Case Rep. 2019, 1, 249–250. [Google Scholar] [CrossRef]
- Yuce, M.; Sari, I.; Davutoglu, V.; Ozer, O.; Usalan, C. Bubbles around the Heart: Pneumopericardium 10 Days after Pericardiocentesis. Echocardiography 2010, 27, E115–E116. [Google Scholar] [CrossRef] [PubMed]
- Satyavolu, B.; Lodhi, H.A.; Mathews, A.; Bansal, P.; Altaii, H.; Morcos, R.; Desai, A.; Maini, B.; Khalili, H. A Rare Iatrogenic Trio: Pneumopericardium, pneumoperitoneum, and pericarditis. JACC Case Rep. 2021, 3, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Varol, E.; Ozaydin, M.; Ağçal, C. Iatrogenic pneumopericardium. Anatol. J. Cardiol. 2006, 6, 298. [Google Scholar]
- Choi, W.H.; Hwang, Y.M.; Park, M.Y.; Lee, S.J.; Lee, H.Y.; Kim, S.W.; Jun, B.Y.; Min, J.S.; Shin, W.S.; Lee, J.M.; et al. Pneumopericardium as a Complication of Pericardiocentesis. Korean Circ. J. 2011, 41, 280. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Hayashi, T.; Mitsuhashi, T.; Fujita, H. Tension pneumopericardium after pericardiocentesis: Useful echocardiographic obscured heart sign and effective postural change during air aspiration. Heart Rhythm 2018, 15, 1116. [Google Scholar] [CrossRef]
- Narins, C.R.; Lee, J.; Cole, M.; Ling, F.S. Pneumopericardium Following Pericardiocentesis. Am. J. Med. 2016, 129, e181–e182. [Google Scholar] [CrossRef]
- Agstam, S.; Gupta, A.; Gupta, P.; Agarwal, T. Iatrogenic Pneumopericardium During Pericardiocentesis. J. Invasive Cardiol. 2020, 32, E299. [Google Scholar] [CrossRef]
- Adrover López, P.A.; Diago Blanco, D.M.; Santiago Rodríguez, P.; González Sanabria, B.; Rivera Berrios, R.J. Complicated Iatrogenic Pneumopericardium in a Patient With Suspected Multiple Myeloma. Cureus 2022, 14, 24483. [Google Scholar] [CrossRef]
- Kim, H.R.; Choi, D.; Chung, J.W.; Youn, Y.N.; Shim, C.Y. Tension pneumopericardium after removal of pericardiocentesis drainage catheter. Cardiol. J. 2009, 16, 477–478. [Google Scholar]
- Kenzaka, T.; Miyoshi, K.; Nakaji, H. Iatrogenic pneumopericardium in the setting of carcinomatous pericarditis. Case Rep. 2018, bcr-2018. [Google Scholar] [CrossRef]
- Namboodiri, N.; Banavalikar, B.; Gopalakrishnan, A.; Prasad, S.; Valaparambil, A.; Tharakan, J. Iatrogenic Pneumopericardium After Pericardiocentesis. J. Invasive Cardiol. 2016, 28, E225–E226. [Google Scholar]
- Mandal, A. Pneumopericardium a Rare Complication of Pericardiocentesis. Int. J. Cardiovasc. Med. 2024, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, B.S.; Kim, C.; Choi, H.J. Tension Pneumopericardium after Pericardiocentesis. J. Korean Med. Sci. 2016, 31, 470. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, S.K.; Devenraj, V.; Kumar, S.; Singh, V. Pneumopericardium: A rare complication following pericardiocentesis. Indian J. Thorac. Cardiovasc. Surg. 2019, 35, 493–495. [Google Scholar] [CrossRef]
- Bedotto, J.B.; McBride, W.; Abraham, M.; Taylor, A.L. Echocardiographic Diagnosis of Pneumopericardium and Hydropneumopericardium. J. Am. Soc. Echocardiogr. 1988, 1, 359–361. [Google Scholar] [CrossRef]
- Delgado-Montero, A.; Carbonell, A.; Camino, A.; Jimenez-Mena, M.; Zamorano, J.L. An unexpected outcome after pericardiocentesis. Intensive Care Med. 2013, 39, 1845–1846. [Google Scholar] [CrossRef]
- Mullens, W.; Dupont, M.; De Raedt, H. Pneumopericardium after pericardiocentesis. Int. J. Cardiol. 2007, 118, e57. [Google Scholar] [CrossRef]
- Methachittiphan, N.; Boonyaratavej, S.; Kittayarak, C.; Bhumimuang, K.; Mankongpaisarnrung, C.; Pinyoluksana, K.O.; Puwanant, S. Pneumohydropericardium with cardiac tamponade after pericardiocentesis. Heart 2012, 98, 93. [Google Scholar] [CrossRef]
- Alonso-Ventura, V.; Gomollón García, J.P.; Ruiz Aranjuelo, A.; Álvarez Roy, L.; Juez Jiménez, Á.; Miñano Oyarzábal, A.; Aured Guallar, C.; Simón Paracuellos, T.; Solana Hidalgo, M.P. Hydropneumopericardium: A rare complication of pericardiocentesis. Echocardiography 2022, 39, 109–111. [Google Scholar] [CrossRef]
- Jansen, G.; Irmscher, L.; Borgstedt, R.; Rehberg, S.W. Tension pneumopericardium and pneumoperitoneum: A rare complication of pericardiocentesis. Can. J. Anesth. Can. Anesth. 2021, 68, 1564–1565. [Google Scholar] [CrossRef]
- Kawanami, Y.; Kawahara, H.; Endo, A.; Yoshitomi, H.; Tanabe, K. Pneumopericardium Resulting After Pericardiocentesis. Circ. Rep. 2023, 5, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Abrahan, L.L., IV; Obillos, S.M.O.; Aherrera, J.A.M.; Magno, J.D.A.; Uy-Agbayani, C.C.C.; Gopez, U.K.G.; Baldonado, J.J.A.R. A Rare Case of Pneumopericardium in the Setting of Tuberculous Constrictive Pericarditis. Case Rep. Cardiol. 2017, 2017, 4257452. [Google Scholar] [CrossRef] [PubMed]
- Vijay, S.K.; Joshi, L.M. Tension Pneumohydropericardium: A Heart Under Stress. Echocardiography 2016, 33, 802–803. [Google Scholar] [CrossRef]
- Shah, H.; Salahudin, M.; Altaf, A. Asymptomatic Pneumopericardium with Atrial Fibrillation after Pericardiocentesis: A Case Report. J. Tehran. Heart Cent. 2019, 14, 134–137. [Google Scholar] [CrossRef]
- Garcia-Izquierdo, E.; Vazquez Lopez-Ibor, J.; Mingo, S.; Escudier, J.M. An echocardiographic artifact gave us the diagnosis. Intensive Care Med. 2017, 43, 263–264. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, W.H.; Lee, S.R.; Rhee, K.S.; Chae, J.K.; Ko, J.K. Cardiac Tamponade by Iatrogenic Pneumopericardium. J. Cardiovasc. Ultrasound. 2008, 16, 26. [Google Scholar] [CrossRef]
- Peters, F.; Patel, A.; Essop, R. Iatrogenic hydropneumopericardium. Cardiovasc. J. Afr. 2012, 23, e1–e2. [Google Scholar] [CrossRef]
- Planchat, A.; Stierlin, F.; Juillet De Saint-Lager-Lucas, A.; Peloso, A.; Mauler-Wittwer, S.; Noble, S. Hydropneumopericardium after pericardiocentesis in a transplant patient. Cardiol. J. 2023, 30, 335–336. [Google Scholar] [CrossRef]
- Vohra, S.; Pradhan, A.; Vishwakarma, P.; Sethi, R. Hydropneumopericardium: A rare complication of pericardiocentesis. Natl. Med. J. India 2021, 34, 158–160. [Google Scholar] [CrossRef]
- Cosyns, B.; Plein, S.; Nihoyanopoulos, P.; Smiseth, O.; Achenbach, S.; Andrade, M.J.; Pepi, M.; Ristic, A.; Imazio, M.; Paelinck, B.; et al. European Association of Cardiovascular Imaging (EACVI) position paper: Multimodality imaging in pericardial disease. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 12–31. [Google Scholar] [CrossRef]
- Tsang, T.S.; Enriquez-Sarano, M.; Freeman, W.K.; Barnes, M.E.; Sinak, L.J.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Consecutive 1127 Therapeutic Echocardiographically Guided Pericardiocenteses: Clinical Profile, Practice Patterns, and Outcomes Spanning 21 Years. Mayo Clin. Proc. 2002, 77, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Duvernoy, O.; Borowiec, J.; Helmius, G.; Erikson, U. Complications of percutaneous pericardiocentesis under fluoroscopic guidance. Acta Radiol. 1992, 33, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.M.; Patel, N.; Biner, S.; Eshaghian, S.; Mendoza, F.; Cercek, B.; Siegel, R.J. Frequency of Recurrence of Pericardial Tamponade in Patients With Extended Versus Nonextended Pericardial Catheter Drainage. Am. J. Cardiol. 2011, 108, 1820–1825. [Google Scholar] [CrossRef]
- Buchanan, C.L.; Sullivan, V.V.; Lampman, R.; Kulkarni, M.G. Pericardiocentesis with extended catheter drainage: An effective therapy. Ann. Thorac. Surg. 2003, 76, 817–820. [Google Scholar] [CrossRef]
- Vilela, E.M.; Ruivo, C.; Guerreiro, C.E.; Silva, M.P.; Ladeiras-Lopes, R.; Caeiro, D.; Morais, G.P.; Primo, J.; Braga, P.; Ferreira, N.; et al. Computed tomography-guided pericardiocentesis: A systematic review concerning contemporary evidence and future perspectives. Ther. Adv. Cardiovasc. Dis. 2018, 12, 299–307. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, T.; Seabra, D.; Moreno, N. The “airgap” and “swirling bubbles” signs in a patient with esophageal carcinoma. Echocardiography 2023, 40, 252–258. [Google Scholar] [CrossRef]
- Rivkind, A.I.; Meretyk, S.; Lebensart, P.; Krausz, M.M. Pneumopericardium—A life-threatening complication of central venous catheterisation. Clin. Nutr. 1991, 10, 128–130. [Google Scholar] [CrossRef]
- Kern, R.; Godfrey, E. Pneumohydropericardium: A report of 3 cases. US Navy Med. Bull. 1943, 41, 1001. [Google Scholar]
- Reid, C.L.; Chandraratna, P.A.N.; Kawanishi, D.; Bezdek, W.D.; Schatz, R.; Nanna, M.; Rahimtoola, S.H. Echocardiographic detection of pneumomediastinum and pneumopericardium: The air gap sign. J. Am. Coll. Cardiol. 1983, 1, 916–921. [Google Scholar] [CrossRef]
- Kerut, E.K.; Hannawalt, C.; Everson, C.T.; Nanda, N.C. The Air Gap Sign. Echocardiography 2014, 31, 400–401. [Google Scholar] [CrossRef]
- Allgood, N.L.; Brownlee, J.R.; Green, G.A. Inability to view the heart through the subxiphoid echocardiographic window: A harbinger of disaster. Pediatr. Cardiol. 1994, 15, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, S.; Gharahbaghian, L.; Perera, P.; Joshi, N. Spontaneous Pneumomediastinum on Bedside Ultrasound: Case Report and Review of the Literature. West J. Emerg. Med. 2015, 16, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Antonini-Canterin, F.; Nicolosi, G.L.; Mascitelli, L.; Zanuttini, D. Direct demonstration of an air-fluid interface by two-dimensional echocardiography: A new diagnostic sign of hydropneumopericardium. J. Am. Soc. Echocardiogr. 1996, 9, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Abbara, S.; Agler, D.A.; Appleton, C.P.; Asher, C.R.; Hoit, B.; Hung, J.; Garcia, M.J.; Kronzon, I.; Oh, J.K.; et al. American Society of Echocardiography Clinical Recommendations for Multimodality Cardiovascular Imaging of Patients with Pericardial Disease. J. Am. Soc. Echocardiogr. 2013, 26, 965–1012.e15. [Google Scholar] [CrossRef]
- Fadl, S.A.; Nasrullah, A.; Harris, A.; Edwards, R.; Kicska, G. Comprehensive review of pericardial diseases using different imaging modalities. Int. J. Cardiovasc. Imaging 2020, 36, 947–969. [Google Scholar] [CrossRef]
- Ristić, A.D.; Imazio, M.; Adler, Y.; Anastasakis, A.; Badano, L.P.; Brucato, A.; Caforio, A.L.; Dubourg, O.; Elliott, P.; Gimeno, J.; et al. Triage strategy for urgent management of cardiac tamponade: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2014, 35, 2279–2284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merz, A.; Ran, H.; Chiu, C.-Y.; Dreger, H.; Morris, D.A.; Schneider-Reigbert, M. Iatrogenic Pneumopericardium After Pericardiocentesis: A Systematic Review and Case Report. J. Cardiovasc. Dev. Dis. 2025, 12, 246. https://doi.org/10.3390/jcdd12070246
Merz A, Ran H, Chiu C-Y, Dreger H, Morris DA, Schneider-Reigbert M. Iatrogenic Pneumopericardium After Pericardiocentesis: A Systematic Review and Case Report. Journal of Cardiovascular Development and Disease. 2025; 12(7):246. https://doi.org/10.3390/jcdd12070246
Chicago/Turabian StyleMerz, Andreas, Hong Ran, Cheng-Ying Chiu, Henryk Dreger, Daniel Armando Morris, and Matthias Schneider-Reigbert. 2025. "Iatrogenic Pneumopericardium After Pericardiocentesis: A Systematic Review and Case Report" Journal of Cardiovascular Development and Disease 12, no. 7: 246. https://doi.org/10.3390/jcdd12070246
APA StyleMerz, A., Ran, H., Chiu, C.-Y., Dreger, H., Morris, D. A., & Schneider-Reigbert, M. (2025). Iatrogenic Pneumopericardium After Pericardiocentesis: A Systematic Review and Case Report. Journal of Cardiovascular Development and Disease, 12(7), 246. https://doi.org/10.3390/jcdd12070246








