Beyond Conventional Meta-Analysis: A Meta-Learning Model to Predict Cohort-Level Mortality After Transcatheter Aortic Valve Replacement (TAVR)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Research Question
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Study Design: RCTs, cohort studies (prospective or retrospective), and analyses of national or multicenter registries.
- Population: Cohorts of adult patients (>18 years) undergoing TAVR for severe aortic stenosis.
- Required Data: Studies that reported the following:
- Baseline and procedural characteristics of the cohort in aggregate format (mean, median, or percentage).
- The 1-year all-cause mortality rate in extractable form. This endpoint was chosen due to its consistent reporting across the widest range of study designs and publication eras, maximizing the available data for robust model training.
- Cohort Size: Predominantly studies with a cohort size greater than 100 patients to ensure stability of estimates.
2.3.2. Exclusion Criteria
- Articles without original data (narrative reviews, editorials, letters, meta-analyses).
- Case reports or small case series (generally N < 100).
- Studies that did not allow extraction of aggregate baseline characteristics or 1-year mortality.
- Duplicate publications of the same cohort (in which case, the most complete or recent report was selected).
- Data published only as conference abstracts or pre-prints without a complete manuscript available.
2.4. Data Sources and Search Strategy
2.5. Information Selection and Extraction
- Study Identifiers: First author, publication year, study design (RCT, registry, cohort), and country/geographic region.
- Cohort Baseline Characteristics: Size of the analyzed cohort, average age, percentage of women, and prevalence of key comorbidities (% of diabetes mellitus, % of atrial fibrillation, % of chronic kidney disease).
- Risk Scores and Hemodynamic Data: Average STS-PROM score, average EuroSCORE II, and average Left Ventricular Ejection Fraction (LVEF).
- Procedural Characteristics: Percentage of transfemoral approach and percentage of self-expanding valve use.
- Methodological Variables: Follow-up duration, Valve Academic Research Consortium criteria used, and study quality score (derived from NOS/Jadad scales).
- Outcome Variables (Endpoints): All-cause mortality rate (at 1 year and other follow-ups), myocardial infarction (%), stroke (%), rehospitalization for heart failure (%), and composite Major Adverse Cardiovascular Events (%).
2.6. Risk of Bias Assessment
2.6.1. Randomized Controlled Trials (RCTs)
2.6.2. Observational Studies
2.7. Descriptive Analysis and Traditional Meta-Analysis/Meta-Regression
2.8. Meta-Learning: Development, Validation, and Interpretation of Predictive Models
2.8.1. Methodology for Calculating the Normalized Quality Score for Studies Used in Meta-Learning
2.8.2. Analytical Dataset and Preprocessing for Modelling
2.8.3. Phase 1: Initial Algorithm Training and Comparison
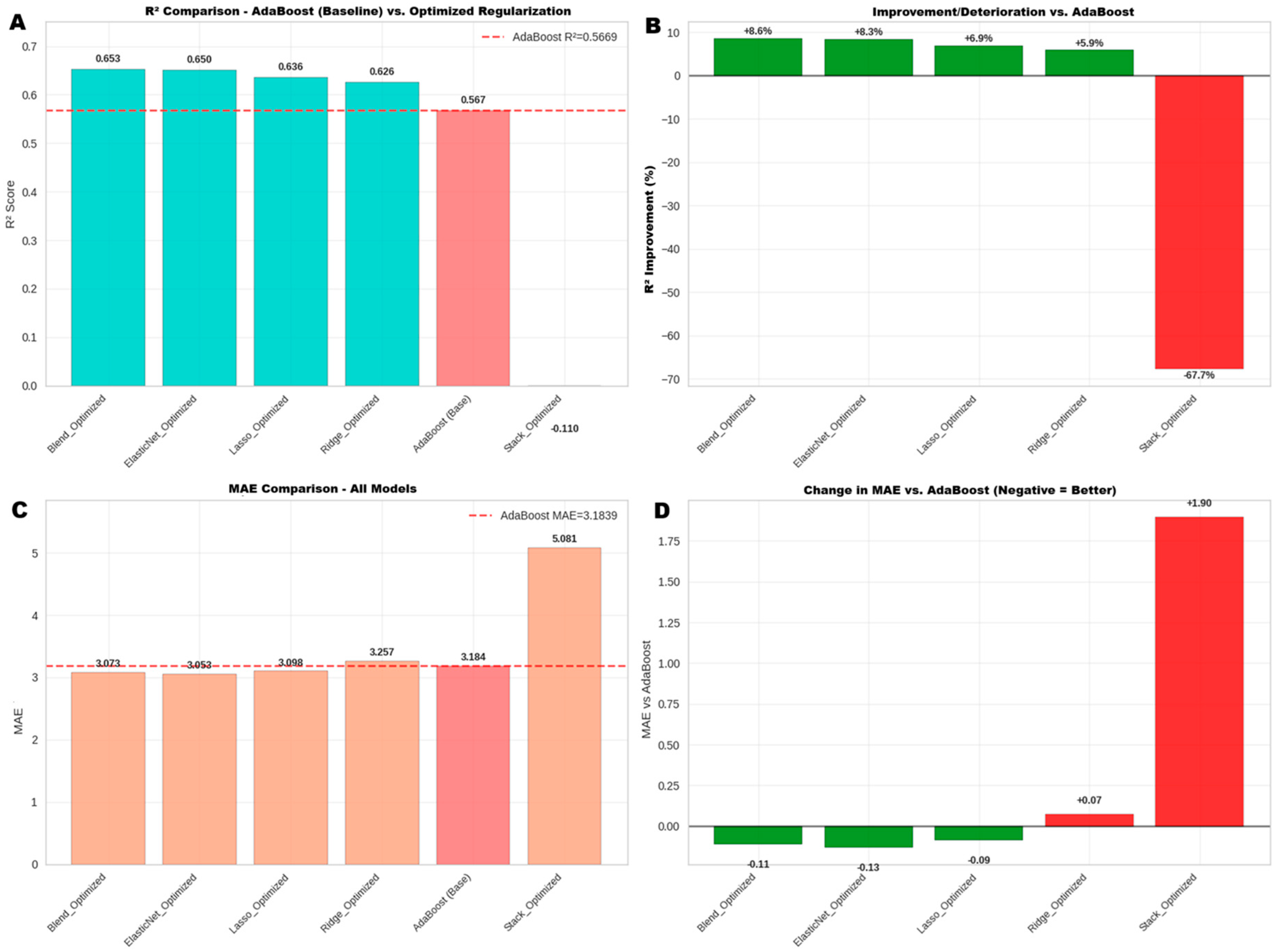
2.8.4. Phase 2: Advanced Optimization and Final Model Selection
- Regularization Models: Ridge, Lasso, and Elastic Net models were individually optimized through extended searches in the parameter space (e.g., logarithmic search for α, two-dimensional search for α and l1_ratio).
- Ensemble Model (Blending): A Blend_Optimized meta-model was constructed that weighted and combined the predictions of the already optimized regularization models.
- Stacking Model: Additionally, a Stack_Optimized model was evaluated to compare its performance against the blending approach.
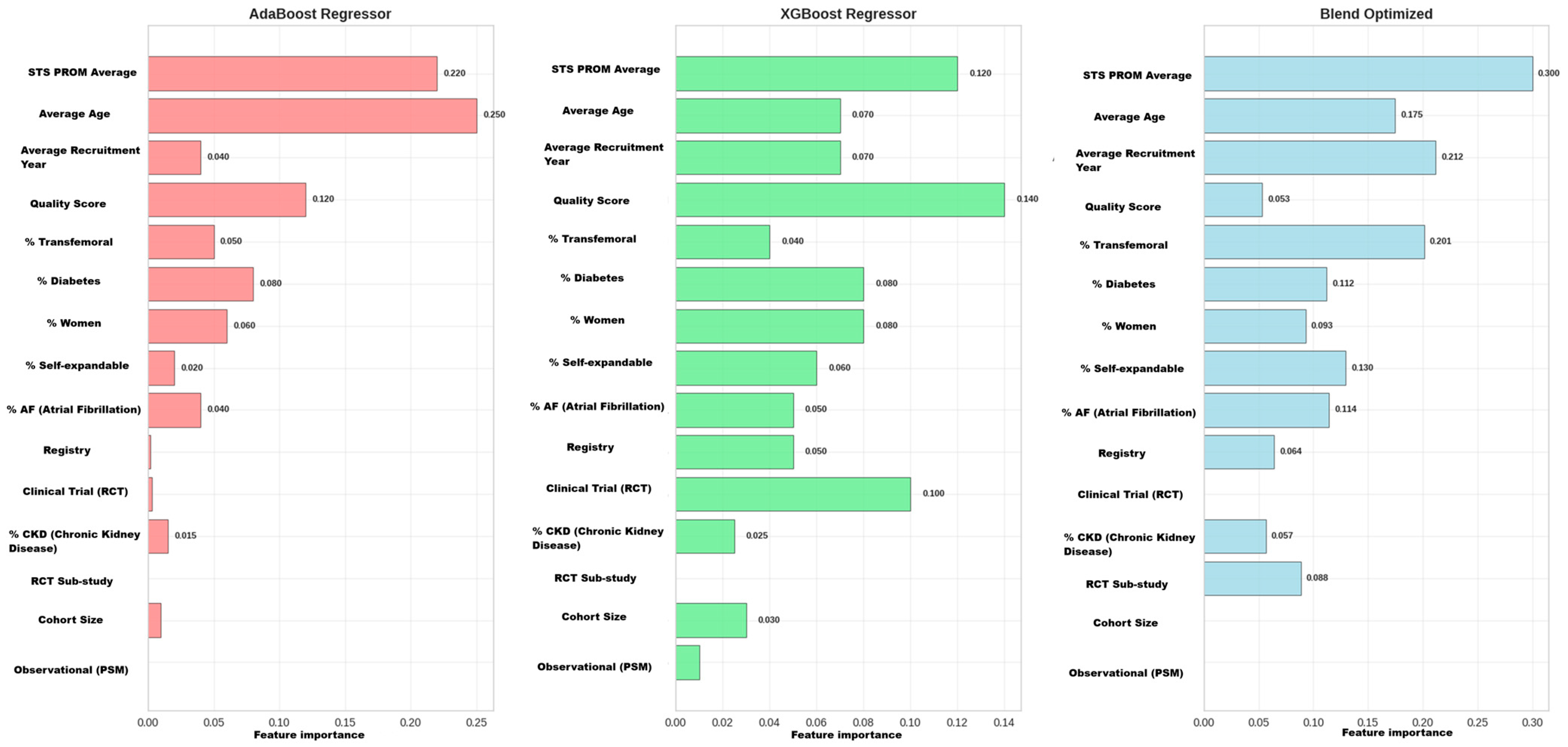
2.8.5. Comparative Model Interpretability Analysis
- Global Feature Importance: The ranking of the global influence of variables from each model was extracted to understand their predictive priorities.
- Comparative Visualization: Results were visualized both in separate bar charts for each model and in a consolidated heatmap. This visual matrix allowed for a direct comparison of how each algorithm weighted the same features, facilitating the identification of robust predictors (important across all models), model-dependent predictors, and synergies or the unique findings of the ensemble model.
2.8.6. Considerations on Ecological Fallacy and Model Limitations
2.9. Ethical Considerations and Software Used for Meta-Analysis, Meta-Regression, and Meta-Learning
3. Results
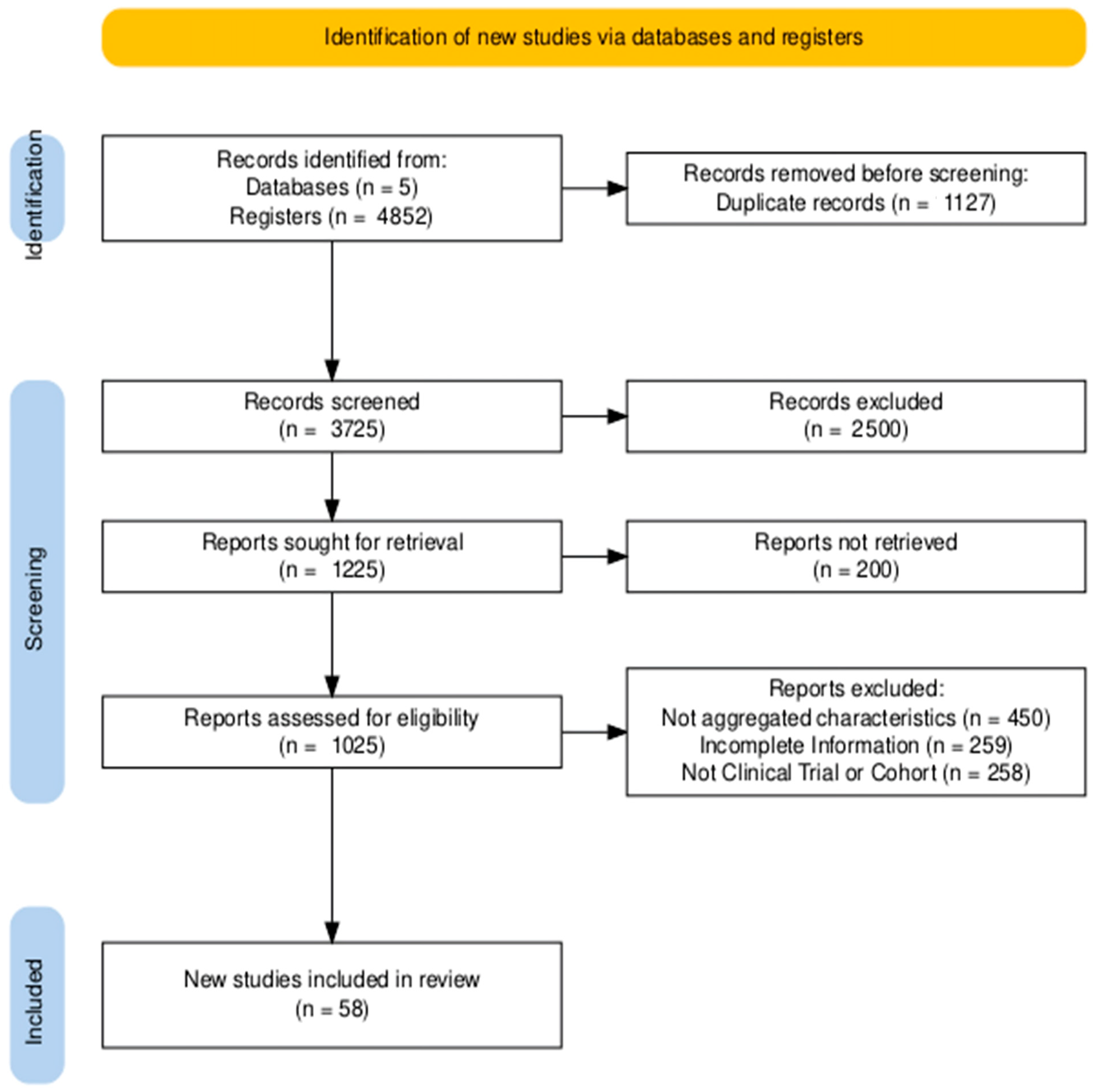
3.1. Studies Identified for Review
- n = 450 for not presenting aggregate characteristics.
- n = 259 for being preprints or other incomplete studies.
- n = 258 for not being RCTs or cohort studies.
3.2. Characteristics of Studies Included in the Review
3.3. Results of Risk of Bias Assessment
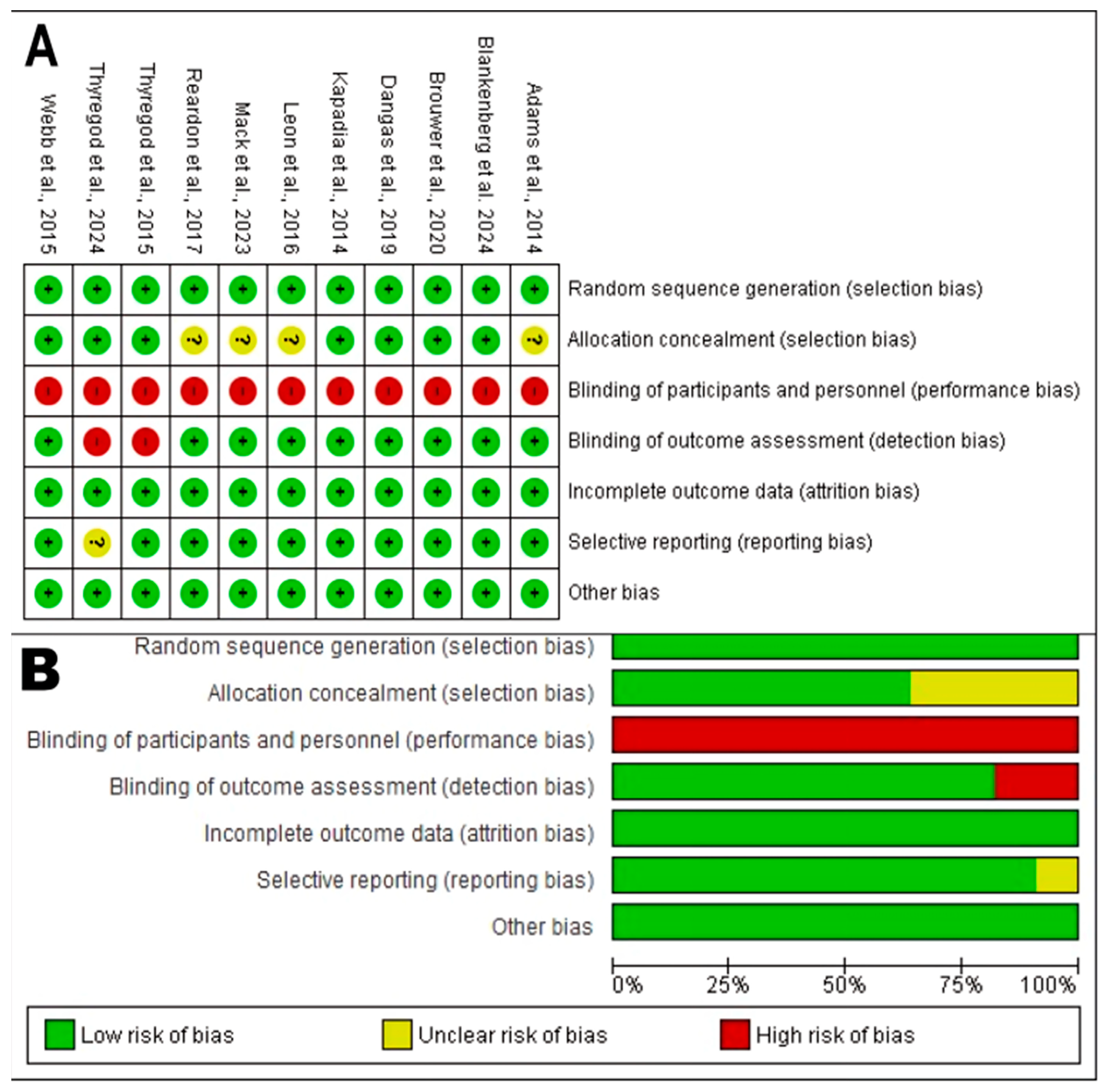
3.3.1. Risk of Bias in RCTs
- Random Sequence Generation (Selection Bias): The generation of an adequate random sequence was considered low risk in all 11 analyzed RCTs. All studies (e.g., Reardon et al., 2017 [58]; Dangas et al., 2019 [35]; Leon et al., 2016 [51]) described an appropriate randomization method, such as centralized or computer-generated systems, achieving the maximum score in this domain. This provides high confidence that group allocation was truly random.
- Allocation Concealment: The description of robust randomization methods in all trials suggests a low to unclear risk of bias. Most protocols for these large trials typically include adequate concealment mechanisms.
- Blinding of Participants and Personnel (Performance Bias): This domain represented the primary and universal source of bias risk. All 11 RCTs [18,27,30,35,44,51,54,58,61,67,73] were rated with high performance bias risk, receiving a score of 0 in the blinding item. This is an inherent and expected limitation in trials comparing device interventions like TAVR versus surgery, where the nature of the procedure prevents blinding of both patients and clinical personnel.
- Blinding of Outcome Assessment (Detection Bias): Despite the lack of participant blinding, the risk of bias in objective outcome assessment (such as mortality) is considered low. This is because hard endpoints are less susceptible to observer influence, and many of these pivotal trials use blinded clinical event adjudication committees.
- Handling of Incomplete Outcome Data (Attrition Bias): This was a domain of methodological strength. All 11 trials adequately described losses during follow-up and withdrawals; thus, they were classified with low attrition bias risk.
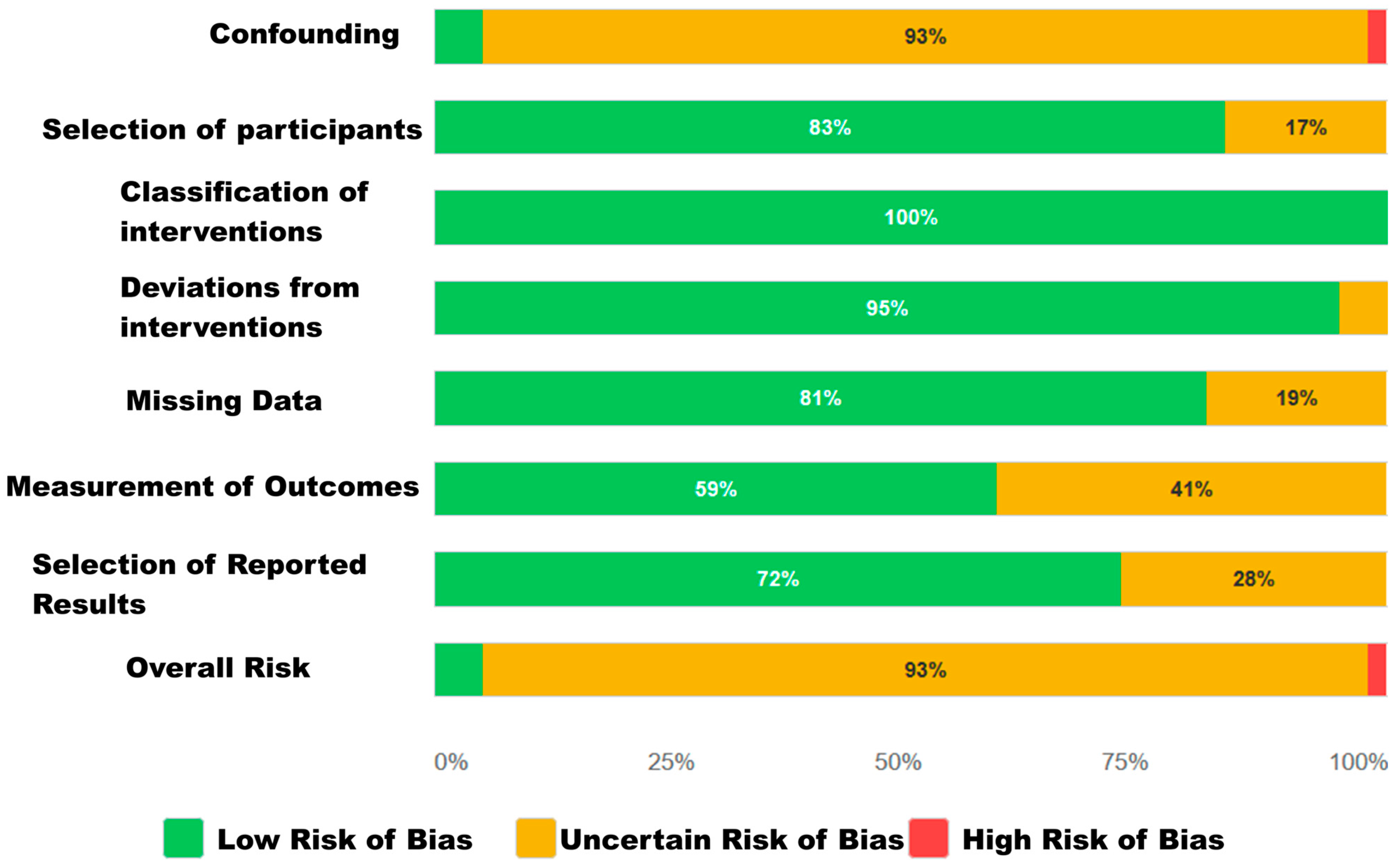
3.3.2. Risk of Bias for Observational Studies
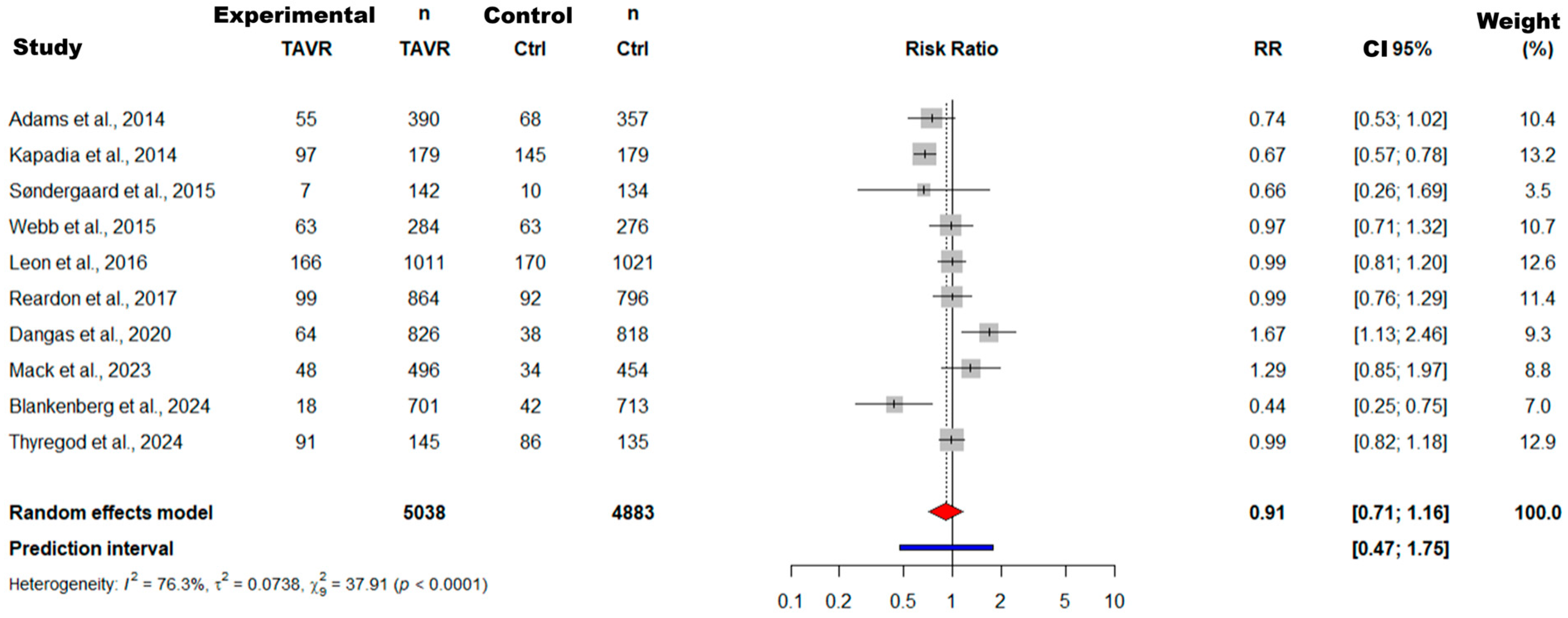
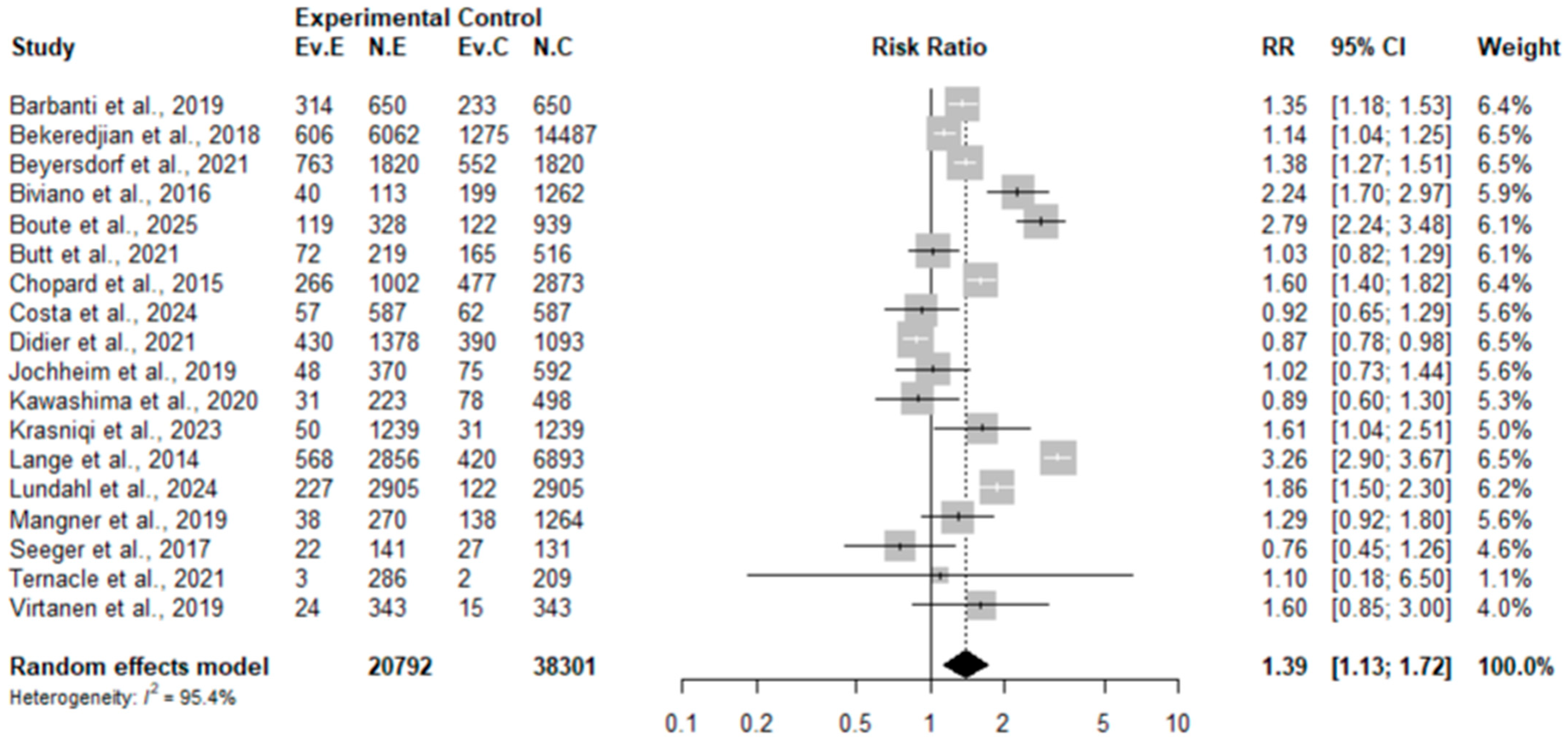
3.4. Results of Meta-Analysis and Meta-Regression
3.4.1. Results of Meta-Analysis and Meta-Regression for the RCT Subgroup
3.4.2. Results of Meta-Analysis and Meta-Regression for the Observational Study Subgroup
3.4.3. Comparative Synthesis of RCTs and Observational Studies
3.5. Meta-Learning Model Results
3.5.1. Phase 1 of Meta-Learning: Screening and Model Comparison
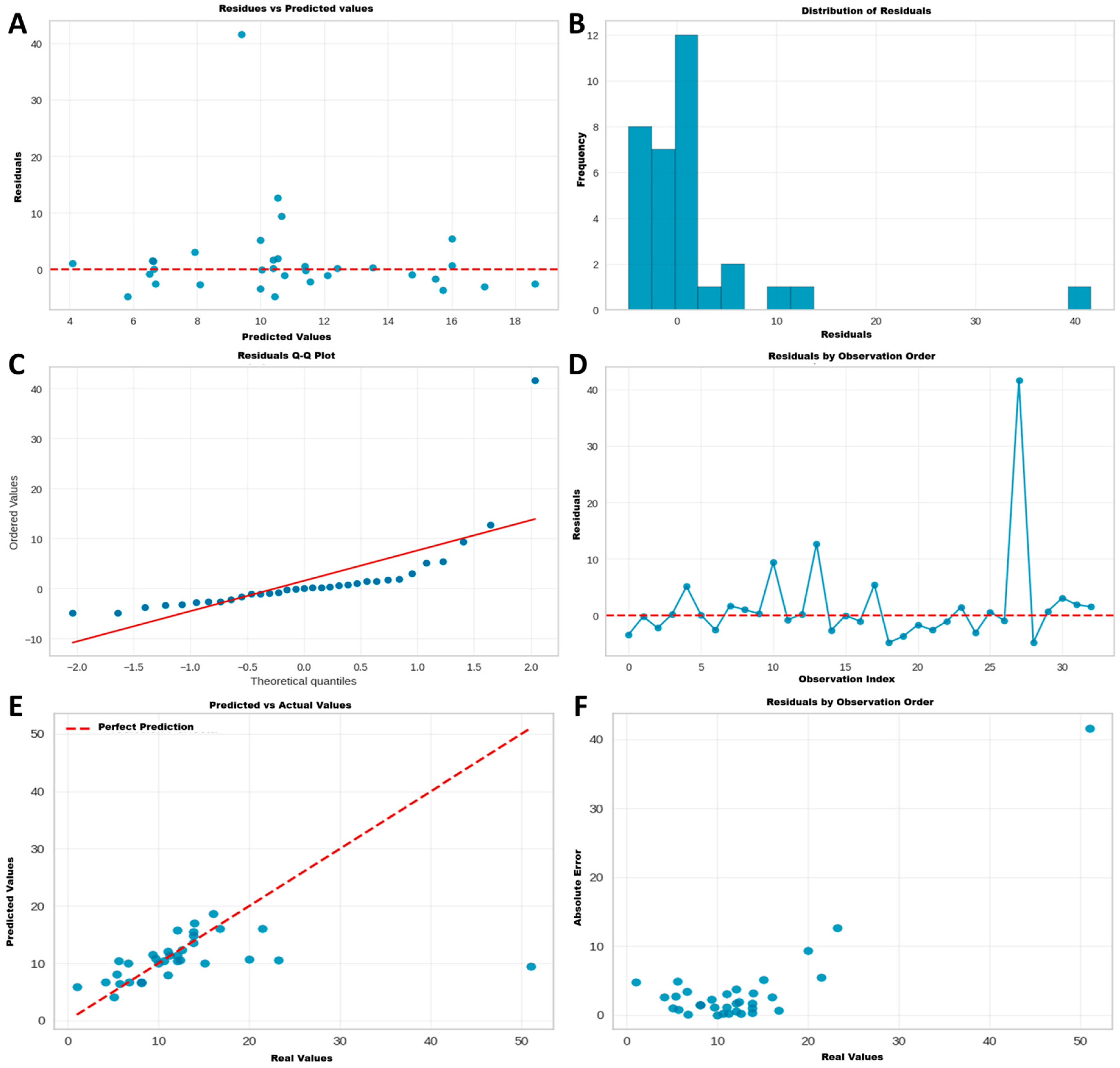
3.5.2. Phase 2: Validation of the Reference Model (AdaBoost)
3.5.3. Phase 3: Advanced Optimization and Final Model Selection
3.5.4. Phase 4: Comparative Model Interpretability Analysis
4. Discussion
4.1. Main Findings
4.2. Contextualization of Findings in Current Literature
4.3. Study Limitations
4.4. Implications for Research and Clinical Practice
4.5. Recommendations for Future Research
- Implement consistent reporting of a minimum set of variables (STS-PROM, EuroSCORE II, exact follow-up duration).
- Adopt standardized definitions based on more recent VARC criteria.
- Develop specific protocols for implementing meta-learning in cardiovascular evidence synthesis.
- Establish international collaborative registries that facilitate future analyses with standardized data.
- Diversity Expansion and Validation:
- Prioritize high-quality studies from underrepresented regions (Asia, Latin America, Africa) to evaluate the generalizability of the findings.
- Validate the identified associations using registries with individual patient data.
- Confirm whether cohort-level relationships remain consistent at the individual level through IPD analysis.
- Explore the applicability of the methodology in other complex cardiovascular procedures.
- Advanced Methodological Development:
- Incorporate new study-level variables (institutional volume, operator experience, specific quality metrics).
- Develop algorithms specifically designed for systematic review data that better handle heterogeneity.
- Explore deep learning techniques that can capture even more complex interactions.
- Investigate methods to integrate unstructured text data from original articles.
- Impact and Utility Assessment:
- Conduct economic evaluations comparing the efficiency of meta-learning versus traditional methods.
- Measure the impact on clinical decision making and the development of clinical practice guidelines.
- Establish standard metrics to evaluate the reduction of unexplained heterogeneity.
- Develop implementation tools that allow clinicians to apply these models in practice.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAVR | Transcatheter Aortic Valve Replacement |
| STS-PROM | Society of Thoracic Surgeons Predicted Risk of Mortality |
| LVEF | Left Ventricular Ejection Fraction |
| MACE | Major Adverse Cardiac Events |
| RCT | Randomized Controlled Trial |
| NOS | Newcastle–Ottawa Scale |
| ROBINS-E | Risk of Bias in Non-randomised Studies—of Exposures |
| RR | Risk Ratio |
| MAE | Mean Absolute Error |
| RMSE | Root Mean Squared Error |
| AI | Artificial Intelligence |
| IPD | Individual Patient Data |
| VARC | Valve Academic Research Consortium |
| AF | Atrial Fibrillation |
| CKD | Chronic Kidney Disease |
| CAD | Coronary Artery Disease |
References
- Mas-Peiro, S.; Fichtlscherer, S.; Walther, C.; Vasa-Nicotera, M. Current Issues in Transcatheter Aortic Valve Replacement. J. Thorac. Dis. 2020, 12, 1665–1680. [Google Scholar] [CrossRef]
- Montenegro-Palacios, J.F.; Vidal-Cañas, S.; Murillo-Benítez, N.E.; Quintana-Ospina, J.; Cardona-Murillo, C.A.; Liscano, Y. Common Risk Factors for Atrial Fibrillation After Transcatheter Aortic Valve Implantation: A Systematic Review from 2009 to 2024. J. Cardiovasc. Dev. Dis. 2025, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Patterson, T.; Redwood, S.R.; Prendergast, B.D. Future Directions. Transcatheter Aortic Valve Implantation for Low-Risk Patients: Inevitable Evolution or a Step Too Far? Rev. Esp. Cardiol. 2019, 8, 664–671. [Google Scholar] [CrossRef]
- Zaka, A.; Mustafiz, C.; Mutahar, D.; Sinhal, S.; Gorcilov, J.; Muston, B.; Evans, S.; Gupta, A.; Stretton, B.; Kovoor, J.; et al. Machine-Learning versus Traditional Methods for Prediction of All-Cause Mortality after Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. Open Heart 2025, 12, e002779. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; DeFrain, M.; Muppala, M.; et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 81, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Ziegelmann, P.K.; Munn, Z.; Falavigna, M. Meta-analysis of Prevalence: I2 Statistic and How to Deal with Heterogeneity. Res. Synth. Methods 2022, 13, 363–367. [Google Scholar] [CrossRef]
- Sattar, Y.; Song, D.; Riasat, M.; Singh, G.; Shah, R.P.; Elgendy, I.Y.; Mehmood, A.; Mir, T.; Zghouzi, M.; Ullah, W.; et al. Quality Assessment of Transcatheter Aortic Valve Replacement Meta Analysis: Is It Worth Reading? Circulation 2021, 144 (Suppl. S1), A14445. [Google Scholar] [CrossRef]
- Sazzad, F.; Ler, A.A.L.; Furqan, M.S.; Tan, L.K.Z.; Leo, H.L.; Kuntjoro, I.; Tay, E.; Kofidis, T. Harnessing the Power of Artificial Intelligence in Predicting All-Cause Mortality in Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2024, 11, 1343210. [Google Scholar] [CrossRef]
- Gupta, A.K.; Zaka, A.; Mutahar, D.; Muston, B.; Tyagi, D.; Farag, M.; Lombardo, A.; Parvez, R.; Stretton, B.; Kovoor, J.G.; et al. Abstract 4142671: Machine-Learning versus Traditional Risk Scores for Predicting Clinical Outcomes after Coronary Artery Bypass Graft Surgery: A Systematic Review and Meta-Analysis. Circulation 2024, 50 (Suppl. S1), A4142671. [Google Scholar] [CrossRef]
- Zisiopoulou, M.; Berkowitsch, A.; Redlich, L.; Walther, T.; Fichtlscherer, S.; Leistner, D.M. Personalised Preinterventional Risk Stratification of Mortality, Length of Stay and Hospitalisation Costs in Transcatheter Aortic Valve Implantation Using a Machine Learning Algorithm: A Pilot Trial. Open Heart 2024, 11, e002540. [Google Scholar] [CrossRef]
- Kent, D.M.; Paulus, J.K.; van Klaveren, D.; D’Agostino, R.B.; Goodman, S.; Hayward, R.A.; Ioannidis, J.P.A.; Patrick-Lake, B.; Morton, S.C.; Pencina, M.J.; et al. The Predictive Approaches to Treatment Effect Heterogeneity (PATH) Statement. Ann. Intern. Med. 2019, 172, 35–45. [Google Scholar] [CrossRef]
- Requena, C.C.; Muriel, A.; Peñuelas, Ó. Analysis of Causality from Observational Studies and Its Application in Clinical Research in Intensive Care Medicine. Med. Intensiva 2018, 42, 292–300. [Google Scholar]
- Steyerberg, E.W.; Nieboer, D.; Debray, T.P.A.; Van Houwelingen, H.C. Assessment of Heterogeneity in an Individual Participant Data Meta-analysis of Prediction Models: An Overview and Illustration. Stat. Med. 2019, 38, 4290–4309. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Lancellotti, P.; Habib, G.; Garbi, M.; Dahl, J.S.; Barbanti, M.; Vannan, M.A.; Vassiliou, V.S.; Dudek, D.; Chioncel, O.; et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2023, 82, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Austin, P.C.; Ross, H.J.; Abdel-Qadir, H.; Freitas, C.; Tomlinson, G.; Chicco, D.; Mahendiran, M.; Lawler, P.R.; Billia, F.; et al. Machine Learning vs. Conventional Statistical Models for Predicting Heart Failure Readmission and Mortality. ESC Heart Failure 2021, 8, 106–115. [Google Scholar] [CrossRef]
- Steele, A.J.; Cakiroglu, S.A.; Shah, A.D.; Denaxas, S.C.; Hemingway, H.; Luscombe, N.M. Machine Learning Models in Electronic Health Records Can Outperform Conventional Survival Models for Predicting Patient Mortality in Coronary Artery Disease. PLoS ONE 2018, 13, e0202344. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Alasnag, M.; AlMerri, K.; Almoghairi, A.; Alenezi, A.; Bardooli, F.; Al-Sheikh, S.; Alanazi, N.; AlHarbi, W.; Al Lawati, H.; Al Faraidy, K.; et al. One-Year Outcomes for Patients Undergoing Transcatheter Aortic Valve Replacement: The Gulf TAVR Registry. Cardiovasc. Revasc. Med. 2022, 41, 19–26. [Google Scholar] [CrossRef]
- Alnabti, A.; Abujalala, S.; Al-Hijji, M.; Othman, K.; Rafie, I.; Al Suwaidi, J.; Yalcin, H.C.; Sulaiman, R.; Seri, A.; Hamid, T. Outcomes of the Qatar Transcatheter Aortic Valve Implantation- Registry (QATAVI-Registry) –First Report 24/7/2024. Int. J. Cardiol. 2025, 424, 133029. [Google Scholar] [CrossRef]
- Arnold, S.V.; Manandhar, P.; Vemulapalli, S.; Kosinski, A.; Desai, N.D.; Bavaria, J.E.; Carroll, J.D.; Mack, M.J.; Thourani, V.H.; Cohen, D.J. Impact of Short-Term Complications of Transcatheter Aortic Valve Replacement on Longer-Term Outcomes: Results from the STS/ACC Transcatheter Valve Therapy Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 208–213. [Google Scholar] [CrossRef]
- Azevedo, F.S.; Correa, M.G.; Paula, D.H.G.; Felix, A.D.S.; Belém, L.H.J.; Mendes, A.P.C.; Silva, V.G.; Marques, B.M.; Monteiro, A.J.D.O.; Weksler, C.; et al. Transcatheter Aortic Valve Replacement: The Experience of One Brazilian Health Care Center. Braz. J. Cardiovasc. Surg. 2018, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Tamburino, C.; D’Errigo, P.; Biancari, F.; Ranucci, M.; Rosato, S.; Santoro, G.; Fusco, D.; Seccareccia, F.; for the OBSERVANT Research Group. Five-Year Outcomes of Transfemoral Transcatheter Aortic Valve Replacement or Surgical Aortic Valve Replacement in a Real World Population: Final Results from the OBSERVANT Study. Circ. Cardiovasc. Interv. 2019, 12, e007825. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian, R.; Szabo, G.; Balaban, Ü.; Bleiziffer, S.; Bauer, T.; Ensminger, S.; Frerker, C.; Herrmann, E.; Beyersdorf, F.; Hamm, C.; et al. Patients at Low Surgical Risk as Defined by the Society of Thoracic Surgeons Score Undergoing Isolated Interventional or Surgical Aortic Valve Implantation: In-Hospital Data and 1-Year Results from the German Aortic Valve Registry (GARY). Eur. Heart J. 2019, 40, 1323–1330. [Google Scholar] [CrossRef]
- Beyersdorf, F.; Bauer, T.; Freemantle, N.; Walther, T.; Frerker, C.; Herrmann, E.; Bleiziffer, S.; Möllmann, H.; Landwehr, S.; Ensminger, S.; et al. Five-Year Outcome in 18 010 Patients from the German Aortic Valve Registry. Eur. J. Cardiothorac. Surg. 2021, 60, 1139–1146. [Google Scholar] [CrossRef]
- Biviano, A.B.; Nazif, T.; Dizon, J.; Garan, H.; Hassan, D.; Kapadia, S.; Babaliaros, V.; Xu, K.; Parvataneni, R.; Rodes-Cabau, J.; et al. Atrial Fibrillation Is Associated with Increased Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights from the PARTNER Trial. Circ. Cardiovasc. Interv. 2017, 9, e002766. [Google Scholar]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.-H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef]
- Bouleti, C.; Himbert, D.; Iung, B.; Alos, B.; Kerneis, C.; Ghodbane, W.; Messika-Zeitoun, D.; Brochet, E.; Fassa, A.-A.; Depoix, J.-P.; et al. Long-Term Outcome after Transcatheter Aortic Valve Implantation. Heart 2015, 101, 936–942. [Google Scholar] [CrossRef]
- Boute, M.; De Azevedo, D.; De Terwangne, C.; Pouleur, A.-C.; Pasquet, A.; Gerber, B.L.; De Kerchove, L.; Beauloye, C.; Kefer, J.; Maes, F.; et al. Surgical and Transcatheter Aortic Valve Replacement Align Survival with General Population Expectations: Insights from Standardized Mortality Ratios. Front. Cardiovasc. Med. 2025, 12, 1547456. [Google Scholar] [CrossRef]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; Van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef]
- Butt, J.H.; De Backer, O.; Olesen, J.B.; Gerds, T.A.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Søndergaard, L.; Køber, L.; Fosbøl, E.L. Vitamin K Antagonists vs. Direct Oral Anticoagulants after Transcatheter Aortic Valve Implantation in Atrial Fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 11–19. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Chopard, R.; Teiger, E.; Meneveau, N.; Chocron, S.; Gilard, M.; Laskar, M.; Eltchaninoff, H.; Iung, B.; Leprince, P.; Chevreul, K.; et al. Baseline Characteristics and Prognostic Implications of Pre-Existing and New-Onset Atrial Fibrillation After Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2015, 8, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Saia, F.; Pilgrim, T.; Abdel-Wahab, M.; Garot, P.; Sammartino, S.; Gandolfo, C.; Branca, L.; Latib, A.; Amat-Santos, I.; et al. One-Year Clinical Outcomes of Transcatheter Aortic Valve Implantation with the Latest Iteration of Self-Expanding or Balloonexpandable Devices: Insights from the OPERA-TAVI Registry. EuroIntervention 2024, 20, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Tijssen, J.G.P.; Wöhrle, J.; Søndergaard, L.; Gilard, M.; Möllmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Didier, R.; Lhermusier, T.; Auffret, V.; Eltchaninoff, H.; Le Breton, H.; Cayla, G.; Commeau, P.; Collet, J.P.; Cuisset, T.; Dumonteil, N.; et al. TAVR Patients Requiring Anticoagulation. JACC Cardiovasc. Interv. 2021, 14, 1704–1713. [Google Scholar] [CrossRef]
- Duncan, A.; Ludman, P.; Banya, W.; Cunningham, D.; Marlee, D.; Davies, S.; Mullen, M.; Kovac, J.; Spyt, T.; Moat, N. Long-Term Outcomes After Transcatheter Aortic Valve Replacement in High-Risk Patients with Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2015, 8, 645–653. [Google Scholar] [CrossRef]
- Frerker, C.; Bestehorn, K.; Schlüter, M.; Bestehorn, M.; Hamm, C.W.; Möllmann, H.; Katus, H.A.; Kuck, K.-H. In-Hospital Mortality in Propensity-Score Matched Low-Risk Patients Undergoing Routine Isolated Surgical or Transfemoral Transcatheter Aortic Valve Replacement in 2014 in Germany. Clin. Res. Cardiol. 2017, 106, 610–617. [Google Scholar] [CrossRef]
- Gilard, M.; Eltchaninoff, H.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; Teiger, E.; et al. Late Outcomes of Transcatheter Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2016, 68, 1637–1647. [Google Scholar] [CrossRef]
- Goel, K.; Shah, P.; Jones, B.M.; Korngold, E.; Bhardwaj, A.; Kar, B.; Barker, C.; Szerlip, M.; Smalling, R.; Dhoble, A. Outcomes of Transcatheter Aortic Valve Replacement in Patients with Cardiogenic Shock. Eur. Heart J. 2023, 44, 3181–3195. [Google Scholar] [CrossRef]
- Holmes, D.R.; Nishimura, R.A.; Grover, F.L.; Brindis, R.G.; Carroll, J.D.; Edwards, F.H.; Peterson, E.D.; Rumsfeld, J.S.; Shahian, D.M.; Thourani, V.H.; et al. Annual Outcomes with Transcatheter Valve Therapy. J. Am. Coll. Cardiol. 2015, 66, 2813–2823. [Google Scholar] [CrossRef]
- Jochheim, D.; Barbanti, M.; Capretti, G.; Stefanini, G.G.; Hapfelmeier, A.; Zadrozny, M.; Baquet, M.; Fischer, J.; Theiss, H.; Todaro, D.; et al. Oral Anticoagulant Type and Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1566–1576. [Google Scholar] [CrossRef]
- Kaneko, T.; Vemulapalli, S.; Kohsaka, S.; Shimamura, K.; Stebbins, A.; Kumamaru, H.; Nelson, A.J.; Kosinski, A.; Maeda, K.; Bavaria, J.E.; et al. Practice Patterns and Outcomes of Transcatheter Aortic Valve Replacement in the United States and Japan: A Report From Joint Data Harmonization Initiative of STS/ACC TVT and J-TVT. J. Am. Heart Assoc. 2022, 11, e023848. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Tuzcu, E.M.; Makkar, R.R.; Svensson, L.G.; Agarwal, S.; Kodali, S.; Fontana, G.P.; Webb, J.G.; Mack, M.; Thourani, V.H.; et al. Long-Term Outcomes of Inoperable Patients with Aortic Stenosis Randomly Assigned to Transcatheter Aortic Valve Replacement or Standard Therapy. Circulation 2014, 130, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Karra, N.; Sharon, A.; Massalha, E.; Fefer, P.; Maor, E.; Guetta, V.; Ben-Zekry, S.; Kuperstein, R.; Matetzky, S.; Beigel, R.; et al. Temporal Trends in Patient Characteristics and Clinical Outcomes of TAVR: Over a Decade of Practice. J. Clin. Med. 2024, 13, 5027. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Watanabe, Y.; Hioki, H.; Kozuma, K.; Kataoka, A.; Nakashima, M.; Nagura, F.; Nara, Y.; Yashima, F.; Tada, N.; et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists in Patients with Atrial Fibrillation After TAVR. JACC Cardiovasc. Interv. 2020, 13, 2587–2597. [Google Scholar] [CrossRef]
- Khashaba, A.A.; Adel, W.; Roshdi, A.; Gafar, A.; Essam, S.; Algendy, M.A.S. First Egyptian Experience of Transcatheter Aortic Valve Implantation: Immediate Results and One Year Follow Up. Egypt. Heart J. 2014, 66, 17–21. [Google Scholar] [CrossRef]
- Krasniqi, L.; Brandes, A.; Mortensen, P.E.; Gerke, O.; Riber, L. Severe Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement with Perimount in Western Denmark 2016–2022: A Nationwide Retrospective Study. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae122. [Google Scholar] [CrossRef]
- Lachonius, M.; Nielsen, S.J.; Giang, K.W.; Backes, J.; Bjursten, H.; Hagström, H.; James, S.; Settergren, M.; Skoglund, K.; Jeppsson, A.; et al. Mortality and Morbidity after Transcatheter Aortic Valve Implantation Compared to an Age- and Sex-Matched Control Population: A Population-Based Study from the SWEDEHEART Registry. Eur. Heart J.—Qual. Care Clin. Outc. 2025, 11, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.; Beckmann, A.; Neumann, T.; Krane, M.; Deutsch, M.-A.; Landwehr, S.; Kötting, J.; Welz, A.; Zahn, R.; Cremer, J.; et al. Quality of Life After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2016, 9, 2541–2554. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Li, Y.-M.; Xiong, T.-Y.; Xu, K.; Fang, Z.-F.; Jiang, L.; Jin, J.; He, S.-H.; Yang, Y.-N.; He, J.-J.; Jia, Y.-H.; et al. Characteristics and Outcomes Following Transcatheter Aortic Valve Replacement in China: A Report from China Aortic Valve Transcatheter Replacement Registry (CARRY). Chin. Med. J. 2021, 134, 2678–2684. [Google Scholar] [CrossRef]
- Lundahl, C.; Kragholm, K.; Tayal, B.; Karasoy, D.; Andersen, N.H.; Strange, J.E.; Olesen, J.B.; Østergaard, L.; Fosbøl, E.; Torp-Pedersen, C.; et al. Temporal Trends in Patient Characteristics and Outcomes of Transcatheter Aortic Valve Implantation and Surgical Aortic Valve Replacement: A Nationwide Study. J. Am. Coll. Cardiol. 2024, 211, 299–306. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Maeda, K.; Kumamaru, H.; Kohsaka, S.; Shimamura, K.; Mizote, I.; Yamashita, K.; Kawamura, A.; Mukai, T.; Nakamura, D.; Takeda, Y.; et al. A Risk Model for 1-Year Mortality After Transcatheter Aortic Valve Replacement From the J-TVT Registry. J. Am. Coll. Cardiol: Asia 2022, 2, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Crusius, L.; Haussig, S.; Woitek, F.J.; Kiefer, P.; Stachel, G.; Leontyev, S.; Schlotter, F.; Spindler, A.; Höllriegel, R.; et al. Continued Versus Interrupted Oral Anticoagulation During Transfemoral Transcatheter Aortic Valve Implantation and Impact of Postoperative Anticoagulant Management on Outcome in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 123, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Alaour, B.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Reuthebuch, O.; Toggweiler, S.; et al. Long-Term Risk of Stroke After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Schaafsma, E.; Weich, H.; Scherman, J.; Tsabedze, N.; Ntsekhe, M. Outcomes in the South African SHARE-TAVI Registry: Comparison of Mortality and Risk between Treated and Untreated Cohort, and Recent 1-Year Outcomes in the Maturing Local TAVR Programme. Eur. Heart J. 2023, 44, ehad655.3028. [Google Scholar] [CrossRef]
- Seeger, J.; Gonska, B.; Rodewald, C.; Rottbauer, W.; Wöhrle, J. Apixaban in Patients with Atrial Fibrillation After Transfemoral Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 66–74. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or Surgical Aortic Valve Implantation: 10-Year Outcomes of the NOTION Trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Stortecky, S.; Franzone, A.; Heg, D.; Tueller, D.; Noble, S.; Pilgrim, T.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Nietlispach, F.; et al. Temporal Trends in Adoption and Outcomes of Transcatheter Aortic Valve Implantation: A SwissTAVI Registry Analysis. Eur. Heart J.—Qual. Care Clin. Outc. 2019, 5, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Strange, J.E.; Fosbøl, E.L.; Sindet-Pedersen, C.; Havers-Borgersen, E.; Køber, L.; Gislason, G.H.; Olesen, J.B. Mortality at One Year after Transcatheter Aortic Valve Replacement—Relation of Age and Comorbidities. Int. J. Cardiol. Heart Vasc. 2022, 43, 101157. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Barbanti, M.; D’Errigo, P.; Ranucci, M.; Onorati, F.; Covello, R.D.; Santini, F.; Rosato, S.; Santoro, G.; Fusco, D.; et al. 1-Year Outcomes After Transfemoral Transcatheter or Surgical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2015, 66, 804–812. [Google Scholar] [CrossRef]
- Ternacle, J.; Al-Azizi, K.; Szerlip, M.; Potluri, S.; Hamandi, M.; Blanke, P.; Leipsic, J.; Dahou, A.; Salaun, E.; Vincent, F.; et al. Impact of Predilation During Transcatheter Aortic Valve Replacement: Insights from the PARTNER 3 Trial. Circ. Cardiovasc. Interv. 2021, 14, e010336. [Google Scholar] [CrossRef]
- Thogata, H.; Garikipati, S.; Reddy, S.S.; Abhinav Reddy, P.; Kumar Jella, H. Long-Term Prognosis and Predictors of Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Retrospective Analysis. Cureus 2023, 15, e44432. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef]
- Tomii, D.; Lanz, J.; Heg, D.; Möllmann, H.; Kim, W.-K.; Burgdorf, C.; Linke, A.; Redwood, S.; Hilker, M.; Joner, M.; et al. The Impact of Frailty on VARC-3 Integrated Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Adv. 2025, 4, 101594. [Google Scholar] [CrossRef]
- Van Bergeijk, K.; Venema, S.; Van Den Heuvel, A.; Van Der Werf, R.; Bouma, W.; Douglas, Y.; Medendorp, N.; Timmermans, M.; Voors, A.; Wykrzykowska, J.J.; et al. Long-Term Age-Stratified Outcomes after Surgical and Transcatheter Aortic Valve Replacement: A Dutch Cohort Study. Neth. Heart J. 2025, 33, 172–179. [Google Scholar] [CrossRef]
- Vekstein, A.M.; Wegermann, Z.K.; Manandhar, P.; Mack, M.J.; Cohen, D.J.; Hughes, G.C.; Harrison, J.K.; Kaneko, T.; Kapadia, S.R.; Stathogiannis, K.; et al. Outcomes of Transcatheter Aortic Valve Replacement in Low-Risk Patients in the United States: A Report From the STS/ACC TVT Registry. Circulation 2025, 151, 1134–1146. [Google Scholar] [CrossRef]
- Virtanen, M.P.O.; Eskola, M.; Jalava, M.P.; Husso, A.; Laakso, T.; Niemelä, M.; Ahvenvaara, T.; Tauriainen, T.; Maaranen, P.; Kinnunen, E.-M.; et al. Comparison of Outcomes After Transcatheter Aortic Valve Replacement vs Surgical Aortic Valve Replacement Among Patients with Aortic Stenosis at Low Operative Risk. JAMA Netw. Open 2019, 2, e195742. [Google Scholar] [CrossRef]
- Wang, M.; Song, G.; Chen, M.; Feng, Y.; Wang, J.; Liu, X.; Zhou, S.; Fang, Z.; Han, Y.; Xu, K.; et al. Twelve-Month Outcomes of the TaurusOne Valve for Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis. EuroIntervention 2022, 17, 1070–1076. [Google Scholar] [CrossRef]
- Webb, J.G.; Doshi, D.; Mack, M.J.; Makkar, R.; Smith, C.R.; Pichard, A.D.; Kodali, S.; Kapadia, S.; Miller, D.C.; Babaliaros, V.; et al. A Randomized Evaluation of the SAPIEN XT Transcatheter Heart Valve System in Patients with Aortic Stenosis Who Are Not Candidates for Surgery. JACC Cardiovasc. Interv. 2015, 8, 1797–1806. [Google Scholar] [CrossRef]
- Yu, C.W.; Kim, W.-J.; Ahn, J.-M.; Kook, H.; Kang, S.H.; Han, J.-K.; Ko, Y.-G.; Choi, S.-H.; Koo, B.-K.; Chang, K.; et al. Trends and Outcomes of Transcatheter Aortic Valve Implantation (TAVI) in Korea: The Results of the First Cohort of Korean TAVI Registry. Korean Circ. J. 2018, 48, 382. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, T.J.; Usman, M.S.; Khan, M.S.; Khan, M.A.A.; Riaz, H.; Khan, S.U.; Murad, M.H.; Kavinsky, C.J.; Doukky, R.; Kalra, A.; et al. Systematic Review and Meta-Analysis of Current Risk Models in Predicting Short-Term Mortality after Transcatheter Aortic Valve Replacement. EuroIntervention 2020, 15, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Barili, F.; Brophy, J.M.; Ronco, D.; Myers, P.O.; Uva, M.S.; Almeida, R.M.S.; Marin-Cuartas, M.; Anselmi, A.; Tomasi, J.; Verhoye, J.-P.; et al. Risk of Bias in Randomized Clinical Trials Comparing Transcatheter and Surgical Aortic Valve Replacement: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2249321. [Google Scholar] [CrossRef]
- Adams, C.; Singh, K. Geographic Variation in the Statin Trials: Underrepresentation of Asian Populations. Int. J. Cardiol. 2020, 316, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Hsueh, L.; Kim, H.; Vidovich, M.I. Global Geographical Variation in Patient Characteristics in Percutaneous Coronary Intervention Clinical Trials: A Systematic Review and Meta-Analysis. Am. Heart J. 2018, 195, 39–49. [Google Scholar] [CrossRef]
- Lertsanguansinchai, P.; Chokesuwattanaskul, R.; Petchlorlian, A.; Suttirut, P.; Buddhari, W. Machine Learning-Based Predictive Risk Models for 30-Day and 1-Year Mortality in Severe Aortic Stenosis Patients Undergoing Transcatheter Aortic Valve Implantation. Int. J. Cardiol. 2022, 374, 20–26. [Google Scholar] [CrossRef]
- Kawahara, T.; Fukuda, M.; Oba, K.; Sakamoto, J.; Buyse, M. Meta-Analysis of Randomized Clinical Trials in the Era of Individual Patient Data Sharing. Int. J. Clin. Oncol. 2018, 23, 403–409. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Seitidis, G.; Tsivgoulis, G.; Katsanos, A.H.; Mavridis, D. An Introduction to Individual Participant Data Meta-Analysis. Neurology 2023, 100, 1102–1110. [Google Scholar] [CrossRef]
| Author, Year (Reference) | Study Design | Country/Region | Number of TAVR Patients (Analyzed Cohort) | Mean Age (SD) (Years) | % Female | % Hypertension | % Diabetes Mellitus | % Coronary Artery Disease (CAD) | % Atrial Fibrillation (AF) | % Chronic Kidney Disease (CKD, eGFR < 60) | Mean STS-PROM (SD) (%) | Mean LVEF (SD) (%) | % Transfemoral Approach | % Self-Expanding Valve | All-Cause Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams et al., 2014 [18] | RCT (TAVR arm) | USA (45 centers) | 390 | 83.1 | 46.9 | 95.1 | 34.9 | 75.4 | 40.9 | 12.2 | 7.3 | NA | 82.8 | 100 | 14.2 |
| Alasnag et al., 2022 [19] | Multicenter retrospective cohort registry | Gulf Region (8 centers) | 795 | 74.6 | 43.8 | 83.5 | 61.6 | 47.9 | 18.1 | 39.3 | 4.9 | 52.7 | 95.8 | 61.8 | 5.4 |
| Alnabti et al., 2025 [20] | Cohort registry (prospective and retrospective) | Qatar (1 center) | 241 | 73.7 | 47.7 | 90.0 | 76.3 | 41.9 | 27.4 | 30.3 | NA | NA | 99.2 | 77.2 | 8.7 |
| Arnold et al., 2020 [21] | Registry analysis (STS/ACC TVT) | USA (513 sites) | 45,884 | 83.0 | 46.7 | NA | 36.5 | 58.5 | 40.6 | NA | 5.8 | 58.0 | 91.5 | NA | 12.4 |
| Azevedo et al., 2018 [22] | Prospective cohort (part of multicenter study) | Brazil (1 center) | 58 | 77.8 | 62.1 | 93.1 | 24.4 | 56.9 | 22.4 | 72.4 | NA | 57.4 | 81.0 | 68.9 | 17.2 |
| Barbanti et al., 2019 [23] | Multicenter prospective observational cohort study | Italy (93 centers) | 650 | 80.5 | 58.9 | NA | 24.8 | 19.7 | NA | NA | NA | 53.6 | 100 | 55.1 | 13.8 |
| Bekeredjian et al., 2019 [24] | Registry analysis (GARY: patients with STS < 4%) | Germany (78 hospitals) | 6062 | 78.9 | 39.1 | 86.9 | 21.4 | NA | 18.8 | NA | 2.9 | 54.6 | 83.2 | NA | 10.0 |
| Beyersdorf et al., 2021 [25] | Prospective observational cohort registry (German Aortic Valve Registry—GARY) with Propensity score Matching analysis | Germany | 8942 | 80.9 | 53.2 | 89.7 | 34.4 | 53.0 | 29.1 | 5.4 | 6.3 | NA | 72.9 | NA | 15.0 |
| Biviano et al., 2017 [26] | Observational study | USA, Canada (PARTNER Trial sites) | 1879 | 85.5 | 49.7 | 92.7 | 37.7 | 25.3 | 25.5 | 17.0 | 10.9 | 57.0 | 58.4 | 0 | 35.7 |
| Blankenberg et al., 2024 [27] | RCT (DEDICATE-DZHK6: TAVI vs SAVR, low-intermediate risk) | Germany (38 sites) | 701 | 74.3 | 44.0 | 84.7 | 33.8 | 34.3 | 28.9 | 30.0 | 1.8 | 57.8 | 97.3 | 35.1 | 2.6 |
| Bouleti et al., 2015 [28] | Single-center prospective registry | France (Paris, 1 center) | 123 | 81.5 | 43.9 | 73.2 | 23.6 | 48.8 | 41.5 | 4.1 | 7.1 | 50.1 | 68.3 | 9.7 | 16.0 |
| Boute et al., 2025 [29] | Cohort study (SAVR and TAVR stratified by frailty) | Belgium (Brussels, 1 center) | 449 | 85.3 | 51.2 | 83.8 | 17.7 | 30.8 | 29.9 | 60.1 | NA | 59.6 | 91.8 | 82.3 | 10.0 |
| Brouwer et al., 2020 [30] | RCT (POPular TAVI—Cohort A) | Europe (17 sites, e.g., Netherlands, Belgium) | 665 | 80.0 | 48.7 | 74.8 | 24.5 | 40.9 | NA | 57.7 | 2.5 | 50.0 | NA | NA | 5.7 |
| Butt et al., 2021 [31] | National observational cohort study (Danish registries) | Denmark | 735 | 82.0 | 46.3 | 88.2 | 22.3 | 54.4 | 100 | 11.7 | NA | NA | 78.7 | NA | 9.7 |
| Carroll et al., 2020 [32] | Registry report (STS/ACC TVT Registry) | USA | 276,316 | 80.0 | 44.2 | NA | NA | NA | NA | NA | 4.4 | NA | 95.3 | 26.7 | 13.6 |
| Chopard et al., 2015 [33] | Multicenter prospective registry analysis (FRANCE-2) | France (and onee center in Monaco) | 3875 | 82.8 | 49.4 | 68.8 | 25.4 | 47.6 | 25.8 | 8.6 | 14.1 | 53.2 | 73.0 | 33.5 | 26.5 |
| Costa et al., 2024 [34] | Multicenter registry (OPERA—TAVI), propensity score matching analysis | Europe and North America (15 centers) | 1174 | 82.0 | 56.4 | 85.5 | 28.3 | 38.9 | 25.0 | 9.7 | 3.2 | 60.0 | 100 | 50.0 | 9.7 |
| Dangas et al., 2020 [35] | RCT, open-label, event-driven (GALILEO) | Multicenter, 16 countries | 1644 | 80.6 | 49.5 | 86.2 | 28.7 | 38.3 | NA | 73.3 | 4.2 | 57.8 | NA | 45.9 | 8.5 |
| Didier et al., 2021 [36] | Registry analyses (FRANCE-2 and France-TAVI) linked to administrative databases | France | 8962 | 83.1 | 48.0 | NA | 26.6 | 37.5 | 69.6 | 49.4 | NA | 54.2 | NA | 34.4 | 33.4 |
| Duncan et al., 2015 [37] | National prospective registry analysis (U.K. TAVI Registry) | United Kingdom (England and Wales) | 870 | 82.0 | 48.0 | NA | 22.9 | 47.2 | 23.9 | 6.5 | NA | 50.0 | 68.4 | 52.5 | 21.4 |
| Frerker et al., 2020 [38] | Observational, retrospective, single-center cohort study | Germany (Hamburg) | 2075 | 78.0 | 45.6 | NA | 32.1 | 43.4 | 23.5 | 25.8 | NA | 50.0 | 100 | NA | 7.4 |
| Gilard et al., 2016 [39] | Multicenter prospective national registry analysis (FRANCE-2, long-term follow-up) | France (34 centers) | 3848 | 82.8 | 48.1 | 68.8 | 25.4 | 47.7 | 26.4 | 2.5 | NA | 55.0 | 73.0 | 33.7 | 20.5 |
| Goel et al., 2023 [40] | Observational, retrospective, single-center cohort study | USA (New Orleans) | 1042 | 81.0 | 50.7 | 92.6 | 31.4 | 57.9 | 25.8 | 36.7 | 7.4 | 53.6 | 100 | 29.8 | 22.3 |
| Holmes et al., 2015 [41] | Registry analysis (STS/ACC TVT Registry linked to Medicare) | USA | 12,182 | 84.1 | 50.4 | 89.7 | 39.9 | 69.1 | 33.6 | 60.7 | 11.2 | 52.4 | 64.5 | 31.9 | 23.7 |
| Jochheim et al., 2019 [42] | International, multicenter, observational, prospective registry (OCEAN-TAVI, DOAC vs VKA) | International (Europe, Israel) | 962 | 78.0 | 42.4 | 82.7 | 21.7 | 44.3 | 22.6 | 3.6 | 3.9 | 50.0 | 89.0 | 48.3 | 12.9 |
| Kaneko et al., 2022 [43] | Observational, retrospective, multicenter cohort study (Japanese OCEAN-TAVI cohort) | Japan (16 centers) | 2446 | 78.7 | 42.5 | 91.1 | 42.9 | 67.3 | 58.9 | 5.7 | 6.9 | 56.1 | 91.5 | 53.8 | 2.1 |
| Kapadia et al., 2015 [44] | RCT (PARTNER I—Cohort B, inoperable), 5-year follow-up of TAVR arm | USA, Canada, Germany (21 sites) | 179 | 83.2 | 55.9 | 87.7 | 43.0 | 73.2 | 40.2 | 9.5 | 11.6 | 51.8 | NA | 0 | 30.7 |
| Karra et al., 2024 [45] | Observational, retrospective, single-center cohort study | United Kingdom (London) | 398 | 81.8 | 47.2 | 74.3 | 24.6 | 55.0 | 36.9 | 52.4 | 5.8 | 55.0 | 81.8 | 50.9 | 15.0 |
| Kawashima et al., 2020 [46] | Multicenter, observational, prospective registry (OCEAN-TAVI, AF patients) | Japan (18 centers) | 721 | 85.1 | 70.0 | 86.3 | 29.7 | 30.2 | 28.2 | 7.7 | 5.7 | 63.4 | 93.6 | 29.7 | 9.7 |
| Khashaba et al., 2014 [47] | Observational, retrospective, single-center cohort study | Egypt | 1007 | 80.5 | 48.8 | 85.4 | 36.6 | 53.7 | 41.5 | 43.9 | 7.1 | 54.0 | 50.0 | 26.8 | 10.4 |
| Krasniqi et al., 2024 [48] | Observational, retrospective, single-center cohort study | Germany (Essen) | 1239 | 81.4 | 51.1 | 86.7 | 30.3 | 51.6 | 26.6 | 68.1 | 5.3 | 53.8 | 89.4 | 22.3 | 8.6 |
| Lachonious et al., 2023 [49] | Observational, retrospective cohort study (SWEDEHEART registry) | Sweden | 1566 | 81.7 | 51.5 | 87.3 | 33.9 | 56.4 | 39.5 | 67.1 | 4.3 | 53.1 | 93.4 | 29.3 | 9.5 |
| Lange et al., 2016 [50] | Registry analysis (GARY), focused on quality of life | Germany | 9749 | 80.9 | 45.4 | 89.0 | 33.2 | 52.2 | 33.7 | 5.2 | 6.3 | 50.0 | 73.0 | 42.4 | 19.9 |
| Leon et al., 2016 [51] | RCT (PARTNER—Cohort B, inoperable, TAVR vs standard therapy) | USA, Canada, Germany (21 sites) | 179 | 83.2 | 55.9 | 87.7 | 43.0 | 73.2 | 40.2 | 9.5 | 11.6 | 51.8 | 100 | 0 | 30.7 |
| Li et al., 2021 [52] | Observational, retrospective, single-center cohort study | China (Beijing) | 293 | 77.8 | 39.1 | 96.3 | 54.7 | 57.8 | 31.7 | 100 | 6.8 | 50.0 | 91.3 | 19.3 | 7.0 |
| Lundahl et al., 2024 [53] | Observational, retrospective, multicenter cohort study (SWEDEHEART registry) | Sweden | 2905 | 82.0 | 51.0 | 81.0 | 24.0 | 45.0 | 35.0 | 65.0 | 5.5 | 53.0 | 89.0 | 59.0 | 7.8 |
| Mack et al., 2023 [54] | RCT (PARTNER I—Cohort A, high risk, TAVR vs SAVR), 5-year follow-up | USA, Canada, Germany (25 sites) | 348 | 84.0 | 45.1 | 91.1 | 46.8 | 75.3 | 39.9 | 45.4 | 11.7 | NA | 70.1 | 0 | 1.0 |
| Maeda et al., 2022 [55] | Observational, prospective, multicenter cohort study (OPTIMAL-TAVI Japan) | Japan (six hospitals) | 302 | 84.8 | 70.0 | 82.0 | 26.0 | 24.0 | 32.0 | 64.0 | 6.9 | 66.3 | 100 | 0 | 4.6 |
| Mangner et al., 2019 [56] | Observational, retrospective, single-center cohort study (low-flow, low-gradient aortic stenosis) | Germany (Leipzig) | 270 | 78.8 | 40.7 | 82.3 | 23.0 | 48.2 | 24.3 | 54.2 | 3.9 | 54.0 | 90.2 | 42.6 | 14.0 |
| Okuno et al., 2023 [57] | Cohort study | Switzerland (Bern) | 101 | 85.9 | 68.6 | 88.3 | 31.0 | 36.8 | 31.4 | 79.3 | 5.7 | 52.0 | 92.9 | 34.2 | 8.0 |
| Reardon et al., 2017 [58] | RCT (SURTAVI Trial—TAVR vs SAVR, intermediate risk) | USA, Europe, Canada (87 centers) | 864 | 79.8 | 45.1 | 89.8 | 32.2 | 52.5 | 24.5 | 39.0 | 4.4 | 56.0 | 83.6 | 100 | 4.9 |
| Schaafsma et al., 2023 [59] | Observational, retrospective, single-center cohort study | Netherlands (Zwolle) | 648 | 81.1 | 50.1 | 81.4 | 28.7 | 54.0 | 37.4 | 65.1 | 4.4 | 54.0 | 83.4 | 60.0 | 5.7 |
| Seeger et al., 2017 [60] | Observational, retrospective, single-center cohort study (Apixaban vs VKA in AF post-TAVI) | Germany (Ulm) | 272 | 81.5 | 46.0 | 87.1 | 30.1 | 53.7 | 100 | 80.5 | 5.0 | 52.0 | 100 | 48.9 | 15.8 |
| Thyregod et al., 2024 [61] | RCT (NOTION Trial—TAVR vs SAVR in low risk), 5-year follow-up | Denmark and Sweden (three centers) | 139 | 79.2 | 44.6 | 77.0 | 15.8 | 20.1 | 5.8 | 20.9 | 2.9 | 58.0 | 100 | 100 | 1.0 |
| Stortecky et al., 2019 [62] | Observational, prospective, single-center cohort study (impact of AF) | Switzerland (Bern) | 389 | 82.9 | 45.5 | 78.6 | 24.2 | 53.2 | 33.7 | 36.5 | 6.5 | 52.6 | 75.3 | 49.3 | 17.6 |
| Strange et al., 2022 [63] | National registry analysis (UK TAVI Registry) | United Kingdom | 20,001 | 82.0 | 46.6 | 77.5 | 22.2 | 40.4 | 33.2 | 58.4 | 4.3 | NA | 88.2 | 48.9 | 6.9 |
| Tamburino et al., 2015 [64] | Prospective, observational, multicenter registry | Italy (14 centers) | 663 | 80.8 | 55.1 | 74.1 | 31.4 | 52.9 | 21.1 | 10.3 | 11.5 | NA | 75.7 | 71.5 | 21.3 |
| Ternacle et al., 2021 [65] | Observational, retrospective, single-center cohort study | France (Caen) | 675 | 83.1 | 49.8 | 84.5 | 25.9 | 42.1 | 25.3 | 59.0 | NA | NA | 82.6 | 35.0 | 5.8 |
| Thogata et al., 2023 [66] | Retrospective analysis | India (Nellore) | 200 | 75.2 | 36.7 | 87.1 | 42.7 | 63.9 | 48.8 | 67.6 | NA | NA | 92.4 | NA | 18.5 |
| Thyregod et al., 2015 [67] | RCT (NOTION Trial—low risk), 10-year follow-up | Denmark and Sweden (three centers) | 145 | 79.1 | 45.5 | 73.9 | 16.6 | 21.6 | 6.5 | 19.4 | 3.0 | 58.0 | 100 | 100 | 4.3 |
| Tomii et al., 2025 [68] | Observational study | Europe (Germany, Switzerland, United Kingdom) | 739 | 82.2 | 60.4 | 85.1 | 28.6 | 37.3 | 25.2 | 10.9 | 3.2 | 60.0 | 100 | 63.2 | 2.6 |
| Van Bergeijk et al., 2025 [69] | Retrospective cohort study (Netherlands Heart Registry) | Netherlands | 7823 | 80.7 | 52.8 | 82.1 | 29.5 | 48.2 | 35.6 | 65.8 | 4.9 | 54.0 | 86.5 | 51.8 | 8.9 |
| Vekstein et al., 2025 [70] | Registry Analysis (STS/ACC TVT Registry) in low-risk patients | USA | 102,774 | 84.7 | 50.3 | 90.7 | 33.3 | 46.2 | 29.0 | 61.8 | 7.3 | 53.5 | 100 | 22.0 | 15.2 |
| Virtanen et al., 2019 [71] | Observational, retrospective, national cohort study (FinnValve registry), TAVR vs SAVR | Finland | 343 | 82.2 | 56.3 | 84.3 | 26.6 | 47.8 | 43.3 | 63.0 | 6.0 | 55.3 | 86.2 | 47.1 | 7.0 |
| Wang et al., 2022 [72] | Prospective, multicenter, single-arm study (TaurusOne valve) | China | 120 | 76.1 | 45.1 | 76.6 | 30.1 | 39.1 | 15.7 | 28.3 | 5.7 | 58.3 | 85.5 | 59.8 | 38.2 |
| Webb et al., 2015 [73] | RCT (PARTNER II—SAPIEN XT inoperable cohort) | USA and Canada (57 centers) | 497 | 82.4 | 46.7 | 86.7 | 38.7 | 64.0 | 37.3 | 10.0 | 7.3 | 54.0 | 66.7 | 0 | 15.0 |
| Witberg et al., 2019 [3] | International, multicenter, retrospective registry (AMTRAC registry), patients < 70 years rejected for surgery | International (Europe, Israel, Canada) | 354 | 81.0 | 48.3 | 84.4 | 41.0 | 60.8 | 45.1 | 100 | 4.9 | 36.8 | 93.7 | 27.1 | 12.4 |
| Yu et al., 2018 [74] | Prospective, multicenter, national registry (K-TAVI Registry) | South Korea (14 centers) | 1,038 | 78.0 | 34.0 | 88.0 | 57.5 | 61.4 | 45.5 | 100 | 7.7 | NA | 89.4 | 41.0 | 7.6 |
| Study | Randomization (0–2) | Blinding (0–2) | Withdrawals/Dropouts (0–1) | Total Score (de 5) |
|---|---|---|---|---|
| Reardon et al., 2017 [58] | 2 | 0 | 1 | 3 |
| Dangas et al., 2020 [35] | 2 | 0 | 1 | 3 |
| Leon et al., 2016 [51] | 2 | 0 | 1 | 3 |
| Thyregod et al., 2015 [67] | 2 | 0 | 1 | 3 |
| Mack et al., 2023 [54] | 2 | 0 | 1 | 3 |
| Brouwer et al., 2020 [30] | 2 | 0 | 1 | 3 |
| Adams et al., 2014 [18] | 2 | 0 | 1 | 3 |
| Blankenberg et al., 2024 [27] | 2 | 0 | 1 | 3 |
| Kapadia et al., 2014 [44] | 2 | 0 | 1 | 3 |
| Thyregod et al., 2024 [61] | 2 | 0 | 1 | 3 |
| Webb et al., 2015 [73] | 2 | 0 | 1 | 3 |
| Study | Confounding | Participant Selection | Classification of Interventions | Deviations from Interventions | Missing Data | Measurement of Outcomes | Selection of Reported Outcomes | Overall Risk |
|---|---|---|---|---|---|---|---|---|
| Alasnag et al., 2022 [19] | Low | Low | Low | Low | Low | Low | Low | Low |
| Alnabti et al., 2025 [20] | Moderate | Low | Low | Low | Moderate | Low | Moderate | Moderate |
| Arnold et al., 2021 [21] | Low | Low | Low | Low | Moderate | Low | Low | Low |
| Azevedo et al., 2018 [22] | Moderate | Moderate | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Barbanti et al., 2019 [23] | Moderate | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Bekeredjian et al., 2019 [24] | Moderate | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Beyersdorf et al., 2021 [25] | Moderate | Moderate | Low | Low | Low | Moderate | Moderate | Moderate |
| Biviano et al., 2017 [26] | Moderate | Low | Low | Moderate | Moderate | Low | Moderate | Moderate |
| Bouleti et al., 2015 [28] | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Boute et al., 2025 [29] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Butt et al., 2021 [31] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Carroll et al., 2020 [32] | Low | Low | Low | Low | Low | Low | Low | Low |
| Chopard et al., 2015 [33] | Moderate | Low | Low | Moderate | Low | Moderate | Moderate | Moderate |
| Costa et al., 2024 [34] | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Didier et al., 2021 [36] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Duncan et al., 2015 [37] | Moderate | Moderate | Low | Low | Low | Moderate | Moderate | Moderate |
| Frerker et al., 2017 [38] | Moderate | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Gilard et al., 2016 [39] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Goel et al., 2023 [40] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Holmes Jr. et al., 2015 [41] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Jochheim et al., 2019 [42] | Moderate | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Kaneko et al., 2022 [43] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Karra et al., 2024 [45] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Kawashima et al., 2020 [46] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Khashaba et al., 2014 [47] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Krasniqi et al., 2024 [48] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Lachonius et al., 2025 [49] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Lange et al., 2016 [50] | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Li et al., 2021 [52] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Lundahl et al., 2024 [53] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Maeda et al., 2022 [55] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Mangner et al., 2019 [56] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Okuno et al., 2023 [57] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Reardon et al., 2017 [58] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Schaafsma et al., 2023 [59] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Seeger et al., 2017 [60] | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Stortecky et al., 2019 [62] | Moderate | Low | Low | Moderate | Low | Moderate | Moderate | Moderate |
| Strange et al., 2022 [63] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Tamburino et al., 2015 [64] | Moderate | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Ternacle et al., 2021 [65] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Thogata et al., 2023 [66] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Tomii et al., 2025 [68] | Moderate | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Van Bergeijk et al., 2025 [69] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Vekstein et al., 2025 [70] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Virtanen et al., 2019 [71] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Wang et al., 2022 [72] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Witberg et al., 2019 [3] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Yu et al., 2018 [74] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Study | Selection (0–4★) | Comparability (0–2★) | Outcome (0–3★) | Total (0–9★) | Quality |
|---|---|---|---|---|---|
| Alasnag et al., 2022 [19] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Alnabti et al., 2025 [20] | ★★★★ | ★★ | ★ | 7★ | Good |
| Arnold et al. 2021 [21] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Azevedo et al., 2018 [22] | ★★★ | ★ | ★★ | 6★ | Good |
| Barbanti et al., 2019 [23] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Bekeredjian et al., 2019 [24] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Beyersdorf et al., 2021 [25] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Biviano et al., 2017 [26] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Bouleti et al., 2015 [28] | ★★★ | ★ | ★★★ | 7★ | Good |
| Boute et al., 2025 [29] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Butt et al., 2021 [31] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Carroll et al., 2020 [32] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Chopard et al., 2015 [33] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Costa et al., 2024 [34] | ★★★ | ★★ | ★★ | 7★ | Good |
| Didier et al., 2021 [36] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Duncan et al., 2015 [37] | ★★★ | ★ | ★★★ | 7★ | Good |
| Frerker et al., 2017 [38] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Gilard et al., 2016 [39] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Goel et al., 2023 [40] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Holmes Jr. et al., 2015 [41] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Jochheim et al., 2019 [42] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Kaneko et al., 2022 [43] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Karra et al., 2024 [45] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Kawashima et al., 2020 [46] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Khashaba et al., 2014 [47] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Krasniqi et al., 2024 [48] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Lachonius et al., 2025 [49] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Lange et al., 2016 [50] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Li et al., 2021 [52] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Lundahl et al., 2024 [53] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Maeda et al., 2022 [55] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Mangner et al., 2019 [56] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Okuno et al., 2023 [57] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Reardon et al., 2017 [58] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Schaafsma et al., 2023 [59] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Seeger et al., 2017 [60] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Stortecky et al., 2019 [62] | ★★★ | ★ | ★★★ | 7★ | Good |
| Strange et al., 2022 [63] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Tamburino et al., 2015 [64] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Ternacle et al., 2021 [65] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Thogata et al., 2023 [66] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Tomii et al., 2025 [68] | ★★★ | ★★ | ★★★ | 8★ | Very Good |
| Van Bergeijk et al., 2025 [69] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Vekstein et al., 2025 [70] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Virtanen et al., 2019 [71] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Wang et al., 2022 [72] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Witberg et al., 2019 [3] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Yu et al., 2018 [74] | ★★★★ | ★★ | ★★★ | 9★ | Excellent |
| Study ID | 1-Year Mortality Rate (%) | Average Age | % Women | % Diabetes | % CKD | % AF | Average STS-PROM | Average EuroSCORE II | Average LVEF (%) | % Transfemoral | % Self-Expanding | Average Recruitment Year | Cohort Size | Study Design | Country | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams et al., 2014 [18] | 14.2 | 83.1 | 46.9 | 34.9 | 12.2 | 40.9 | 7.3 | 17.7 | NA | 82.8 | 100.0 | 2013 | 390 | RCT | USA | 6.0 |
| Alasnag et al., 2022 [19] | 5.4 | 74.6 | 44.0 | 61.6 | 8.4 | 18.1 | 4.9 | NA | 53.0 | 95.8 | 61.8 | 2018 | 795 | Registry | Gulf Region | 10.0 |
| Alnabti et al., 2025 [20] | 8.7 | 73.7 | 47.7 | 76.3 | 30.3 | 27.4 | NA | NA | NA | 99.2 | 77.2 | 2018 | 241 | Registry | Qatar | 7.8 |
| Arnold et al., 2020 [21] | 12.4 | 83.0 | 46.7 | 36.5 | NA | 40.6 | 5.8 | NA | 58.0 | 91.5 | NA | 2016 | 45,884 | Registry | USA | 10.0 |
| Azevedo et al., 2018 [22] | 17.2 | 77.8 | 62.1 | 24.4 | 72.4 | 22.4 | NA | 12.7 | 57.4 | 81.0 | 68.9 | 2013 | 58 | Observational | Brazil | 6.7 |
| Barbanti et al., 2019 [23] | 13.8 | 80.5 | 58.9 | 24.8 | 1.4 | NA | NA | 4.9 | 53.6 | 100.0 | 55.1 | 2011 | 650 | Observational (PSM) | Italy | 8.9 |
| Bekeredjian et al., 2019 [24] | 10.0 | 78.9 | 39.1 | 21.4 | 0.0 | 18.8 | 2.9 | 12.9 | 54.6 | 83.1 | NA | 2015 | 6062 | Registry | Germany | 8.9 |
| Beyersdorf et al., 2021 [25] | 20.9 | 80.9 | 53.2 | 34.4 | 5.3 | 29.1 | 6.3 | NA | NA | 72.9 | NA | 2012 | 4157 | Registry | Germany | 8.9 |
| Biviano et al., 2017 [26] | 20.6 | 85.5 | 50.7 | 37.7 | 16.5 | 25.0 | 10.9 | NA | 56.9 | 58.4 | 0.0 | 2010 | 1879 | Post-hoc of RCT | Multinational | 8.9 |
| Blankenberg et al., 2024 [27] | 2.6 | 74.0 | 44.0 | 33.8 | NA | 28.9 | 1.8 | 2.1 | 57.8 | 97.3 | 35.1 | 2019 | 701 | RCT | Germany | 6.0 |
| Bouleti et al., 2015 [28] | 16.0 | 82.0 | 43.9 | 23.6 | 4.1 | 41.5 | 7.1 | 7.8 | 50.1 | 68.3 | NA | 2008 | 123 | Observational | France | 7.8 |
| Boute et al., 2025 [29] | 11.9 | 85.4 | 55.1 | 18.1 | 59.7 | 30.8 | NA | 4.3 | 59.8 | 92.2 | 80.7 | 2017 | 449 | Observational | Belgium | 10.0 |
| Brouwer et al., 2020 [30] | 6.0 | 80.0 | 48.7 | 24.5 | NA | 0.0 | 2.5 | NA | NA | NA | NA | 2016 | 665 | RCT | Europe | 6.0 |
| Butt et al., 2021 [31] | 11.0 | 82.5 | 46.3 | 22.3 | 11.7 | 100.0 | NA | NA | NA | 77.3 | NA | 2014 | 735 | Registry | Denmark | 8.9 |
| Carroll et al., 2020 [32] | 13.2 | 80.0 | 44.2 | NA | NA | NA | 4.4 | NA | NA | 95.3 | 26.7 | 2019 | 276,316 | Registry | USA | 10.0 |
| Chopard et al., 2015 [33] | 19.2 | 82.8 | 49.4 | 25.4 | 8.6 | 25.8 | 14.1 | 21.8 | NA | 73.0 | 33.5 | 2011 | 3875 | Registry | France | 8.9 |
| Costa et al., 2024 [34] | 10.1 | 82.0 | 56.4 | 28.3 | 9.7 | 25.0 | 3.2 | NA | 60.0 | 100.0 | 50.0 | 2019 | 1174 | Registry | Multinational | 7.8 |
| Dangas et al., 2020 [35] | 5.3 | 80.6 | 50.5 | 28.7 | NA | 0.0 | 4.2 | 4.1 | 57.8 | NA | 51.0 | 2017 | 1644 | RCT | Multinational | 6.0 |
| Didier et al., 2021 [36] | 14.9 | 83.2 | 48.9 | 26.2 | 34.7 | 100.0 | 5.0 | NA | 55.0 | 89.2 | NA | 2015 | 1714 | Registry | France | 10.0 |
| Duncan et al., 2015 [37] | 21.4 | 82.0 | 48.0 | 22.9 | 6.5 | 23.9 | NA | 18.5 | NA | 68.4 | 52.5 | 2008 | 850 | Registry | United Kingdom | 7.8 |
| Frerker et al., 2020 [38] | 7.4 | 77.5 | 60.4 | 23.6 | 10.6 | 8.0 | NA | 6.8 | NA | 100.0 | NA | 2014 | 805 | Observational (PSM) | Germany | 8.9 |
| Gilard et al., 2016 [39] | 23.2 | 82.8 | 49.3 | 25.4 | 2.5 | 26.3 | NA | 21.7 | 55.0 | 73.0 | 33.7 | 2011 | 4201 | Registry | France | 10.0 |
| Goel et al., 2023 [40] | 51.0 | 77.0 | 41.2 | 44.9 | 53.9 | 47.9 | 6.9 | NA | 25.0 | 88.5 | 35.6 | 2018 | 1172 | Registry | USA | 10.0 |
| Holmes et al., 2015 [41] | 23.7 | 84.0 | 49.3 | 42.1 | 8.8 | 38.6 | 11.2 | NA | 54.0 | 62.1 | NA | 2012 | 42,982 | Registry | USA | 10.0 |
| Jochheim et al., 2019 [42] | 13.8 | 81.0 | 60.7 | 30.1 | 40.9 | 100.0 | 4.2 | NA | 55.0 | 95.9 | NA | 2012 | 962 | Registry | Europe | 8.9 |
| Kaneko et al., 2022 [43] | 12.1 | 81.6 | 47.4 | 42.6 | 55.4 | 49.3 | 5.0 | NA | NA | 94.7 | 35.8 | 2015 | 64,815 | Registry | USA | 10.0 |
| Kapadia et al., 2015 [44] | 30.7 | 83.1 | 43.6 | 44.7 | 45.3 | 33.0 | 11.8 | 29.3 | 58.0 | 100.0 | 0.0 | 2008 | 179 | RCT | Multinational | 6.0 |
| Karra et al., 2024 [45] | 9.3 | 81.0 | 44.0 | 37.0 | 62.0 | 40.0 | 2.9 | 2.6 | 55.0 | 94.6 | 49.3 | 2014 | 2073 | Registry | Israel | 10.0 |
| Kawashima et al., 2020 [46] | 12.0 | 85.0 | 71.0 | 24.0 | 47.0 | 32.0 | 5.4 | NA | 62.0 | 96.6 | 62.4 | 2014 | 7981 | Registry | Japan | 10.0 |
| Khashaba et al., 2014 [47] | 20.0 | 78.6 | 60.0 | 40.0 | 60.0 | 50.0 | NA | 26.5 | 51.0 | 100.0 | 0.0 | 2012 | 10 | Observational | Egypt | 10.0 |
| Krasniqi et al., 2024 [48] | 6.6 | 82.0 | 51.0 | 23.0 | 36.0 | 38.0 | NA | 3.1 | NA | 100.0 | 58.0 | 2019 | 1146 | Registry | Denmark | 10.0 |
| Lachonious et al., 2023 [49] | 12.0 | 81.2 | 48.0 | 28.0 | 40.2 | 47.9 | NA | 3.8 | NA | 91.1 | 62.4 | 2012 | 3349 | Registry | Sweden | 10.0 |
| Lange et al., 2016 [50] | 20.7 | 81.1 | 58.7 | 32.1 | 35.8 | 36.1 | 7.5 | 23.3 | NA | 100.0 | NA | 2012 | 6563 | Registry | Germany | 8.9 |
| Leon et al., 2016 [51] | 12.3 | 81.6 | 44.9 | 31.6 | 64.1 | 37.0 | 5.8 | NA | NA | 76.3 | 0.0 | 2012 | 1011 | RCT | Multinational | 6.0 |
| Li et al., 2021 [52] | 6.8 | 74.8 | 47.9 | 29.8 | 29.8 | 13.9 | 4.1 | NA | 62.1 | 83.9 | 83.3 | 2016 | 3028 | Registry | China | 10.0 |
| Lundahl et al., 2024 [53] | 11.0 | 82.0 | 47.5 | 23.9 | 44.0 | 48.0 | NA | 2.9 | NA | 96.6 | NA | 2019 | 4847 | Registry | Denmark | 10.0 |
| Mack et al., 2023 [54] | 1.0 | 73.0 | 32.5 | 30.1 | NA | 15.3 | 1.9 | NA | 60.9 | 100.0 | 0.0 | 2017 | 496 | RCT | Multinational | 6.0 |
| Maeda et al., 2022 [55] | 9.7 | 85.3 | 68.3 | 23.3 | 47.9 | 32.1 | 4.6 | NA | 62.0 | 96.6 | NA | 2015 | 16,837 | Registry | Japan | 10.0 |
| Mangner et al., 2019 [56] | 12.6 | 81.5 | 58.3 | 32.6 | 47.6 | 100.0 | 4.2 | NA | 52.5 | 100.0 | NA | 2014 | 598 | Observational | Germany | 8.9 |
| Okuno et al., 2023 [57] | 6.7 | 82.2 | 41.5 | 23.9 | 43.1 | 40.5 | 3.1 | NA | 58.0 | 95.8 | 45.4 | 2016 | 11,889 | Registry | Switzerland | 10.0 |
| Reardon et al., 2017 [58] | 6.7 | 79.8 | 44.1 | 32.8 | 32.2 | 22.8 | 4.4 | NA | NA | 90.7 | 100.0 | 2014 | 864 | RCT | Multinational | 6.0 |
| Schaafsma et al., 2023 [59] | 8.1 | 73.1 | 42.4 | NA | 19.0 | 19.3 | 5.7 | 5.2 | NA | 95.0 | NA | 2017 | 2083 | Registry | South Africa | 10.0 |
| Seeger et al., 2017 [60] | 13.9 | 82.5 | 61.8 | 32.7 | 64.0 | 100.0 | 4.7 | 21.0 | 54.0 | 100.0 | 63.2 | 2013 | 272 | Observational | Germany | 8.9 |
| Thyregod et al., 2024 [61] | 4.9 | 79.1 | 48.2 | 15.6 | 9.9 | 7.1 | 3.0 | 7.5 | 60.1 | 100.0 | 100.0 | 2011 | 145 | RCT | Nordic Countries | 6.0 |
| Stortecky et al., 2019 [62] | 16.7 | 83.0 | 46.1 | 23.9 | 20.4 | 22.8 | 7.5 | 22.8 | 55.0 | 71.8 | 92.5 | 2011 | 697 | Registry | Switzerland | 7.8 |
| Strange et al., 2022 [63] | 8.1 | 82.0 | 48.6 | 22.8 | 43.1 | 47.9 | NA | 2.9 | NA | 96.0 | 62.1 | 2014 | 6,56 | Registry | Denmark | 10.0 |
| Tamburino et al., 2015 [64] | 13.8 | 80.5 | 58.9 | 24.8 | 1.4 | NA | NA | 21.4 | 53.6 | 100.0 | 55.1 | 2011 | 650 | Observational (PSM) | Italy | 8.9 |
| Ternacle et al., 2021 [65] | 1.0 | 73.3 | 32.6 | 30.1 | NA | 15.3 | 1.9 | NA | 60.8 | 100.0 | 0.0 | 2017 | 495 | Sub-study of RCT | Multinational | 10.0 |
| Thogata et al., 2023 [66] | 18.2 | 72.8 | 41.5 | 44.8 | 24.3 | 22.4 | NA | NA | 43.1 | 78.4 | 62.7 | 2019 | 302 | Observational | India | 10.0 |
| Thyregod et al., 2015 [67] | 4.9 | 79.1 | 48.2 | 15.6 | 9.9 | 7.1 | 3.0 | NA | 60.1 | 100.0 | 100.0 | 2011 | 145 | RCT | Nordic Countries | 6.0 |
| Tomii et al., 2025 [68] | 7.7 | 80.0 | 60.5 | 23.3 | 35.3 | 14.1 | 2.9 | NA | 58.0 | 100.0 | 50.0 | 2017 | 739 | Sub-study of RCT | Europe | 8.9 |
| Van Bergeijk et al., 2025 [69] | 5.6 | 81.0 | 48.3 | 21.1 | 33.6 | 29.8 | NA | 2.6 | NA | 95.8 | NA | 2017 | 10,767 | Registry | Netherlands | 10.0 |
| Vekstein et al., 2025 [70] | 5.7 | 75.0 | 37.5 | 32.2 | NA | 22.8 | 2.0 | NA | 58.0 | 100.0 | 32.2 | 2022 | 16,988 | Registry | USA | 10.0 |
| Virtanen et al., 2019 [71] | 4.1 | 75.3 | 40.5 | 20.3 | 6.3 | 19.0 | 1.8 | NA | NA | 100.0 | NA | 2015 | 306 | Observational (PSM) | Finland | 10.0 |
| Wang et al., 2022 [72] | 6.7 | 76.8 | 51.7 | 30.0 | NA | 14.2 | 6.1 | NA | 57.9 | 99.2 | 100.0 | 2020 | 120 | Prospective | China | 10.0 |
| Webb et al., 2015 [73] | 22.0 | 84.1 | 40.2 | 42.1 | 49.6 | 38.6 | 10.8 | NA | 54.0 | 100.0 | 0.0 | 2011 | 271 | RCT | Multinational | 6.0 |
| Witberg et al., 2019 [3] | 5.1 | 65.0 | 30.4 | 38.6 | NA | 10.9 | 2.3 | 1.9 | 50.0 | 96.6 | 62.1 | 2018 | 750 | Registry | Multinational | 10.0 |
| Yu et al., 2018 [74] | 10.6 | 80.0 | 49.3 | 35.5 | 23.3 | 19.3 | 5.8 | 17.5 | 58.6 | 91.6 | NA | 2014 | 1007 | Registry | South Korea | 10.0 |
| Model Abbr. | Model Name | MAE | MSE | RMSE | R2 | RMSLE | MAPE | TT (s) |
|---|---|---|---|---|---|---|---|---|
| ada | AdaBoost Regressor | 41.837 | 588.412 | 58.768 | 0.1908 | 0.4504 | 0.3665 | 0.1230 |
| xgboost | Extreme Gradient Boosting | 44.553 | 690.457 | 63.180 | 0.0308 | 0.4699 | 0.3390 | 0.1010 |
| et | Extra Trees Regressor | 46.728 | 774.489 | 65.289 | −0.0353 | 0.4805 | 0.3757 | 0.1570 |
| rf | Random Forest Regressor | 46.907 | 693.559 | 64.722 | −0.0126 | 0.4933 | 0.4015 | 0.2430 |
| gbr | Gradient Boosting Regressor | 47.496 | 801.806 | 67.985 | −0.1607 | 0.5074 | 0.3751 | 0.1080 |
| br | Bayesian Ridge | 52.424 | 728.165 | 70.859 | −0.5175 | 0.5354 | 0.7187 | 0.0640 |
| lasso | Lasso Regression | 53.373 | 759.845 | 71.738 | −0.5479 | 0.5573 | 0.7428 | 0.0590 |
| llar | Lasso Least Angle Regression | 53.373 | 759.845 | 71.738 | −0.5479 | 0.5573 | 0.7428 | 0.0590 |
| huber | Huber Regressor | 54.631 | 858.332 | 75.655 | −1.1585 | 0.5549 | 0.6779 | 0.0960 |
| en | Elastic Net | 55.280 | 811.797 | 75.077 | −0.7140 | 0.5632 | 0.6957 | 0.0630 |
| dt | Decision Tree Regressor | 55.640 | 783.749 | 74.446 | −0.4875 | 0.5958 | 0.4110 | 0.0850 |
| ridge | Ridge Regression | 58.226 | 1,123.217 | 78.645 | −1.0956 | 0.6054 | 0.8556 | 0.0600 |
| lr | Linear Regression | 60.527 | 959.125 | 84.368 | −1.8527 | 0.6060 | 0.8607 | 0.0610 |
| lightgbm | Light Gradient Boosting Machine | 60.914 | 829.995 | 80.008 | −0.8215 | 0.6483 | 0.7869 | 0.1820 |
| dummy | Dummy Regressor | 60.914 | 929.995 | 81.239 | −0.8433 | 0.6483 | 0.7869 | 0.0000 |
| omp | Orthogonal Matching Pursuit | 65.273 | 950.847 | 82.094 | −1.0051 | 0.6641 | 0.8143 | 0.0570 |
| knn | K Neighbors Regressor | 67.586 | 963.289 | 84.364 | −1.4425 | 0.7194 | 0.8750 | 0.0590 |
| lar | Least Angle Regression | 151.363 | 13,529.595 | 231.197 | −38.9906 | 0.8156 | 15.673 | 0.0660 |
| par | Passive Aggressive Regressor | 1,591.696 | 7,776,826.429 | 3,068.901 | −40,213.4769 | 13.979 | 127.703 | 0.0610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liscano, Y.; Martinez Guevara, D.; Urriago-Osorio, G.A.; Quintana, J. Beyond Conventional Meta-Analysis: A Meta-Learning Model to Predict Cohort-Level Mortality After Transcatheter Aortic Valve Replacement (TAVR). J. Cardiovasc. Dev. Dis. 2025, 12, 376. https://doi.org/10.3390/jcdd12100376
Liscano Y, Martinez Guevara D, Urriago-Osorio GA, Quintana J. Beyond Conventional Meta-Analysis: A Meta-Learning Model to Predict Cohort-Level Mortality After Transcatheter Aortic Valve Replacement (TAVR). Journal of Cardiovascular Development and Disease. 2025; 12(10):376. https://doi.org/10.3390/jcdd12100376
Chicago/Turabian StyleLiscano, Yamil, Darly Martinez Guevara, Gustavo Andrés Urriago-Osorio, and John Quintana. 2025. "Beyond Conventional Meta-Analysis: A Meta-Learning Model to Predict Cohort-Level Mortality After Transcatheter Aortic Valve Replacement (TAVR)" Journal of Cardiovascular Development and Disease 12, no. 10: 376. https://doi.org/10.3390/jcdd12100376
APA StyleLiscano, Y., Martinez Guevara, D., Urriago-Osorio, G. A., & Quintana, J. (2025). Beyond Conventional Meta-Analysis: A Meta-Learning Model to Predict Cohort-Level Mortality After Transcatheter Aortic Valve Replacement (TAVR). Journal of Cardiovascular Development and Disease, 12(10), 376. https://doi.org/10.3390/jcdd12100376










