Specific Premature Ventricular Complex Characteristics in Women: Insights from a Patient Cohort
Abstract
1. Introduction
2. Methods
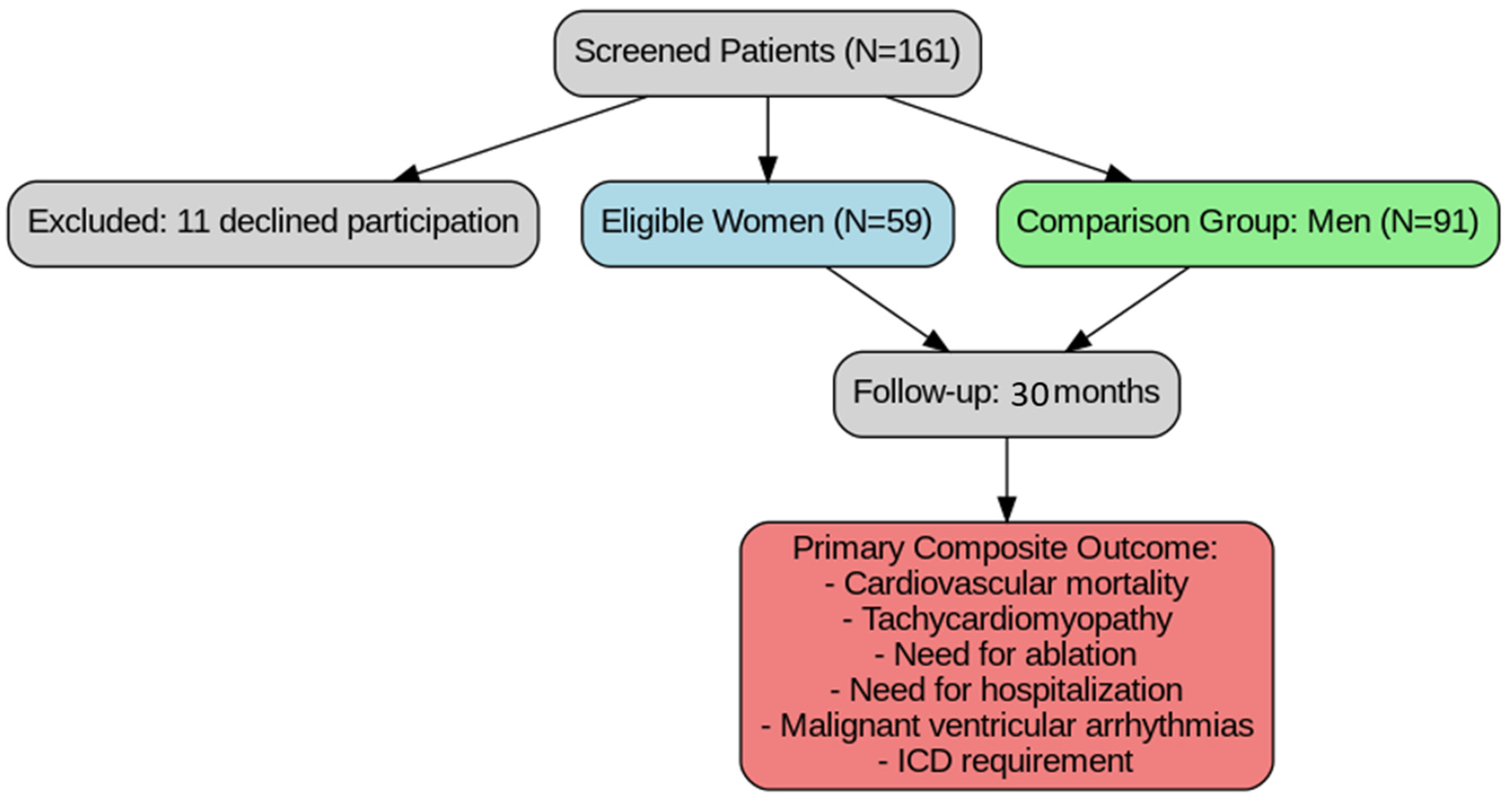
2.1. Patients
2.2. Study Design
2.3. Data Collection and Variables
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Electrocardiographic and PVC Characteristics
3.3. TTE and CMR Characteristics of the Study Population
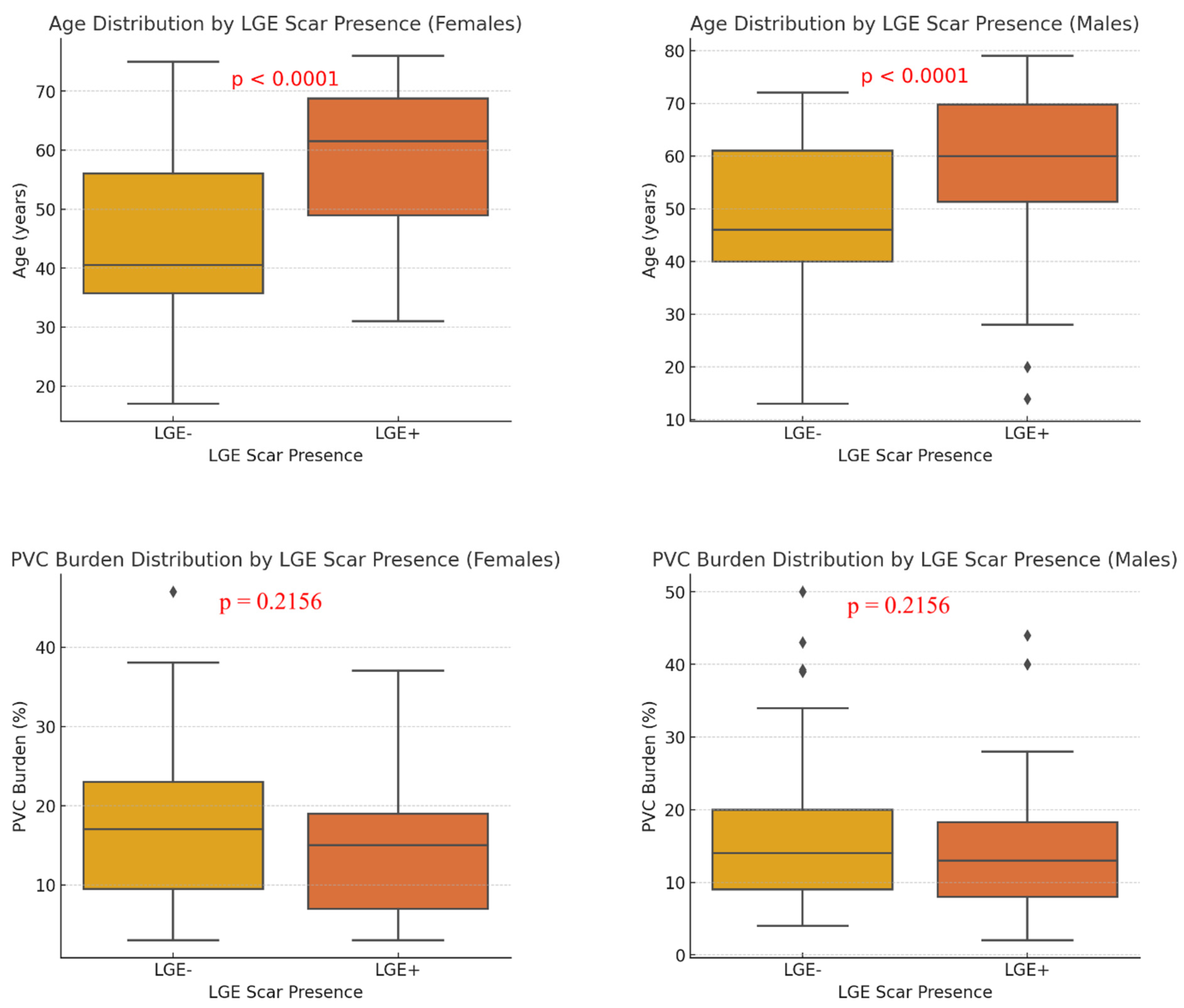
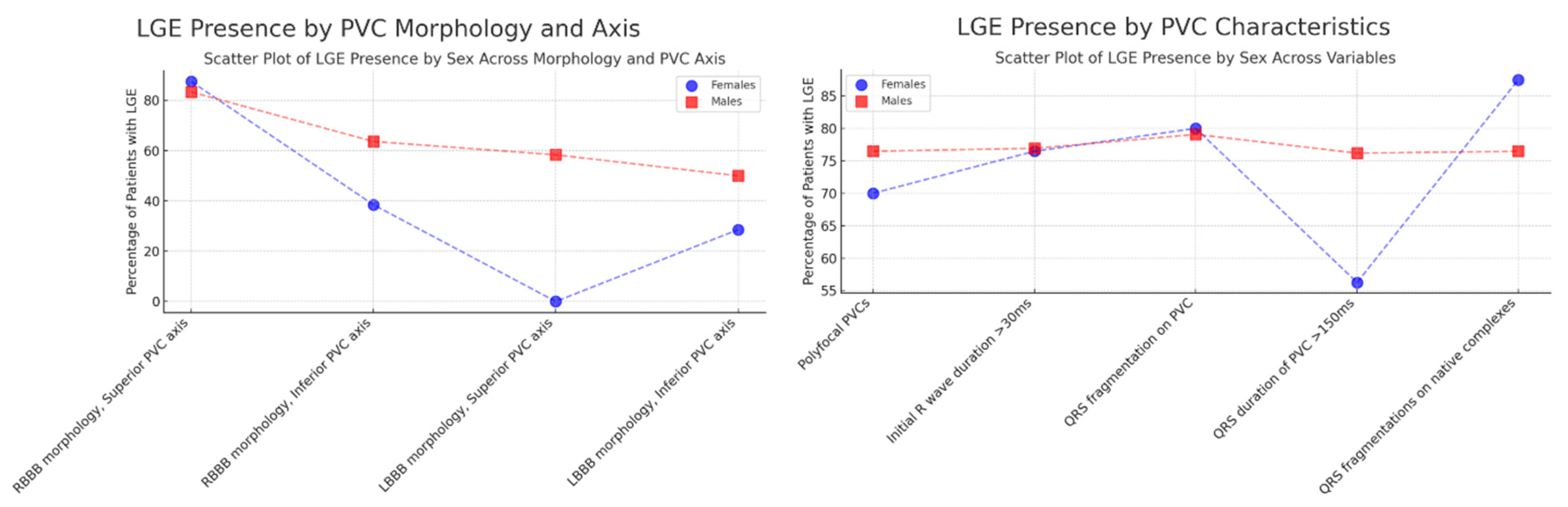
3.4. Correlation of PVC Morphology, Clinical Characteristics, and CMR Scar Presence
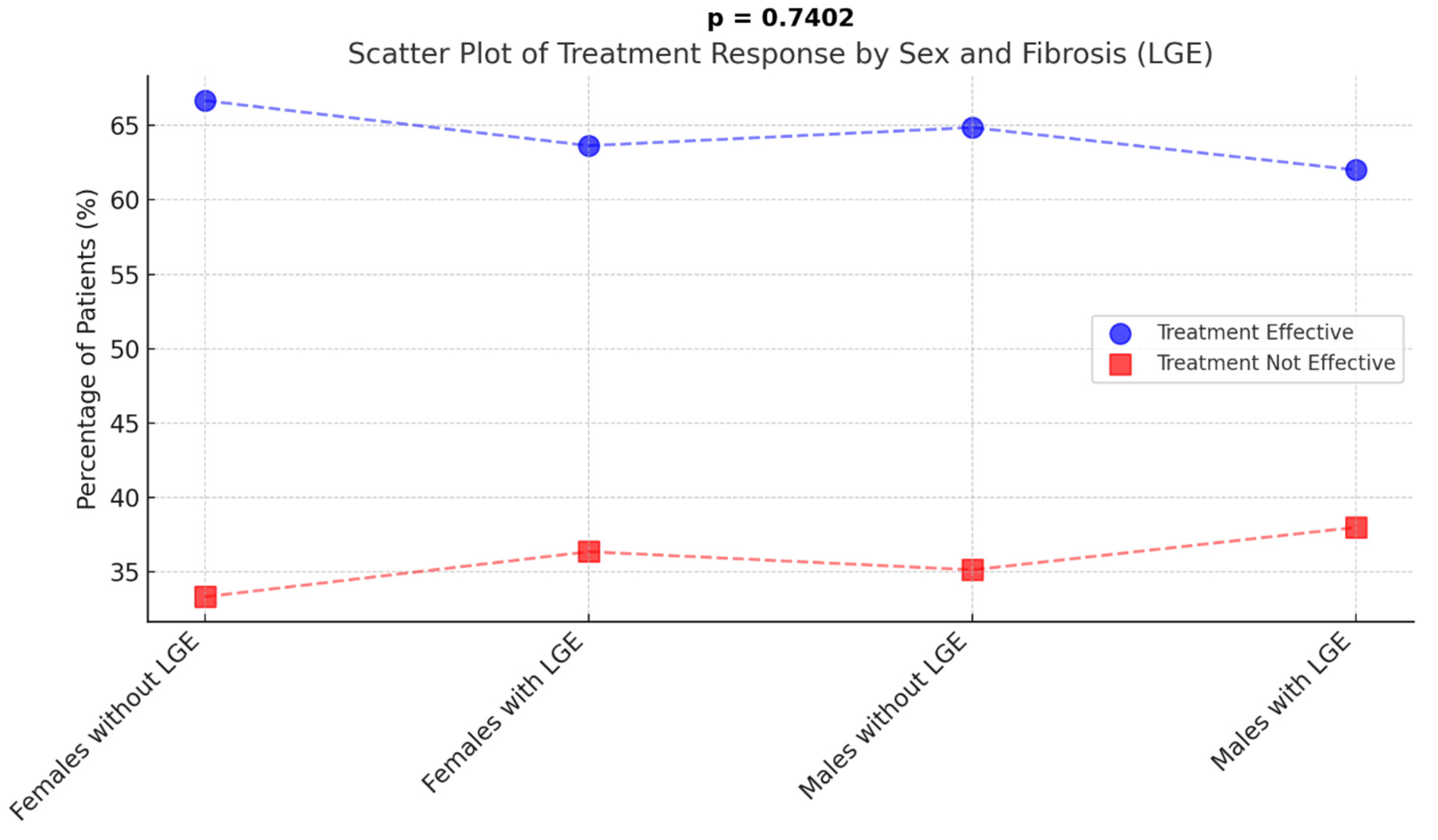
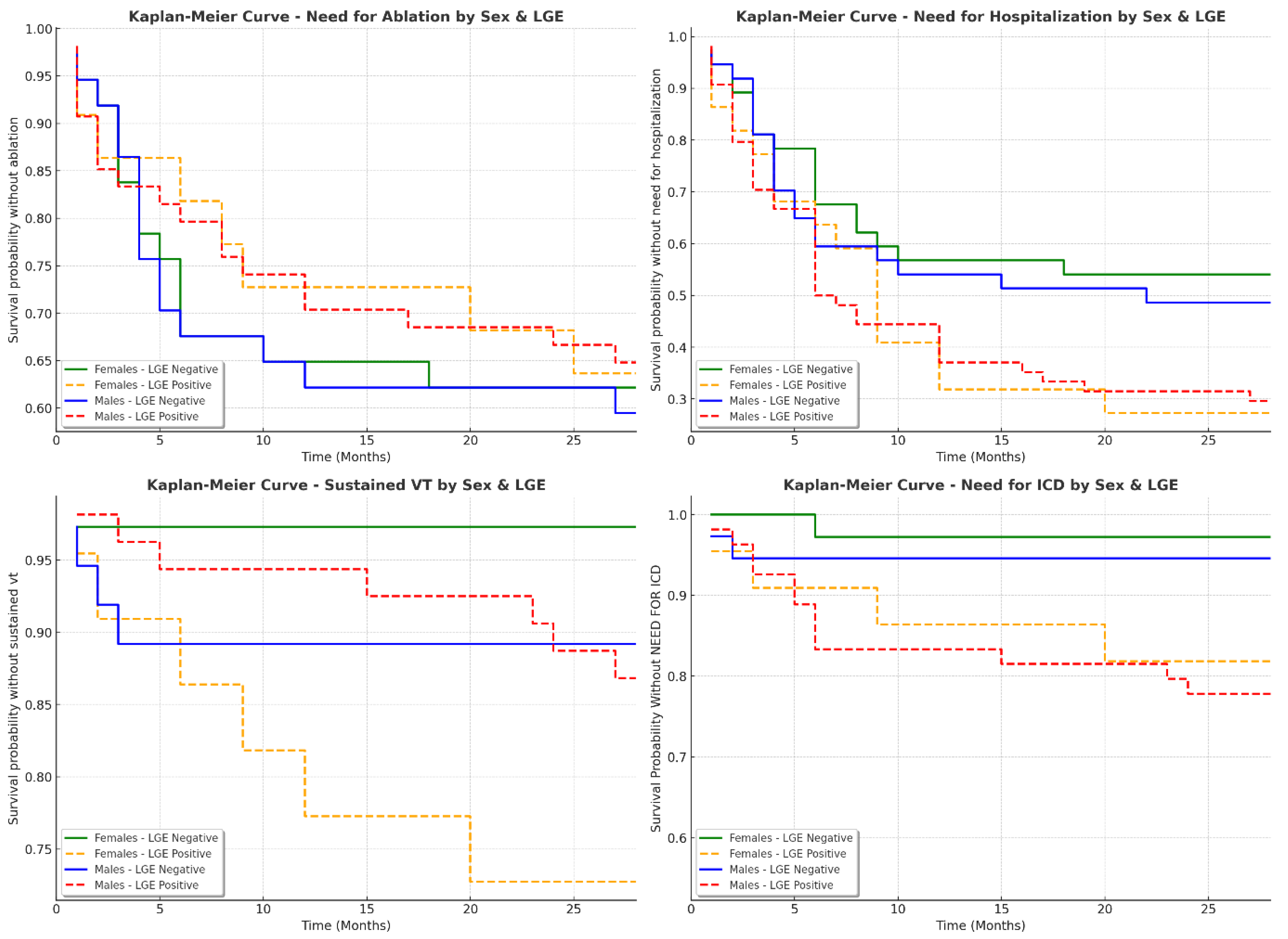
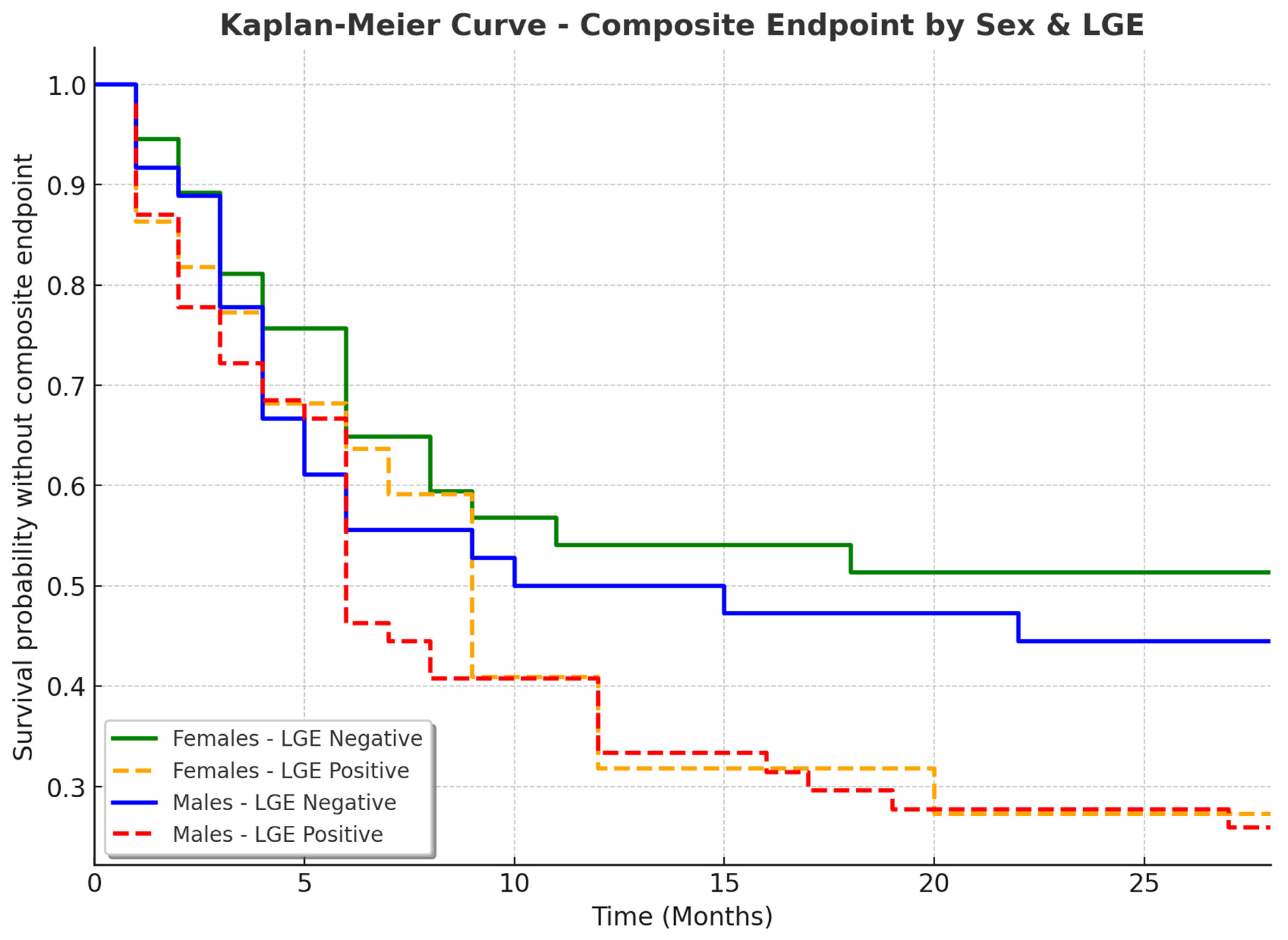
3.5. Sex-Specific Clinical Outcomes in Patients with and Without LGE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CMR | cardiac magnetic resonance |
| ECG | electrocardiogram |
| EF | ejection fraction |
| EDDLV | end-diastolic diameter of the left ventricle |
| EDV RV | end-diastolic volume of the right ventricle |
| ESDLV | end-systolic diameter of the left ventricle |
| FAC | fractional area change |
| GLS | global longitudinal strain |
| IC AADs | class IC antiarrhythmic drugs |
| ICD | implantable cardioverter-defibrillator |
| IOTVAs | idiopathic outflow tract ventricular arrhythmias |
| IVS | interventricular septum |
| LA | left atrium |
| LBBB | left bundle branch block |
| LGE | late gadolinium enhancement |
| LVEF | left ventricular ejection fraction |
| LWT | lateral wall thickness |
| PIC | premature ventricular contraction-induced cardiomyopathy |
| PVCs | premature ventricular contractions |
| RA | right atrium |
| RBBB | right bundle branch block |
| RVEF | right ventricular ejection fraction |
| RV FAC | right ventricular fractional area change |
| SCD | sudden cardiac death |
| TTE | transthoracic echocardiography |
| VT | ventricular tachycardia |
References
- Sciomer, S.; Moscucci, F.; Dessalvi, C.C.; Deidda, M.; Mercuro, G. Gender differences in cardiology: Is it time for new guidelines? J. Cardiovasc. Med. 2018, 19, 685–688. [Google Scholar] [CrossRef]
- Yoder, M.; Dils, A.; Chakrabarti, A.; Driesenga, S.; Alaka, A.; Ghannam, M.; Bogun, F.; Liang, J.J. Gender and race-related disparities in the management of ventricular arrhythmias. Trends Cardiovasc. Med. 2024, 34, 381–386. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar]
- Hosseini, F.; Thibert, M.J.; Gulsin, G.S.; Murphy, D.; Alexander, G.; Andrade, J.G.; Hawkins, N.M.; Laksman, Z.W.; Yeung-Lai-Wah, J.A.; Chakrabarti, S.; et al. Cardiac Magnetic Resonance in the Evaluation of Patients with Frequent Premature Ventricular Complexes. JACC Clin. Electrophysiol. 2022, 8, 1122–1132. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P.; Selvanayagam, J.B.; Nucifora, G. Role of Cardiac Magnetic Resonance Imaging in Patients with Idiopathic Ventricular Arrhythmias. Curr. Cardiol. Rev. 2019, 15, 12–23. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- Ailoaei, Ș.; Șorodoc, L.; Ureche, C.; Sîtari, N.; Sandu, G.; Ceasovschih, A.; Grecu, M.; Sascău, R.A.; Stătescu, C. Predictors of Myocardial Fibrosis Detected by CMR in Patients with Idiopathic PVCs. Ann. Noninvasive Electrocardiol. 2024, 29, e70039. [Google Scholar] [CrossRef]
- Ailoaei, S.; Sorodoc, L.; Ureche, C.; Sandu, G.; Sitari, N.; Ceasovschih, A.; Grecu, M.; Sascau, R.A.; Statescu, C. Role of Cardiac Magnetic Resonance in the Assessment of Patients with Premature Ventricular Contractions: A Narrative Review. Anatol. J. Cardiol. 2024, 28, 467–478. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P.; Castro, S.A.; Casado Arroyo, R.; Maeda, S.; Benhayon, D.A.; Liuba, I.; Liang, J.J.; Sadek, M.M.; Chahal, A.; et al. Risk Stratification of Patients with Apparently Idiopathic Premature Ventricular Contractions: A Multicenter International CMR Registry. JACC Clin. Electrophysiol. 2020, 6, 722–735. [Google Scholar] [CrossRef]
- Santangeli, P.; di Biase, L.; Pelargonio, G.; Natale, A. Outcome of invasive electrophysiological procedures and gender: Are males and females the same? J. Cardiovasc. Electrophysiol. 2011, 22, 605–612. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Basile, E.; Al-Ahmad, A.; Natale, A. Outcomes in Women Undergoing Electrophysiological Procedures. Arrhythm. Electrophysiol. Rev. 2013, 2, 41–44. [Google Scholar] [CrossRef]
- Yang, S.G.; Mlček, M.; Kittnar, O. Gender differences in electrophysiological characteristics of idiopathic ventricular tachycardia originating from right ventricular outflow tract. Physiol. Res. 2014, 63 (Suppl. 4), S451–S458. [Google Scholar] [CrossRef]
- Westerman, S.; Wenger, N.K. Women and heart disease, the underrecognized burden: Sex differences, biases, and unmet clinical and research challenges. Clin. Sci. 2016, 130, 551–563. [Google Scholar] [CrossRef]
- Ghannam, M.; Siontis, K.C.; Cochet, H.; Jais, P.; Juhoor, M.; Attili, A.; Sharaf-Dabbagh, G.; Latchamsetty, R.; Jongnarangsin, K.; Morady, F.; et al. Risk stratification in patients with nonischemic cardiomyopathy and ventricular arrhythmias based on quantification of intramural delayed enhancement on cardiac magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2020, 31, 1762–1769. [Google Scholar] [CrossRef]
- Attachaipanich, T.; Thiravetyan, B.; Tribuddharat, N.; Jaroonpipatkul, S.; Navaravong, L. Premature Ventricular Contraction-Induced Cardiomyopathy: Contemporary Evidence from Risk Stratification, Pathophysiology, and Management. J. Clin. Med. 2024, 13, 2635. [Google Scholar] [CrossRef]
- Amir, M.; Mappangara, I.; Setiadji, R.; Zam, S.M. Characteristics and Prevalence of Premature Ventricular Complex: A Telemedicine Study. Cardiol. Res. 2019, 10, 285–292. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, H.; Xu, C.; Zhou, X.; Cui, B.; Lu, Z. Relationship between sex hormones and idiopathic outflow tract ventricular arrhythmias in adult male patients. Transl. Res. 2009, 154, 265–268. [Google Scholar] [CrossRef]
- Gillis, A.M. Atrial Fibrillation and Ventricular Arrhythmias: Sex Differences in Electrophysiology, Epidemiology, Clinical Presentation, and Clinical Outcomes. Circulation 2017, 135, 593–608. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P.; Liang, J.J. Mechanisms of Ventricular Arrhythmias and Implications for Catheter Ablation. Card. Electrophysiol. Clin. 2022, 14, 547–558. [Google Scholar] [CrossRef]
- You, C.X.; Liu, C.F. Premature Ventricular Contractions and Cardiomyopathy. Cardiol. Rev. 2019, 27, 322–326. [Google Scholar] [CrossRef]
- Andreini, D.; Russo, A.D.; Pontone, G.; Mushtaq, S.; Conte, E.; Perchinunno, M.; Guglielmo, M.; Santos, A.C.; Magatelli, M.; Baggiano, A.; et al. CMR for Identifying the Substrate of Ventricular Arrhythmia in Patients With Normal Echocardiography. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 410–421. [Google Scholar] [CrossRef]
- Baldinger, S.H.; Kumar, S.; Romero, J.; Fujii, A.; Epstein, L.M.; Michaud, G.F.; John, R.; Tedrow, U.B.; Stevenson, W.G. A Comparison of Women and Men Undergoing Catheter Ablation for Sustained Monomorphic Ventricular Tachycardia. J. Cardiovasc. Electrophysiol. 2017, 28, 201–207. [Google Scholar] [CrossRef]
- Del Carpio Munoz, F.; Syed, F.F.; Noheria, A.; Cha, Y.-M.; Friedman, P.A.; Hammill, S.C.; Munger, T.M.; Venkatachalam, K.; Shen, W.-K.; Packer, D.L.; et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J. Cardiovasc. Electrophysiol. 2011, 22, 791–798. [Google Scholar] [CrossRef]
- Dukes, J.W.; Dewland, T.A.; Vittinghoff, E.; Mandyam, M.C.; Heckbert, S.R.; Siscovick, D.S.; Stein, P.K.; Psaty, B.M.; Sotoodehnia, N.; Gottdiener, J.S.; et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. J. Am. Coll. Cardiol. 2015, 66, 101–109. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Vittinghoff, E.; Dewland, T.A.; Dukes, J.W.; Soliman, E.Z.; Stein, P.K.; Gottdiener, J.S.; Alonso, A.; Chen, L.Y.; Psaty, B.M.; et al. Ectopy on a Single 12-Lead ECG, Incident Cardiac Myopathy, and Death in the Community. J. Am. Heart Assoc. 2017, 6, e006028. [Google Scholar] [CrossRef]
- Oebel, S.; Dinov, B.; Arya, A.; Hilbert, S.; Sommer, P.; Bollmann, A.; Hindricks, G.; Paetsch, I.; Jahnke, C. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J. Cardiovasc. Electrophysiol. 2017, 28, 1316–1323. [Google Scholar] [CrossRef]
| Female (n, %) | Male (n, %) | p-Value | |
|---|---|---|---|
| General Characteristics | |||
| Age (mean ± SD) | 49.27 ± 15.71 | 54.21 ± 15.68 | 0.0488 |
| BMI (kg/m2) | 26.02 ± 4.68 | 29.03 ± 3.66 | <0.0001 |
| Family history of SCD | 4 (6.78%) | 4 (4.40%) | 0.7124 |
| Smoker | 9 (15.25%) | 36 (39.56%) | 0.0028 |
| Diabetes mellitus | 9 (15.25%) | 22 (24.18%) | 0.2662 |
| Sedentary | 7 (11.86%) | 13 (14.28%) | 0.002 |
| Symptoms | |||
| Asymptomatic | 2 (3.39%) | 9 (9.89%) | 0.202 |
| Syncope | 5 (8.47%) | 12 (13.19%) | 0.5315 |
| Presyncope | 7 (11.86%) | 2 (2.20%) | 0.0289 |
| Palpitations | 54 (91.53%) | 80 (87.91%) | 0.6675 |
| Medical Therapy | |||
| IC AADs | 39 (66.1%) | 24 (26.37%) | <0.0001 |
| Amiodarone | 15 (25.42%) | 32 (35.16%) | 0.2089 |
| Betablockers | 39 (66.1%) | 74 (81.31%) | 0.0346 |
| Sotalol | 3 (5.08%) | 1 (1.09%) | 0.1388 |
| Female (Mean ± SD or n, %) | Male (Mean ± SD or n, %) | p-Value | |
|---|---|---|---|
| QRS fragmentations on native complexes | 8 (13.79%) | 17 (18.68%) | 0.6088 |
| Baseline ECG microvoltage | 6 (10.34%) | 6 (6.59%) | 0.6088 |
| PVC burden, % | 17.20 ± 10.02 | 15.80 ± 10.53 | 0.2378 |
| Initial R wave duration on PVC, ms | 30.97 ± 14.87 | 35.13 ± 17.30 | 0.0984 |
| QRS duration of PVC, ms | 144.66 ± 17.41 | 144.37 ± 16.44 | 0.9289 |
| Polyfocal PVCs | 10 (16.95%) | 17 (18.68%) | 0.9584 |
| QRS fragmentation on PVC | 16 (27.12%) | 43 (47.25%) | 0.0217 |
| LBBB inferior axis | 35/59 (59.32%) | 48/91 (52.75%) | 0.4532 |
| LBBB superior axis | 2/59 (3.39%) | 12/91 (13.19%) | 0.0481 |
| RBBB inferior axis | 13/59 (22.03%) | 11/91 (12.09%) | 0.1173 |
| RBBB superior axis | 8/59 (13.56%) | 18/91 (19.78%) | 0.3415 |
| Female (Mean ± SD) | Male (Mean ± SD) | p-Value | |
|---|---|---|---|
| LV EDV, mL | 82 ± 12.51 | 110 ± 18.4 | 0.0041 |
| LV EDV indexed, mL/m2 | 47.3 ± 7.23 | 59.45 ± 9.94 | 0.003 |
| IVS, mm | 10.55 ± 6.93 | 12.62 ± 17.10 | 0.3066 |
| EF, % | 54.84 ± 11.01 | 49.65 ± 12.23 | 0.0009 |
| GLS, % | 17.94 ± 3.99 | 17.20 ± 3.33 | 0.0318 |
| LA volume, mL | 50.10 ± 25.49 | 58.50 ± 24.86 | <0.0001 |
| LA volume indexed, mL/m2 | 28.9 ± 14.73 | 31.62 ± 13.43 | 0.0021 |
| RV FAC, % | 49.39 ± 5.36 | 47.56 ± 5.42 | 0.0169 |
| Category | Female (Mean ± SD or n, %) | Male (Mean ± SD or n, %) | p-Value |
|---|---|---|---|
| EDDLV, mm | 45.12 ± 5.76 | 50.34 ± 6.88 | 0.0021 |
| ESDLV, mm | 29.45 ± 6.32 | 34.21 ± 7.45 | 0.0048 |
| IVS, mm | 10.58 ± 2.14 | 12.67 ± 2.98 | 0.0312 |
| LWT, mm | 8.89 ± 1.76 | 9.45 ± 2.01 | 0.2156 |
| EDV LV indexed, mL/m2 | 83.76 ± 21.56 | 87.24 ± 22.7 | 0.003 |
| EDV RV indexed, mL/m2 | 72.45 ± 20.04 | 76.08 ± 21.19 | 0.0298 |
| RVEF, % | 48.12 ± 6.89 | 44.78 ± 7.34 | 0.0412 |
| LVEF, % | 57.23 ± 5.43 | 53.89 ± 6.01 | 0.0185 |
| LA area, cm2 | 21.34 ± 4.56 | 24.89 ± 5.67 | 0.0376 |
| RA area, cm2 | 19.45 ± 3.98 | 21.67 ± 4.32 | 0.0461 |
| LGE scar presence | 22 (37.93%) | 54 (59.34%) | 0.0173 |
| Female with LGE (n, %) | Male with LGE (n, %) | p-Value | |
|---|---|---|---|
| Polyfocal PVCs | 7 (70.00%) | 13 (76.47%) | 0.9965 |
| Initial R wave duration on PVC > 30 ms | 13 (76.47%) | 30 (76.92%) | 0.1359 |
| QRS fragmentation on PVC | 12 (80.00%) | 34 (79.07%) | 0.0147 |
| QRS duration of PVC > 150 ms | 9 (56.25%) | 16 (76.19%) | 0.6696 |
| QRS fragmentations on native complexes | 7 (87.50%) | 13 (76.47%) | 0.5798 |
| RBBB morphology, superior PVC axis | 7 (87.50%) | 15 (83.33%) | 1.0000 |
| RBBB morphology, inferior PVC axis | 5 (38.46%) | 7 (63.64%) | 0.4126 |
| LBBB morphology, superior PVC axis | 0 (0.00%) | 7 (58.33%) | 0.4450 |
| LBBB morphology, inferior PVC axis | 10 (28.57%) | 24 (50.00%) | 0.0828 |
| Variable | Females Without LGE (n, %) | Females with LGE (n, %) | Males Without LGE (n, %) | Males with LGE (n, %) | p-Value |
|---|---|---|---|---|---|
| Composite endpoint | 17 (47.22%) | 16 (72.73%) | 20 (54.05%) | 40 (74.07%) | 0.0520 |
| ICD needed | 1 (2.78%) | 5 (22.73%) | 2 (5.56%) | 15 (28.85%) | 0.0043 |
| Hospitalization needed | 16 (44.44%) | 16 (72.73%) | 19 (51.35%) | 38 (70.37%) | 0.0528 |
| Ablation needed | 13 (36.11%) | 8 (36.36%) | 15 (40.54%) | 19 (35.19%) | 0.7395 |
| VT present | 1 (2.78%) | 6 (28.57%) | 4 (10.81%) | 7 (12.96%) | 0.0736 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailoaei, Ș.; Șorodoc, L.; Ureche, C.; Sîtari, N.; Ceasovschih, A.; Grecu, M.; Sascău, R.A.; Stătescu, C. Specific Premature Ventricular Complex Characteristics in Women: Insights from a Patient Cohort. J. Cardiovasc. Dev. Dis. 2025, 12, 181. https://doi.org/10.3390/jcdd12050181
Ailoaei Ș, Șorodoc L, Ureche C, Sîtari N, Ceasovschih A, Grecu M, Sascău RA, Stătescu C. Specific Premature Ventricular Complex Characteristics in Women: Insights from a Patient Cohort. Journal of Cardiovascular Development and Disease. 2025; 12(5):181. https://doi.org/10.3390/jcdd12050181
Chicago/Turabian StyleAiloaei, Ștefan, Laurențiu Șorodoc, Carina Ureche, Nicolae Sîtari, Alexandr Ceasovschih, Mihaela Grecu, Radu Andy Sascău, and Cristian Stătescu. 2025. "Specific Premature Ventricular Complex Characteristics in Women: Insights from a Patient Cohort" Journal of Cardiovascular Development and Disease 12, no. 5: 181. https://doi.org/10.3390/jcdd12050181
APA StyleAiloaei, Ș., Șorodoc, L., Ureche, C., Sîtari, N., Ceasovschih, A., Grecu, M., Sascău, R. A., & Stătescu, C. (2025). Specific Premature Ventricular Complex Characteristics in Women: Insights from a Patient Cohort. Journal of Cardiovascular Development and Disease, 12(5), 181. https://doi.org/10.3390/jcdd12050181









