Mitral Annular Calcification, a Not So Marginal and Relatively Benign Finding as Many of Us Think: A Review
Abstract
1. Introduction
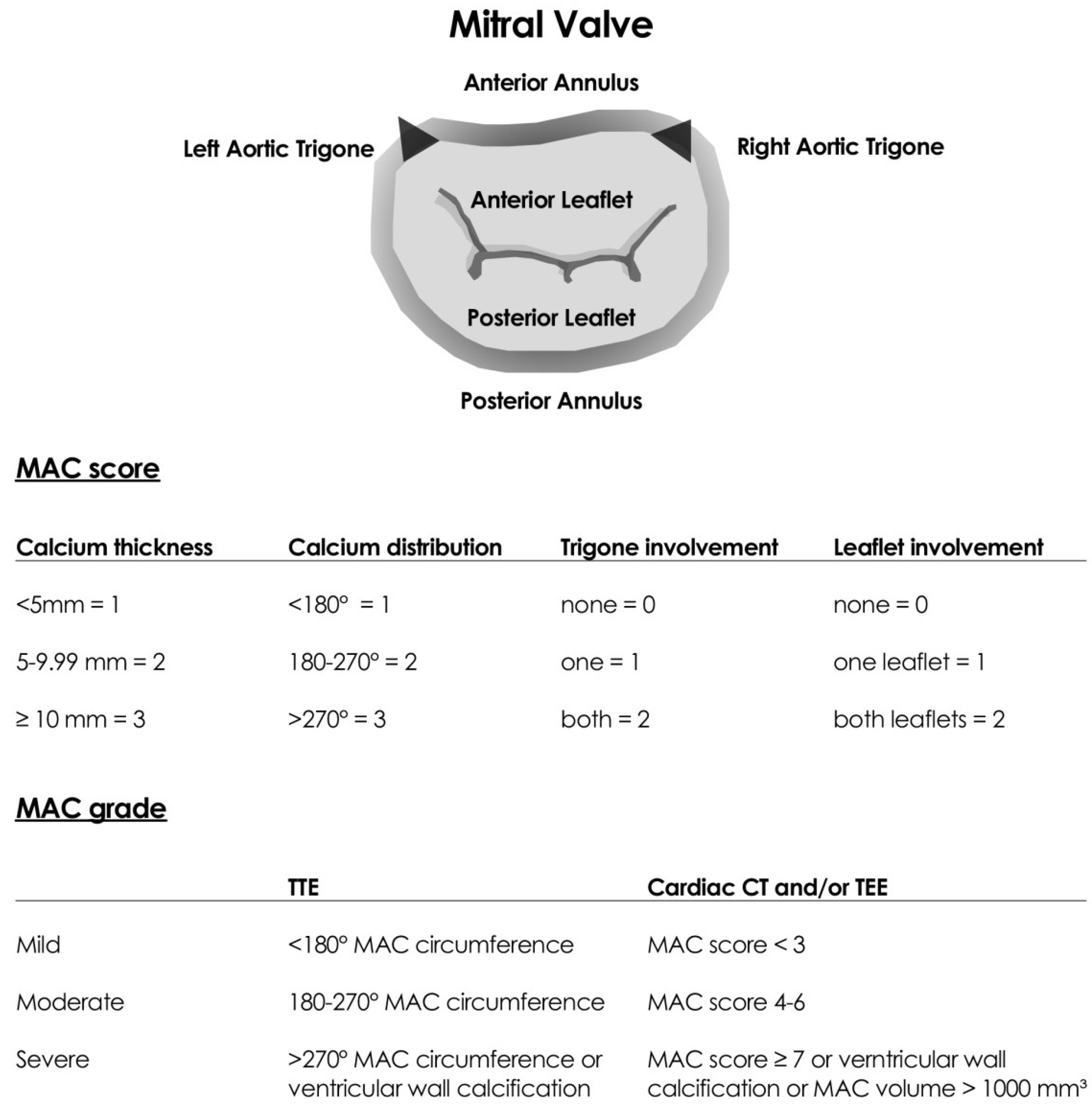
2. Definition
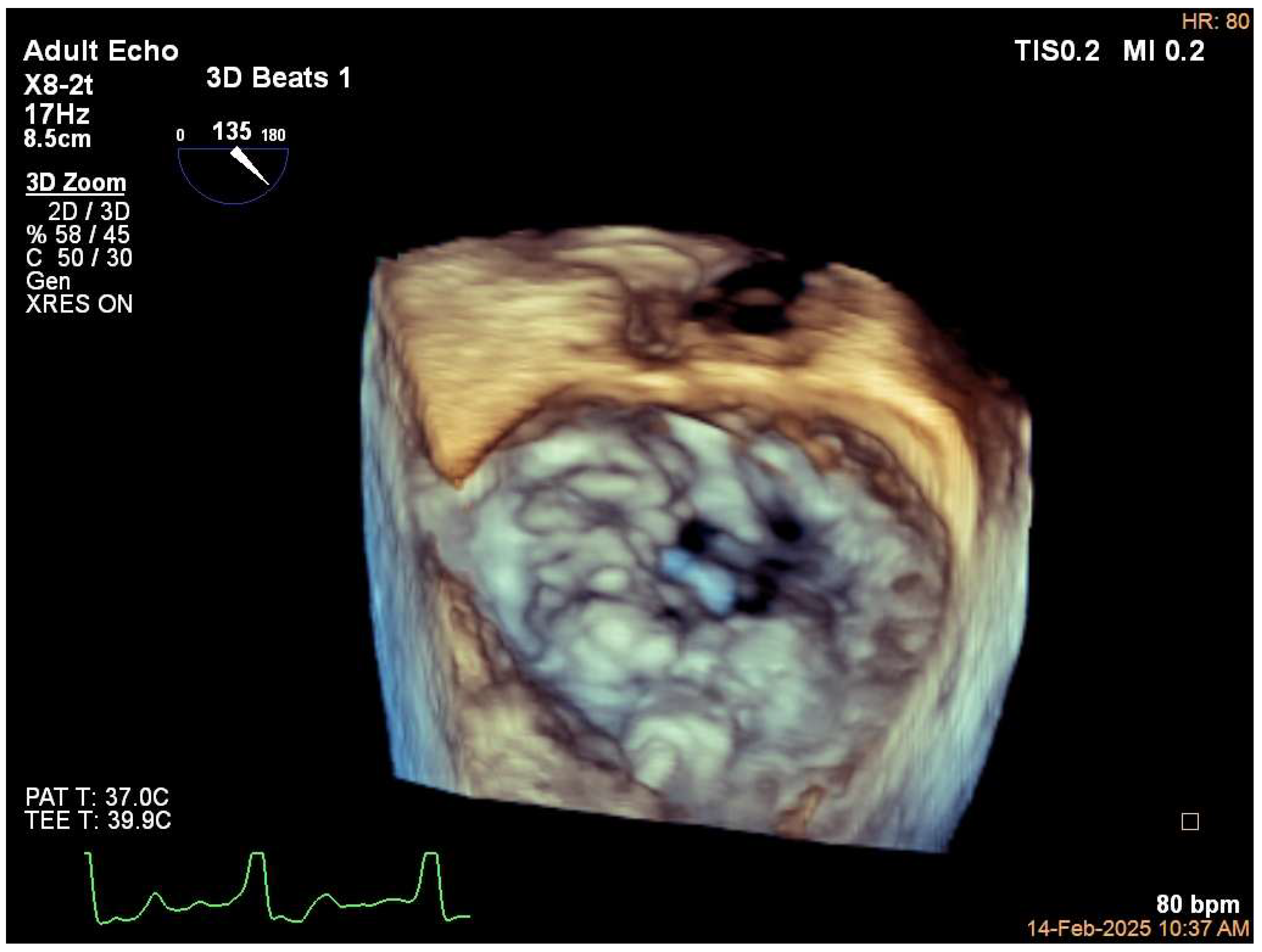
3. Diagnosis
4. Pathogenesis
5. Clinical Implications
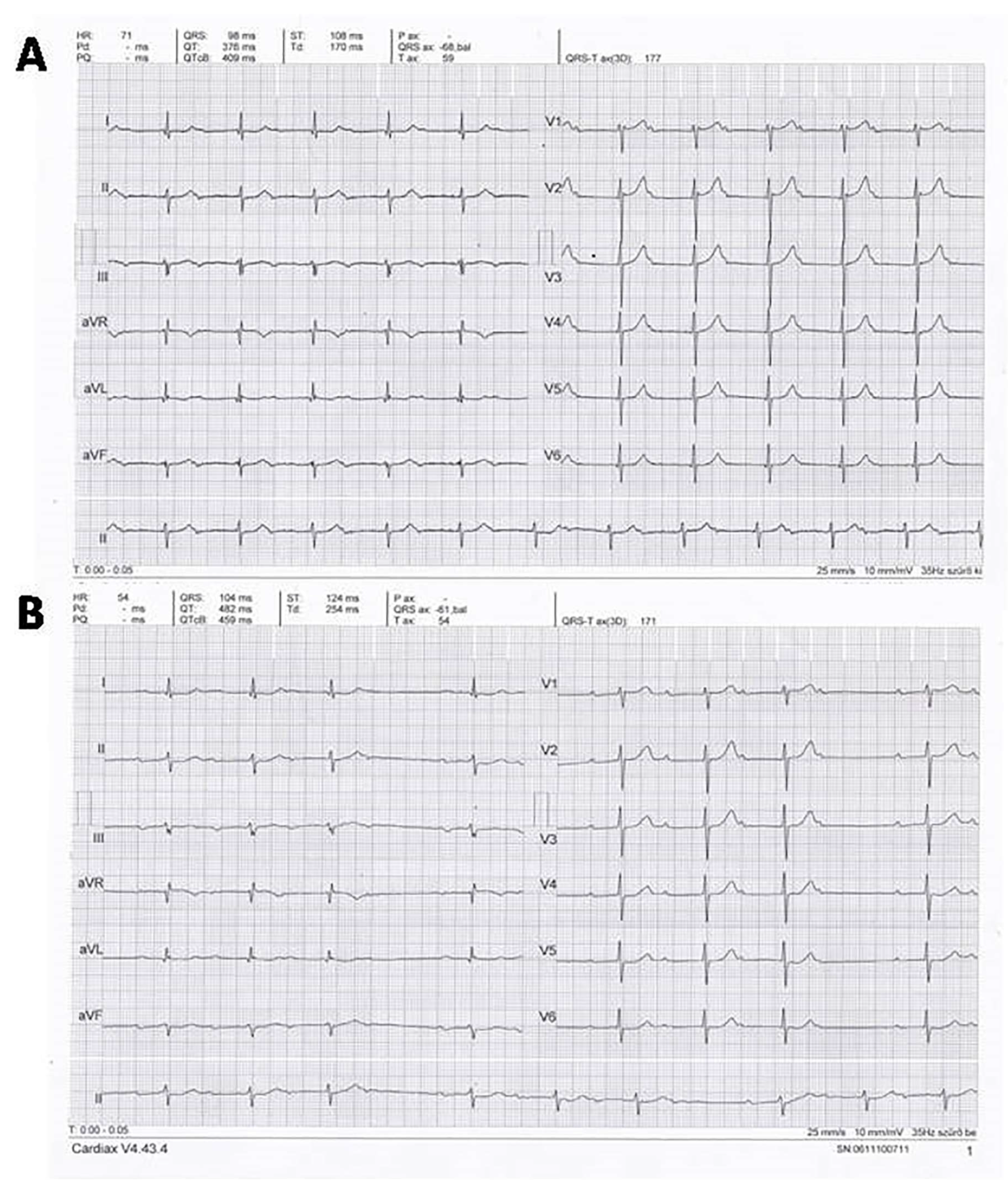
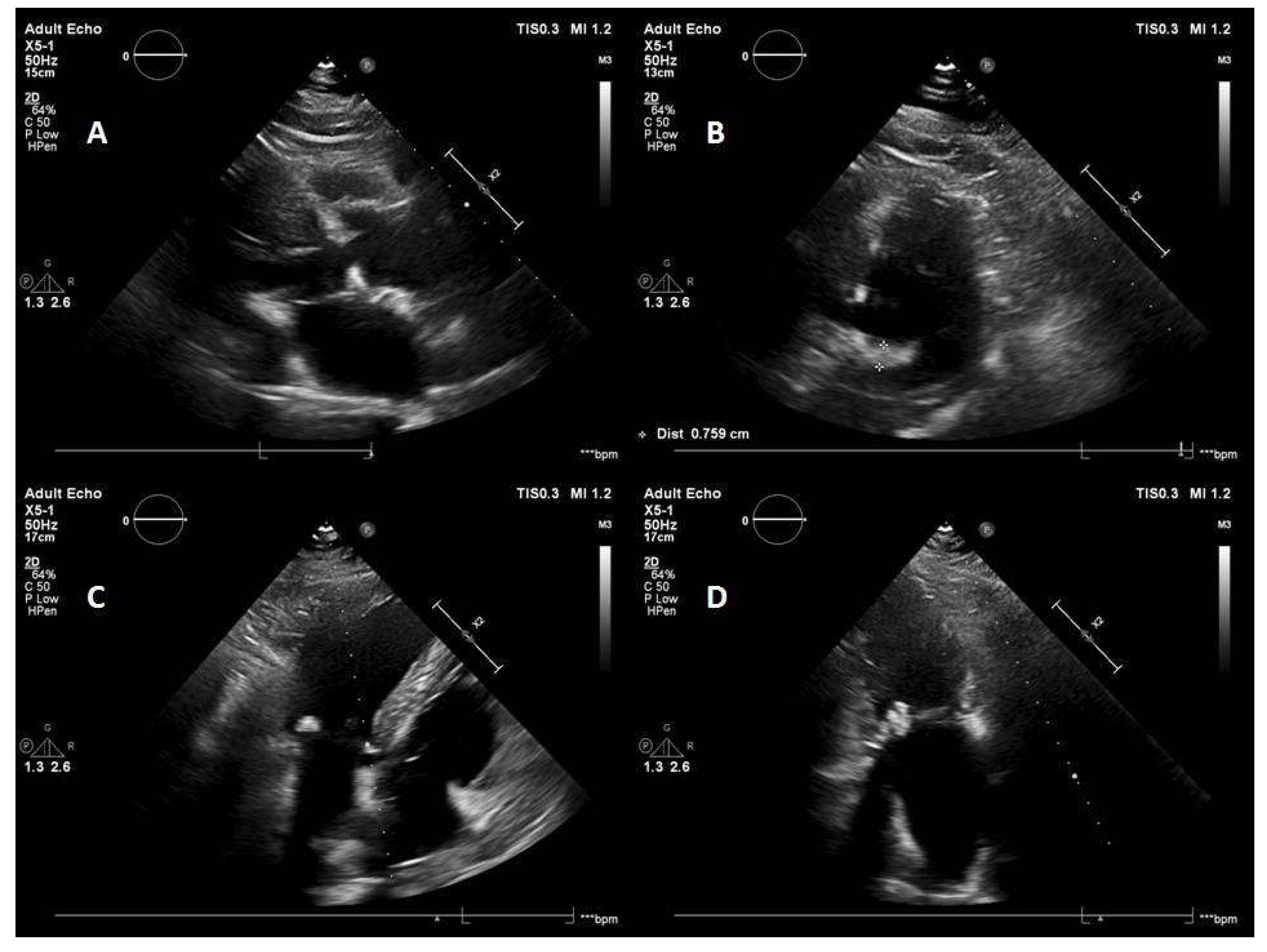
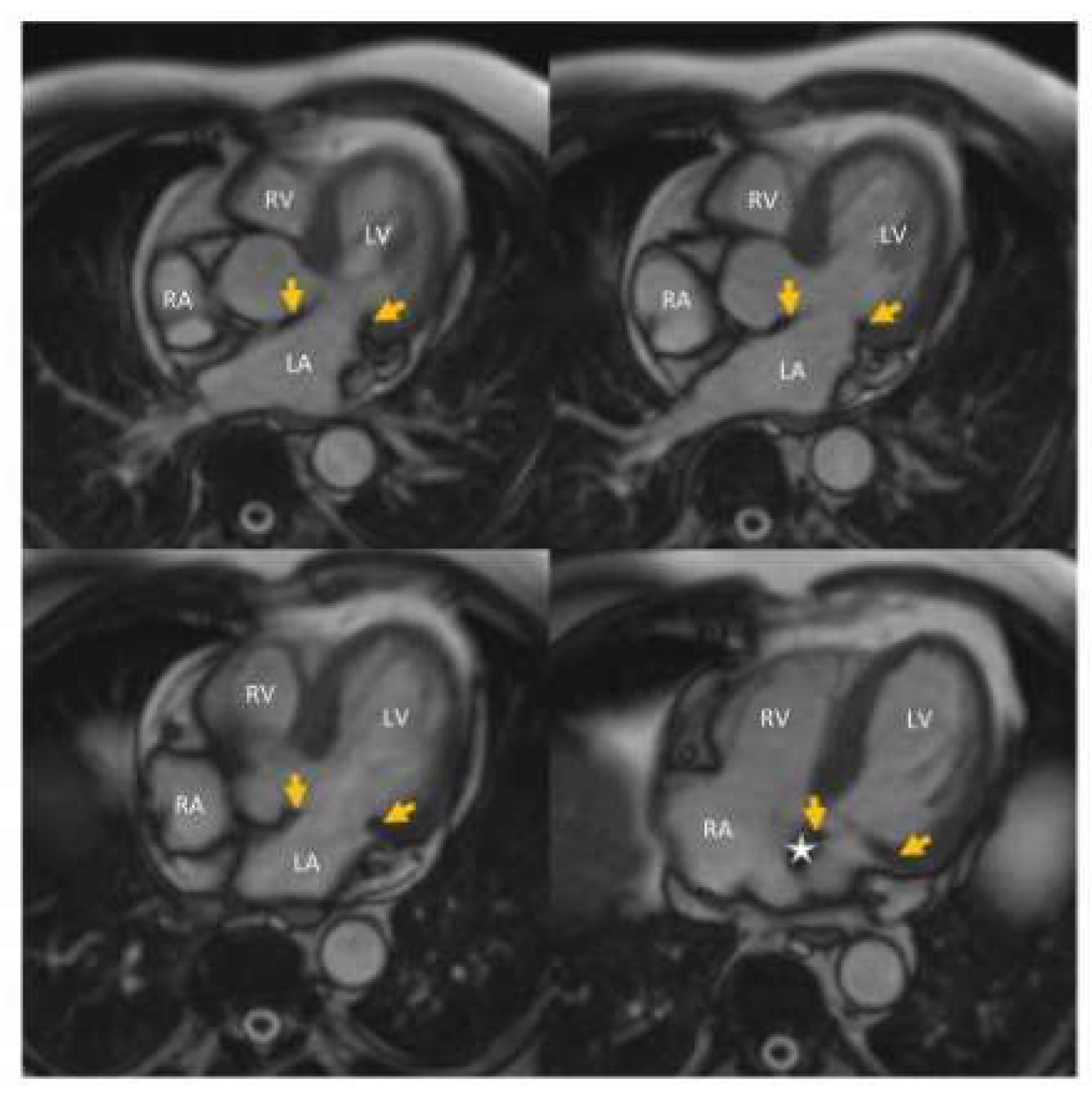
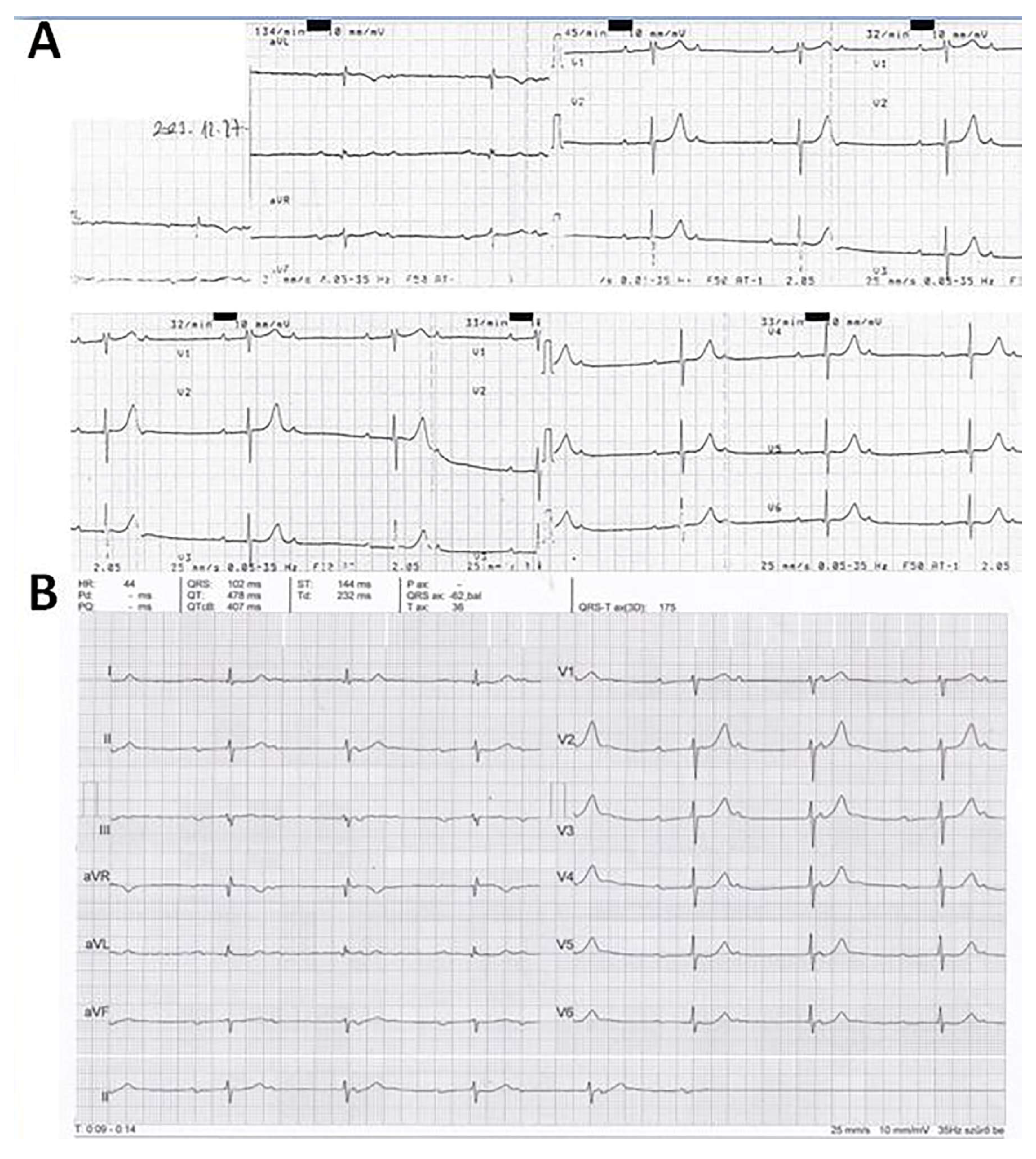
5.1. Case
5.2. Mitral Valve Dysfunction
5.3. Other Cardiovascular Disease, Mortality
5.4. The Effect of MAC on Transcatheter and Surgical Cardiac Interventions
5.5. Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massera, D.; Kizer, J.R.; Dweck, M.R. Mechanisms of mitral annular calcification. Trends Cardiovasc. Med. 2020, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, Y.; Jilaihawi, H.; Chakravarty, T.; Mack, M.J.; Makkar, R.R. Mitral annulus calcification. J. Am. Coll. Cardiol. 2015, 66, 1934–1941. [Google Scholar] [CrossRef]
- Rasmussen, J.H.; Fredgart, M.H.; Linholt, J.S.; Johansen, J.B.; Sangaard, N.; Yousef, A.H.; Hasific, S.; Sonderskov, P.; Steffensen, F.H.; Frost, L.; et al. Mitral annulus calcification and cardiac conduction disturbances: A DANCAVAS Sub-study. J. Cardiovasc. Imaging 2022, 30, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.A.; Cheung, P.; Criqui, M.H.; Langer, R.D.; Wright, C.M. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation 2006, 113, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Asselbergs, F.W.; Mozaffarian, D.; Katz, R.; Kestenbaum, B.; Fried, L.F.; Gottdiener, J.S.; Shlipak, M.G.; Siscovick, D.S. Association of renal function with cardiac calcifications in older adults: The cardiovascular health study. Nephrol. Dial. Transplant. 2009, 24, 834–840. [Google Scholar] [CrossRef]
- Kanjanauthai, S.; Nasir, K.; Katz, R.; Rivera, J.J.; Takasu, J.; Blumenthal, R.S.; Eng, J.; Budoff, M.J. Relationships of mitral annular calcification to cardiovascular risk factors: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2010, 213, 558–562. [Google Scholar] [CrossRef]
- Silbiger, J.J. Anatomy, mechanics and pathophysiology of the mitral annulus. Am. Heart J. 2012, 164, 163–176. [Google Scholar] [CrossRef]
- Oni, E.; Boakye, E.; Pressmann, G.S.; Dardari, Z.; Jha, K.; Szklo, M.; Budoff, M.; Nasir, K.; Hughes, T.M.; Blaha, M.J. The association of mitral annular calcification with cardiovascular and noncardiovascular outcomes: The Multi-Ethnic Study of Atherosclerosis. Am. J. Cardiol. 2024, 225, 75–83. [Google Scholar] [CrossRef]
- Nestico, P.F.; Depace, N.L.; Morganroth, J.; Kotler, M.N.; Ross, J. Mitral annular calcification: Clinical, pathophysiology, and echocardiographic review. Am. Heart J. 1984, 107, 989–996. [Google Scholar] [CrossRef]
- Mellino, M.; Salcedo, E.E.; Lever, H.M.; Vasudevan, G.; Kramer, J.R. Echocardiographic-quantified severity of mitral annulus calcification: Prognostic correlation to related hemodynamic, valvular rhythm and conduction abnormalities. Am. Heart J. 1982, 103, 222–225. [Google Scholar] [CrossRef]
- Guerrero, M.A.; Grayburn, P.; Smith, R.L.; Sorajja, P.; Wang, D.D.; Ahmad, Y.; Blusztein, D.; Cavalcante, J.; Tang, G.H.L.; Ailawadi, G.; et al. Diagnosis, classification and management stretegies for mitral annular calcification. A Heart Valve Collaboratory position statement. J. Am. Coll. Cardiol. Interv. 2023, 16, 2195–2210. [Google Scholar] [CrossRef] [PubMed]
- Barasch, E.; Gottdiener, J.S.; Larsen, E.K.M.; Chaves, P.H.M.; Newman, A.B.; Manolio, T.A. Clinical significance of calcification of the fibrous skeleton of the heart and atherosclerosis in community dwelling elderly. Tha Cardiovascular Health Study (CHS). Am. Heart J. 2006, 151, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chehab, O.; Roberts-Thomson, R.; Bivona, A.; Gill, H.; Patterson, T.; Pursnani, A.; Grigoryan, K.; Vargas, B.; Bokhary, U.; Blauth, C.; et al. Manegement of patients with severe mitral annular calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Budoff, M.J.; Delaney, J.A.C.; Hamirani, Y.; Eng, J.; Fuster, V.; Kronmal, R.A.; Halperin, J.L.; O’Brien, K.D. Risk factors associated with the incidence and progression of mitral annulus calcification: The multi-ethnic study of atherosclerosis. Am. Heart J. 2013, 166, 904–912. [Google Scholar] [CrossRef]
- Massera, D.; Xu, S.; Bartz, T.M.; Bortnick, A.E.; Ix, J.H.; Chonchol, M.; Owens, D.S.; Barasch, E.; Gardin, J.M.; Gottdiener, J.S.; et al. Relationship of bone mineral density with valvular and annular calcification in community-dwelling older people: The Cardiovascular Health Study. Arch. Osteoporos. 2017, 12, 52. [Google Scholar] [CrossRef]
- Smith, J.G.; Luk, K.; Schulz, C.-A.; Engert, J.C.; Do, R.; Hindy, G.; Rukh, G.; Dufresne, L.; Almgren, P.; Owens, D.S.; et al. Thanassoulis G for the Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) Extracoronary Calcium Working Group. Association of Low-Density Lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 2014, 312, 1764–1771. [Google Scholar]
- Afshar, M.; Luk, K.; Do, R.; Dufresne, L.; Owens, D.S.; Harris, T.B.; Pelosos, G.M.; Kerr, K.F.; Wong, Q.; Smith, A.V.; et al. Association of triglyceride-related genetic variants with mitral annular calcification. J. Am. Coll. Cardiol. 2017, 69, 2941–2948. [Google Scholar] [CrossRef]
- Fulkerson, P.K.; Beaver, B.M.; Auseon, J.C.; Graber, H.L. Calcification of the mitral annulus. Am. J. Med. 1979, 66, 967–977. [Google Scholar] [CrossRef]
- Carpentier, A.F.; Pellerin, M.; Fuzellier, J.-F.; Relland, J.Y.M. Extensive calcification of the mitral valve anulus: Pathology and surgical management. J. Thorac. Cardiovasc. Surg. 1996, 111, 718–730. [Google Scholar] [CrossRef]
- Payvandi, L.A.; Rigolin, V.H. Calcific mitral stenosis. Cardiol. Clin. 2013, 31, 193–202. [Google Scholar] [CrossRef]
- Lewis, J.F.; Maron, B.J. Elderly patients with hypertrophic cardiomyopathy: A subset with distinctive left ventricular morphology and progressive clinical course late in life. J. Am. Coll. Cardiol. 1989, 13, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Vasan, R.S.; Parise, H.; Levy, D.; O’Donnell, C.J.; D’Agostino, R.B.; Benjamin, E.J. Mitral annular calcification predicts cardiovascular morbidity and mortality. The Framingham Heart Study. Circulation 2003, 107, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Kizer, J.R.; Wiebers, D.O.; Whisnant, J.P.; Galloway, J.M.; Welty, T.K.; Lee, E.T.; Best, L.G.; Resnick, H.E.; Roman, M.I.; Devereux, R.B. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease. The Strong Heart Study. Stroke 2005, 36, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Durst, R.; Picard, M.H.; Cury, R.C. Progression of mitral annulus calcification to caseous necrosis of the mitral valve: Complementary role of multi-modality imaging. Eur. Heart J. 2009, 30, 304. [Google Scholar] [CrossRef]
- Nair, C.K.; Runco, V.; Everson, G.T.; Boghiari, A.; Mooss, A.N.; Mohiuddin, S.M.; Sketch, M.H. Conduction defects and mitral annulus calcification. Br. Heart J. 1980, 44, 162–167. [Google Scholar] [CrossRef]
- Nair, C.K.; Sketch, M.H.; Desai, R.; Mohiuddin, S.M.; Runco, V. High prevalence of symptomatic bradyarrhythmias due to atrioventricular node-fascicular and sinus node-atrial disease in patients with mitral anular calcification. Am. Heart J. 1982, 103, 226–229. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Efird, J.T.; Nazarian, S.; Alonso, A.; Michos, E.D.; Szklo, M.; Heckbert, S.R.; Soliman, E.Z. Mitral annular calcification progression and the risk of atrial fibrillation: Results from MESA. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 279–284. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Efird, J.T.; Nazarian, S.; Alonso, A.; Heckberg, S.R.; Soliman, E.Z. Mitral annular calcification and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. Europace 2015, 17, 358–363. [Google Scholar] [CrossRef]
- Pressmann, G.S.; Rodriguez-Ziccardi, M.; Gartman, C.H.; Obasare, E.; Melendres, E.; Arguello, V.; Bhalla, V. Mitral annular calcification as a possible nidus for endocarditis: A descriptive series with bacteriological differences noted. J. Am. Soc. Echocardiogr. 2017, 30, 572–578. [Google Scholar] [CrossRef]
- Abramowitz, Y.; Kazuno, Y.; Chakravarty, T.; Kawamori, H.; Maeno, Y.; Anderson, D.; Allison, Z.; Mangat, G.; Cheng, W.; Gopal, A.; et al. Concomitant mitral annular calcification and severe aortic stenosis: Prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 1194–1203. [Google Scholar] [CrossRef]
- Boerlage-Van Dijk, K.; Kooiman, K.M.; Yong, Z.Y.; Wiegerinck, E.M.A.; Damman, P.; Bouma, B.J.; Tijssen, J.G.P.; Piek, J.J.; Knops, R.E.; Baan, J. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin. Electrophysiol. 2014, 37, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Durst, R.; Avelar, E.; McCarty, D.; Poh, K.-K.; Friera, L.F.; Liano, M.F.; Chu, J.; Anumandla, A.K.R.; Rodriguez, L.L.; Mack, M.J.; et al. Outcome an improvement predictors of mitral regirgitation after transcatheter aortic valve implantation. J. Heart Valve Dis. 2011, 20, 272–281. [Google Scholar] [PubMed]
- Brodov, Y.; Konen, E.; Di Segni, M.; Samoocha, D.; Chernomordik, F.; Barbash, I.; Regev, E.; Raanani, E.; Guetta, V.; Segev, A.; et al. Mitral annulus calcium score. An independent predictor of new conduction system abnormalities in patients after transcatheter aortic valve implantation. Circ. Cardiovasc. Imaging 2019, 12, e007508. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y. Surgical management of mitral annular calcification. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 619–625. [Google Scholar] [CrossRef]
- Demal, T.J.; Conradi, L. Management of valve dysfunction in patients with mitral annular calcification. Heart 2023, 109, 1639–1646. [Google Scholar] [CrossRef]
- Lim, D.S.; Herrmann, H.C.; Grayburn, P.; Koulogiannis, K.; Allawadi, G.; Williams, M.; Ng, V.G.; Chau, K.H.; Sorajja, P.; Smith, I.I.R.L.; et al. Consensus document on non-suitability for transcatheter mitral valve repair by edge-to-edge therapy. Struct. Heart 2021, 5, 227–233. [Google Scholar] [CrossRef]
- Cheng, R.; Tat, E.; Siegel, R.J.; Arsanjani, R.; Hussaini, A.; Makar, M.; Mizutani, Y.; Trento, A.; Kar, S. Mitral annular calcification is not associated with decreased procedural success, durability of repair, or left ventricular remodelling in percutaneous edge-to-edge repair of mitral regurgitation. Eurointervention 2016, 12, 1176–1184. [Google Scholar] [CrossRef]
- Fernández-Peregrina, E.; Pascual, I.; Freixa, X.; Tirado-Conte, G.; Estévez-Loureiro, R.; Carrasco-Chinchilla, F.; Benito-González, T.; Asmarats, L.; Sanchís, L.; Jiménez-Quevedo, P.; et al. Transcatheter edge-to-edge mitral valve repair in patients with mitral annulus calcification. Eurointervention 2022, 17, 1300–1309. [Google Scholar] [CrossRef]
- Thaden, J.J.; Wiley, B.M.; Pollak, P.M.; Rihal, C.S. Interventional echocardiogrphy. In The Echo Manual, 4th ed.; Oh, J.K., Kane, G.C., Seward, J.B., Tajik, A.J., Eds.; Wolters Kluwer: Lieaufen, The Netherlands, 2019; pp. 567–593. [Google Scholar]
- Tom, S.K.; Kaira, K.; Perdoncin, E.; Tully, A.; Devireddy, C.M.; Inci, E.; Greenbaum, A.; Grubb, K.J. Transcatheter treatment options for functional mitral regurgitation: Which device for which patients? Interv. Cardiol. 2024, 19, e10. [Google Scholar] [CrossRef]
- Tagliari, A.P.; Saadi, R.P.; Tang, G.H.L. A deep dive into the percutaneous mitral valve data. Curr. Opin. Cardiol. 2024, 39, 226–233. [Google Scholar] [CrossRef]
- Churchill, T.W.; Yucel, E.; Deferm, S.; Levine, R.A.; Hung, J.; Bertrand, P.B. Mitral valve dysfunction in patients with annular calcification. J. Am. Coll. Cardiol. 2022, 80, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Hafiane, A.; Pisaturo, A.; Favari, E.; Bortnick, A.E. Atherosclerosis, calcific aortic valve disease and mitral annular calcification: Same or different? Int. J. Cardiol. 2025, 420, 132741. [Google Scholar] [CrossRef] [PubMed]
- Bortnick, A.E.; Bartz, T.M.; Ix, J.H.; Chonchol, M.; Reiner, A.; Cushman, M.; Owens, D.; Barasch, E.; Siscovick, D.S.; Gottdiener, J.S.; et al. Association of inflammatory, lipid and mineral markers with cardiac calcification in older adults. Heart 2016, 102, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E.; Kim, K.H.; Han, J.Y.; Bae, H.R.; Rha, S.H.; Park, Y. Omega-3 fatty acid supplementation increases 1,25-dihydroxyvitamin D and fetiun A levels in dialysis patients. Nutr. Res. 2012, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereckei, A.; Jenei, Z.; Vágó, H.; Balla, D.; Panajotu, A.; Nagy, A.; Katona, G. Mitral Annular Calcification, a Not So Marginal and Relatively Benign Finding as Many of Us Think: A Review. J. Cardiovasc. Dev. Dis. 2025, 12, 233. https://doi.org/10.3390/jcdd12060233
Vereckei A, Jenei Z, Vágó H, Balla D, Panajotu A, Nagy A, Katona G. Mitral Annular Calcification, a Not So Marginal and Relatively Benign Finding as Many of Us Think: A Review. Journal of Cardiovascular Development and Disease. 2025; 12(6):233. https://doi.org/10.3390/jcdd12060233
Chicago/Turabian StyleVereckei, András, Zsigmond Jenei, Hajnalka Vágó, Dorottya Balla, Alexisz Panajotu, Andrea Nagy, and Gábor Katona. 2025. "Mitral Annular Calcification, a Not So Marginal and Relatively Benign Finding as Many of Us Think: A Review" Journal of Cardiovascular Development and Disease 12, no. 6: 233. https://doi.org/10.3390/jcdd12060233
APA StyleVereckei, A., Jenei, Z., Vágó, H., Balla, D., Panajotu, A., Nagy, A., & Katona, G. (2025). Mitral Annular Calcification, a Not So Marginal and Relatively Benign Finding as Many of Us Think: A Review. Journal of Cardiovascular Development and Disease, 12(6), 233. https://doi.org/10.3390/jcdd12060233





