Non-Robotic Endoscopic-Assisted Internal Mammary Artery Harvest—A Historical Review and Recent Advancements
Abstract
1. Introduction
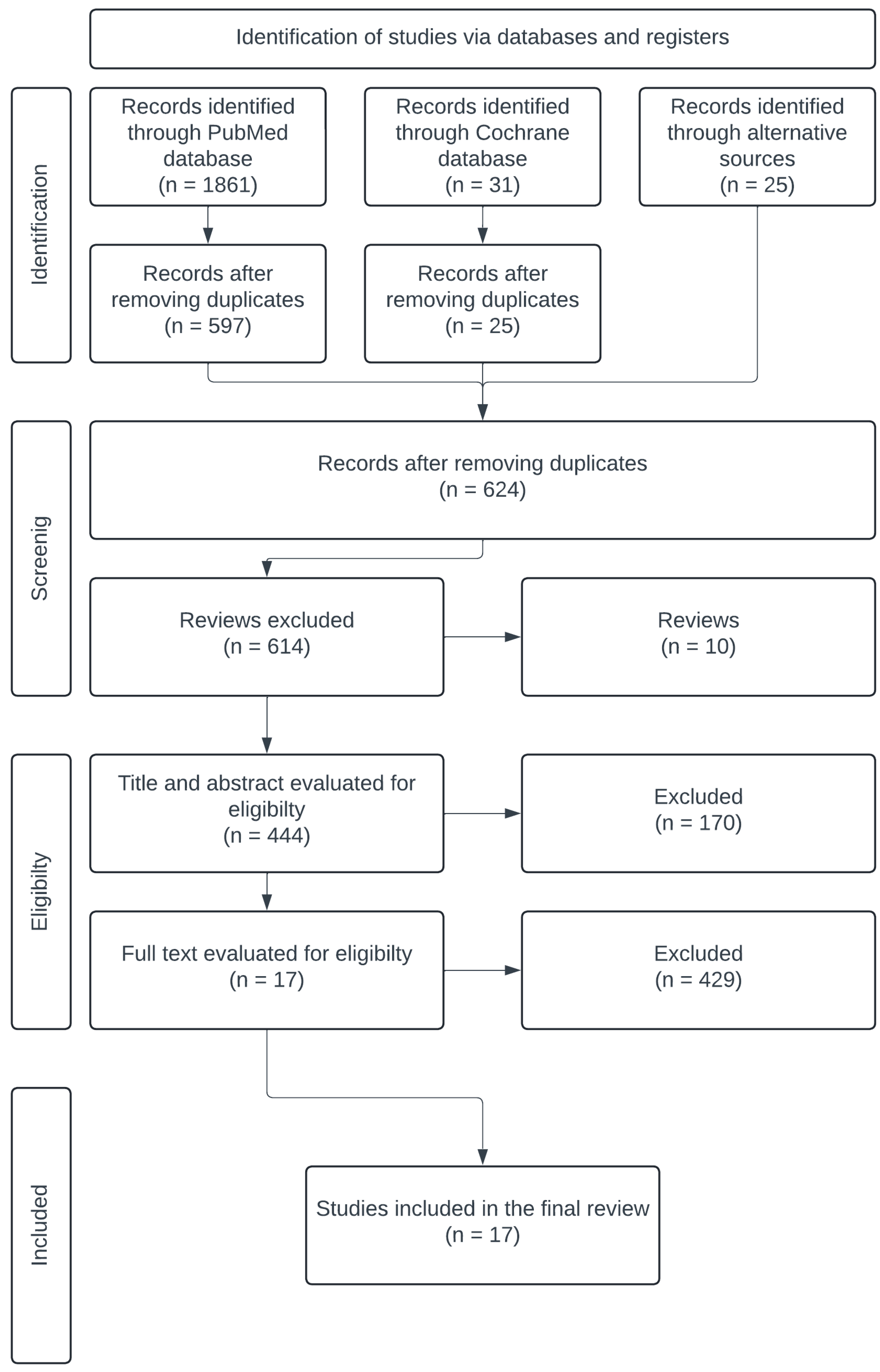
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
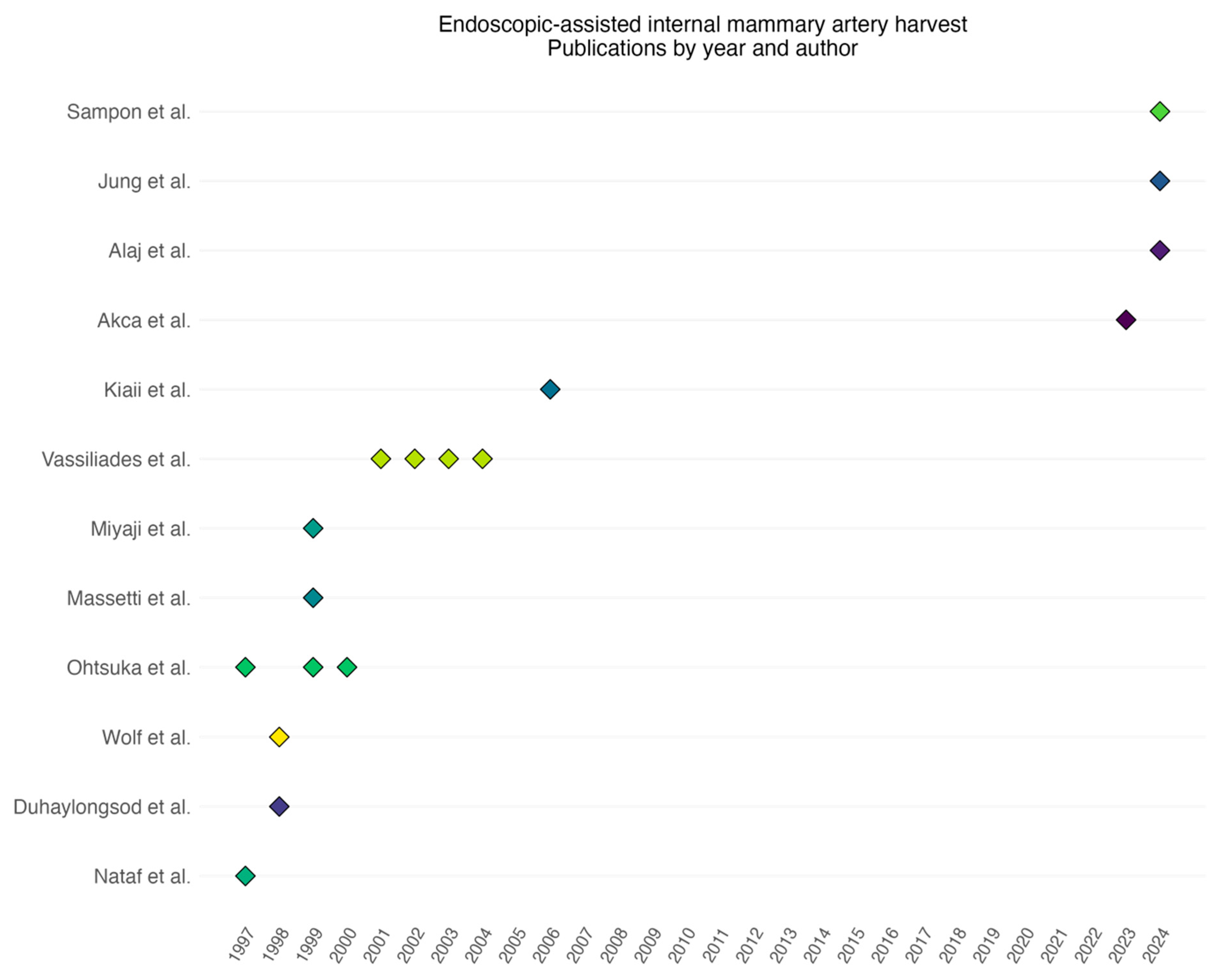
3. Results
| Study | Year of Publication | Study Period | Type of Study | Number of Patients | Single or Multivessel |
|---|---|---|---|---|---|
| Nataf [7] | 1997 | 1995–1996 | Retrospective | 32 | Single |
| Ohtsuka [13] | 1997 | 1995–1996 | Retrospective | 37 | Both |
| Wolf [14] | 1998 | 1995–1997 | Retrospective | 48 | Both |
| Duhaylongsod [9] | 1998 | 1995–1997 | Retrospective | 218 | Both |
| Ohtsuka [15] | 1999 | 1997–1998 | Retrospective | 22 | Single |
| Miyaji [16] | 1999 | 1993–1998 | Retrospective | 73 | Both |
| Massetti [17] | 1999 | 1996–1997 | Retrospective | 30 | Single |
| Ohtsuka [18] | 2000 | 1997–1999 | Retrospective | 38 | Both |
| Vassiliades [19] | 2001 | 1996–2001 | Retrospective | 300 | Both |
| Vassiliades [20] | 2002 | 1996–2001 | Retrospective | 350 | Both |
| Vassiliades [21] | 2003 | 2002–2002 | Retrospective | 18 | Multivessel |
| Vassiliades [11] | 2004 | 1996–2003 | Retrospective | 509 | Both |
| Kiaii [22] | 2006 | NR | Prospective | 50 | Single |
| Akca [8] | 2023 | 2021–2022 | Retrospective | 80 | Single |
| Jung [23] | 2024 | 2019–2023 | Retrospective | 40 | Both |
| Alaj [24] | 2024 | 2021–2022 | Retrospective | 91 | Both |
| Sampon [10] | 2024 | 2018–2023 | Retrospective matched cohort | 266 | Single |
4. Discussion
4.1. Endoscopes Used During LIMA Harvest
4.2. Energy Source During LIMA Harvest
4.3. Pedicled or Skeletonized Harvest
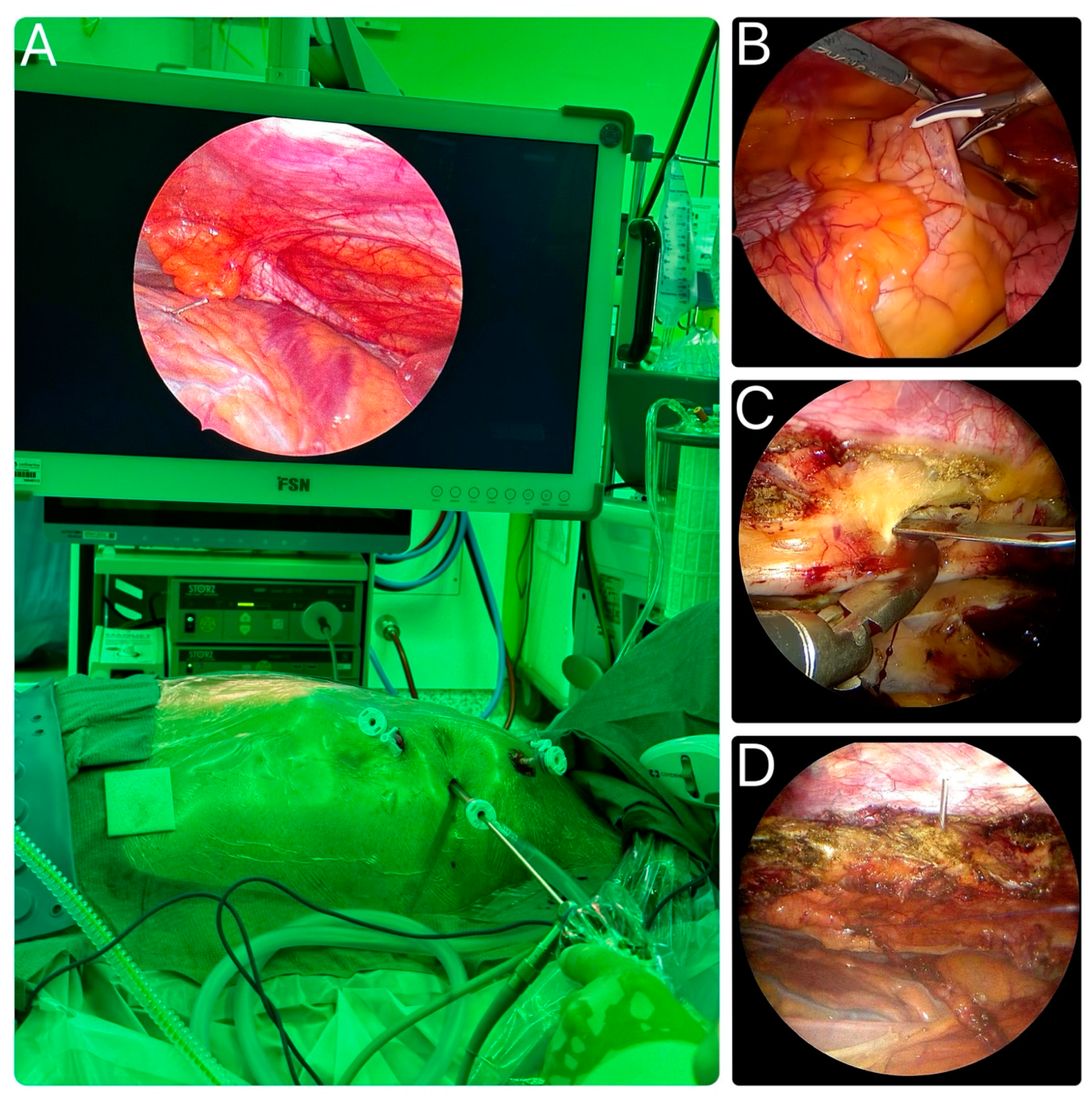
4.4. Overview of the General Technique of Endoscopic LIMA Harvesting
4.5. Patient Selection and Post-Operative Results
4.6. Cost and Availability of Materials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. Patencies of 2,127 Arterial to Coronary Conduits over 15 Years. Ann. Thorac. Surg. 2004, 77, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.J.; Zhao, S.; Tian, D.H.; Taggart, D.P.; Yan, T.D. A Meta-Analysis Comparing Bilateral Internal Mammary Artery with Left Internal Mammary Artery for Coronary Artery Bypass Grafting. Ann. Cardiothorac. Surg. 2013, 2, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Farhat, F.; Metton, O.; Jegaden, O. Benefits and Complications of Total Sternotomy and Ministernotomy in Cardiac Surgery. Surg. Technol. Int. 2004, 13, 199–205. [Google Scholar]
- Soulez, G.; Gagner, M.; Therasse, E.; Basile, F.; Prieto, I.; Pibarot, P.; Laflamme, C.; Lamarre, L.; Shennib, H. Catheter-Assisted Totally Thoracoscopic Coronary Artery Bypass Grafting: A Feasibility Study. Ann. Thorac. Surg. 1997, 64, 1036–1040. [Google Scholar] [CrossRef]
- Zacharias, J.; Glauber, M.; Pitsis, A.; Solinas, M.; Kempfert, J.; Castillo-Sang, M.; Balkhy, H.H.; Perier, P. The 7 Pillars of Starting an Endoscopic Cardiac Surgery Program. Innovations 2024, 19, 107–117. [Google Scholar] [CrossRef]
- Harky, A.; Hussain, S.M.A. Robotic Cardiac Surgery: The Future Gold Standard or An Unnecessary Extravagance? Braz. J. Cardiovasc. Surg. 2019, 34, XII–XIII. [Google Scholar] [CrossRef]
- Nataf, P.; Lima, L.; Regan, M.; Benarim, S.; Ramadan, R.; Pavie, A.; Gandjbakhch, I. Thoracoscopic Internal Mammary Artery Harvesting: Technical Considerations. Ann. Thorac. Surg. 1997, 63, S104–S106. [Google Scholar] [CrossRef]
- Akca, F.; ter Woorst, J. Learning Curve of Thoracoscopic Nonrobotic Harvest of the Left Internal Mammary Artery in Minimally Invasive Coronary Artery Bypass Grafting. Innovations 2023, 18, 262–265. [Google Scholar] [CrossRef]
- Duhaylongsod, F.G.; Mayfield, W.R.; Wolf, R.K. Thoracoscopic Harvest of the Internal Thoracic Artery: A Multicenter Experience in 218 Cases1. Ann. Thorac. Surg. 1998, 66, 1012–1017. [Google Scholar] [CrossRef]
- Sampon, F.; Ter Woorst, J.; Dekker, L.; Akca, F. Thoracoscopic-Assisted, Minimally Invasive versus off-Pump Bypass Grafting for Single Vessel Coronary Artery Disease—A Propensity Matched Analysis. Int. J. Cardiol. 2024, 409, 132175. [Google Scholar] [CrossRef]
- Vassiliades, T.A. Conversion of Endoscopic, Robotically Assisted Coronary Bypass: Incidence, Risk Factors, and Outcome. Heart Surg. Forum 2004, 7, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Vassiliades, T.A. The Cardiopulmonary Effects of Single-Lung Ventilation and Carbon Dioxide Insufflation during Thoracoscopic Internal Mammary Artery Harvesting. Heart Surg. Forum 2002, 5, 22–24. [Google Scholar] [PubMed]
- Ohtsuka, T.; Wolf, R.K.; Hiratzka, L.F.; Wurnig, P.; Flege, J.B. Thoracoscopic Internal Mammary Artery Harvest for MICABG Using the Harmonic Scalpel. Ann. Thorac. Surg. 1997, 63, S107–S109. [Google Scholar] [CrossRef]
- Wolf, R.K.; Ohtsuka, T.; Flege, J.B., Jr. Early Results of Thoracoscopic Internal Mammary Artery Harvest Using an Ultrasonic Scalpel. Eur. J. Cardio-Thorac. Surg. 1998, 14, S54–S57. [Google Scholar] [CrossRef][Green Version]
- Ohtsuka, T.; Imanaka, K.; Endoh, M.; Kohno, T.; Nakajima, J.; Kotsuka, Y.; Takamoto, S. Hemodynamic Effects of Carbon Dioxide Insufflation under Single-Lung Ventilation during Thoracoscopy. Ann. Thorac. Surg. 1999, 68, 29–32, discussion 32–33. [Google Scholar] [CrossRef]
- Miyaji, K.; Wolf, R.K.; Flege, J.B. Surgical Results of Video-Assisted Minimally Invasive Direct Coronary Artery Bypass. Ann. Thorac. Surg. 1999, 67, 1018–1021. [Google Scholar] [CrossRef]
- Massetti, M.; Babatasi, G.; Nataf, P.; Bhoyroo, S.; Le Page, O.; Khayat, A. Minimally Invasive Internal Thoracic Artery Harvest: The Hybrid Approach. Ann. Thorac. Surg. 1999, 67, 632–634. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nakajima, J.; Kotsuka, Y.; Takamoto, S. Hemodynamic Responses to Intrapleural Insufflation with Hemipulmonary Collapse. Surg. Endosc. 2001, 15, 1327–1330. [Google Scholar] [CrossRef]
- Vassiliades, T.A. Atraumatic Coronary Artery Bypass (ACAB): Techniques and Outcome. Heart Surg. Forum 2001, 4, 331–334. [Google Scholar]
- Vassiliades, T.A.; Nielsen, J.L.; Lonquist, J.L. Effects of Obesity on Outcomes in Endoscopically Assisted Coronary Artery Bypass Operations. Heart Surg. Forum 2003, 6, 99–101. [Google Scholar] [CrossRef][Green Version]
- Vassiliades, T.A., Jr. A Unilateral Approach to Bilateral Thoracoscopic Internal Mammary Artery Harvestingq. Interact. Cardiovasc. Thorac. Surg. 2003, 2, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kiaii, B.; McClure, R.S.; Stitt, L.; Rayman, R.; Dobkowski, W.B.; Jablonsky, G.; Novick, R.J.; Boyd, W.D. Prospective Angiographic Comparison of Direct, Endoscopic, and Telesurgical Approaches to Harvesting the Internal Thoracic Artery. Ann. Thorac. Surg. 2006, 82, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Chong, Y.; Kang, M.-W.; Han, S.J.; Cho, H.J.; Park, S.-J.; Shim, M.-S. Clipless Internal Mammary Artery Harvesting for Minimally Invasive Coronary Artery Bypass Grafting Using the Shear-Tip Harmonic Scalpel. J. Thorac. Dis. 2024, 16, 3711–3721. [Google Scholar] [CrossRef]
- Alaj, E.; Seidiramool, V.; Ciobanu, V.; Bakhtiary, F.; Monsefi, N. Short-Term Clinical Results of Minimally Invasive Direct Coronary Artery Bypass (MIDCAB) Procedure. J. Clin. Med. 2024, 13, 3124. [Google Scholar] [CrossRef]
- Nataf, P.; Lima, L.; Regan, M.; Benarim, S.; Pavie, A.; Cabrol, C.; Gandjbakch, I. Minimally Invasive Coronary Surgery with Thoracoscopic Internal Mammary Artery Dissection: Surgical Technique. J. Card. Surg. 1996, 11, 288–292. [Google Scholar] [CrossRef]
- Tevaearai, H.T.; Mueller, X.M.; Stumpe, F.; Ruchat, P.; Segesser, L.K. von Advantages of a Modified Gastroscope for Video-Assisted Internal Mammary Artery Harvesting. Ann. Thorac. Surg. 1999, 67, 872–873. [Google Scholar] [CrossRef]
- Kaneyuki, D.; Patil, S.; Jackson, J.; Ahmad, D.; Plestis, K.A.; Guy, T.S.; Massey, H.T.; Entwistle, J.W.; Morris, R.J.; Tchantchaleishvili, V. Ultrasonic Scalpel versus Electrocautery for Internal Mammary Artery Harvesting: A Meta-Analysis. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lamy, A.; Browne, A.; Sheth, T.; Zheng, Z.; Dagenais, F.; Noiseux, N.; Chen, X.; Bakaeen, F.G.; Brtko, M.; Stevens, L.-M.; et al. Skeletonized vs Pedicled Internal Mammary Artery Graft Harvesting in Coronary Artery Bypass Surgery. JAMA Cardiol. 2021, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, A.; Maniya, M.T.; Duhan, S.; Jamil, A.; Hirji, S.A. Skeletonized versus Pedicled Harvesting of Internal Mammary Artery: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2024, 49, 102160. [Google Scholar] [CrossRef]
- Akca, F. Thoracoscopic (Non-Robotic) Harvesting of Bilateral Internal Mammary Artery Grafts. Multimed. Man. Cardiothorac. Surg. 2023, 2023. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Ninomiya, M.; Maemura, T.; Takamoto, S. Needle-Guided Mini-Entry in Video-Assisted Coronary Artery Bypass. Eur. J. Cardiothorac. Surg. 2003, 24, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Claessens, J.; Rottiers, R.; Vandenbrande, J.; Gruyters, I.; Yilmaz, A.; Kaya, A.; Stessel, B. Quality of Life in Patients Undergoing Minimally Invasive Cardiac Surgery: A Systematic Review. Indian J. Thorac. Cardiovasc. Surg. 2023, 39, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Karangelis, D.; Androutsopoulou, V.; Tzifa, A.; Chalikias, G.; Tziakas, D.; Mitropoulos, F.; Mikroulis, D. Minimally Invasive Cardiac Surgery: In the Pursuit to Treat More and Hurt Less. J. Thorac. Dis. 2021, 13, 6209–6213. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Internal mammary harvest (IMA) | Reviews |
| Thoracoscopic-assisted | Direct-vision harvest |
| Video-assisted | Robotic harvest |
| Endoscopic | Animal training models |
| Perioperative data about IMA harvest | Other thoracic surgery |
| Study | Age (Years) | Sex (Men) | Chronic Obstructive Pulmonary Disease (COPD) | Previous Cardiac Surgery |
|---|---|---|---|---|
| Nataf [7] | ||||
| Ohtsuka [13] | 70.2 [47–89] | 13 (56.5) | ||
| Wolf [14] | 4 (8.7) | |||
| Duhaylongsod [9] | 61.7 [38–89] | 170 (78.0) | 4 (1.8) | |
| Ohtsuka [15] | 71.5 ± 6.5 | 18 (81.8) | ||
| Miyaji [16] | 64.0 ± 12.1 | 43 (58.9) | 11 (10.0) | 7 (7.7) |
| Massetti [17] | 67.0 ± 10.0 | 18 (60.0) | ||
| Ohtsuka [18] | 69.5 ± 11.5 | 32 (84.2) | ||
| Vassiliades [19] | 69.8 [28–85] | 191 (63.7) | 11 (3.7) | |
| Vassiliades [20] | 56.0 ± 11.0 | 219 (62.6) | ||
| Vassiliades [21] | 58.9 ± 13.1 | 12 (66.7) | ||
| Vassiliades [11] | 64.5 | 345 (67.7) | 136 (26.8) | |
| Kiaii [22] | 56.9 ± 11.2 | 44 (88.0) | ||
| Akca [8] | 66.0 ± 9 | 64 (79.0) | ||
| Jung [23] | 70.0 [30–86] | 27 (67.5) | 3 (7.7) | |
| Alaj [24] | 65.1 ± 10.1 | 79 (86.8) | 9 (9.9) | 0 |
| Sampon [10] | 64.0 [58–70] | 111 (81.6) | 3 (2.2) |
| Study | Tool | Skeletonized/Pedicled | LIMA Time (min) | LIMA Flow (mL/min) | Right Internal Mammary Artery (RIMA) Time (min) | RIMA Flow | Total Operation Time | Conversion Rates | Injury to LIMA |
|---|---|---|---|---|---|---|---|---|---|
| Nataf [7] | EC | Pedicled | 58.7 [20–130] | 0 | 0 | ||||
| Ohtsuka [13] | HS | Pedicled | 42 [28–48] | 28 | 0 | 0 | |||
| Wolf [14] | HS | Pedicled | 65 [35–95] | 20–110 | 37 [25–45] | 0 | 1 (2.2) | ||
| Duhaylongsod [9] | EC | Pedicled | 48 [29–95] | 29 [25–45] | 18 (8.3) | 4 (1.8) | |||
| Ohtsuka [15] | HS | Pedicled | 44 ± 12 | 0 | |||||
| Miyaji [16] | HS | Pedicled | 31.2 ± 12.4 | ||||||
| Massetti [17] | EC | Pedicled | 90 | ||||||
| Ohtsuka [18] | HS | Pedicled | 40.8 ± 12.2 | 33.5 ± 8.5 | 0 | ||||
| Vassiliades [19] | EC | Pedicled | 24.4 [17–61] | 96.4 [54–154] | 0 | 2 (0.7) | |||
| Vassiliades [20] | EC | Pedicled | 37.6 ± 12 | 37.6 ± 12 | 126 ± 36 | 9 (2.6) | |||
| Vassiliades [21] | EC | Pedicled | 52.3 ± 17.5 | 61.1 ± 13.0 | 35.6 ± 6.7 | 56.4 ± 14.1 | 211 ± 14 | 0 | 0 |
| Vassiliades [11] | EC | Pedicled | 20 (3.8) | 0 | |||||
| Kiaii [22] | HS | Pedicled | 63.3 ± 20.3 | 33.7 (19.3) | [35–95] | 0 | 0 | ||
| Akca [8] | Ligasure | Skeletonized | 58 ± 19 | 41 ± 25 | 150 ± 39 | 0 | 0 | ||
| Jung [23] | HS | Skeletonized | 87 [25–164] | 22 [5–73] | 24 [19–50] | 22.3 [17–30] | 0 | ||
| Alaj [24] | EC | Pedicled | 156 ± 48 | 0 | |||||
| Sampon [10] | Ligasure | Skeletonized | 48 [37–61] | 125 [104–150] | 1 (0.7) |
| Study | Intensive Care Unit (ICU) Stay (Hours) | Total Hospital Stay (Days) | Transfusion | Atrial Fibrillation (AF) de Novo | 30-Day Mortality | Myocardial Infarction (MI) | Cerebrovascular Accident (CVA) | Wound Reintervention | Pneumonia | Phrenic Nerve Injury |
|---|---|---|---|---|---|---|---|---|---|---|
| Nataf [7] | ||||||||||
| Ohtsuka [13] | 0 | |||||||||
| Wolf [14] | 1 (2.2) | 1 (2.2) | ||||||||
| Duhaylongsod [9] | 5 (2.3) | 1 (0.5) | 6 (2.8) | 1 (0.5) | ||||||
| Ohtsuka [15] | ||||||||||
| Miyaji [16] | 29.0 ± 20.5 | 4.2 ± 2.1 | 2 (2.9) | 2 (2.9) | 2 (2.9) | |||||
| Massetti [17] | ||||||||||
| Ohtsuka [18] | ||||||||||
| Vassiliades [19] | 11.9 | 2.4 | 32 (10.5) | 65 (21.6) | 1 (0.3) | 3 (0.7) | 3 (0.7) | 4 (1.3) | 43 (14.3) | |
| Vassiliades [20] | 5.23 ± 4.33 | 2.3 ± 1.2 | 30 (11.7) | 4 (1.4) | 8 (2.3) | 6 (1.7) | 4 (1.3) | |||
| Vassiliades [21] | 6.9 ± 4.5 | 2.3 ± 0.3 | 0 | |||||||
| Vassiliades [11] | 40.8 | 5.25 | 77 (15.0) | 102 (20.0) | 0 | 0 | 0 | 0 | ||
| Kiaii [22] | 17.8 ± 8.5 | 2 (4.0) | 0 | 0 | 0 | |||||
| Akca [8] | ||||||||||
| Jung [23] | 23.0 | 6 [3–22] | 1 (2.5) | 0 | 2 (5.0) | 1 (2.5) | ||||
| Alaj [24] | 36.0 ± 38.4 | 0 | 1 (1.0) | 0 | 2 (2.2) | |||||
| Sampon [10] | 12 [12–24] | 3.0 [3.0–4.0] | 3 (2.2) | 10 (7.4) | 1 (0.7) | 1 (0.7) | 1 (0.7) | 0 | 1 (0.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Görtzen, D.Q.; Sampon, F.; Ter Woorst, J.; Akca, F. Non-Robotic Endoscopic-Assisted Internal Mammary Artery Harvest—A Historical Review and Recent Advancements. J. Cardiovasc. Dev. Dis. 2025, 12, 68. https://doi.org/10.3390/jcdd12020068
Görtzen DQ, Sampon F, Ter Woorst J, Akca F. Non-Robotic Endoscopic-Assisted Internal Mammary Artery Harvest—A Historical Review and Recent Advancements. Journal of Cardiovascular Development and Disease. 2025; 12(2):68. https://doi.org/10.3390/jcdd12020068
Chicago/Turabian StyleGörtzen, De Qing, Fleur Sampon, Joost Ter Woorst, and Ferdi Akca. 2025. "Non-Robotic Endoscopic-Assisted Internal Mammary Artery Harvest—A Historical Review and Recent Advancements" Journal of Cardiovascular Development and Disease 12, no. 2: 68. https://doi.org/10.3390/jcdd12020068
APA StyleGörtzen, D. Q., Sampon, F., Ter Woorst, J., & Akca, F. (2025). Non-Robotic Endoscopic-Assisted Internal Mammary Artery Harvest—A Historical Review and Recent Advancements. Journal of Cardiovascular Development and Disease, 12(2), 68. https://doi.org/10.3390/jcdd12020068







