Polypharmacy Patterns in Multimorbid Older People with Cardiovascular Disease: Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
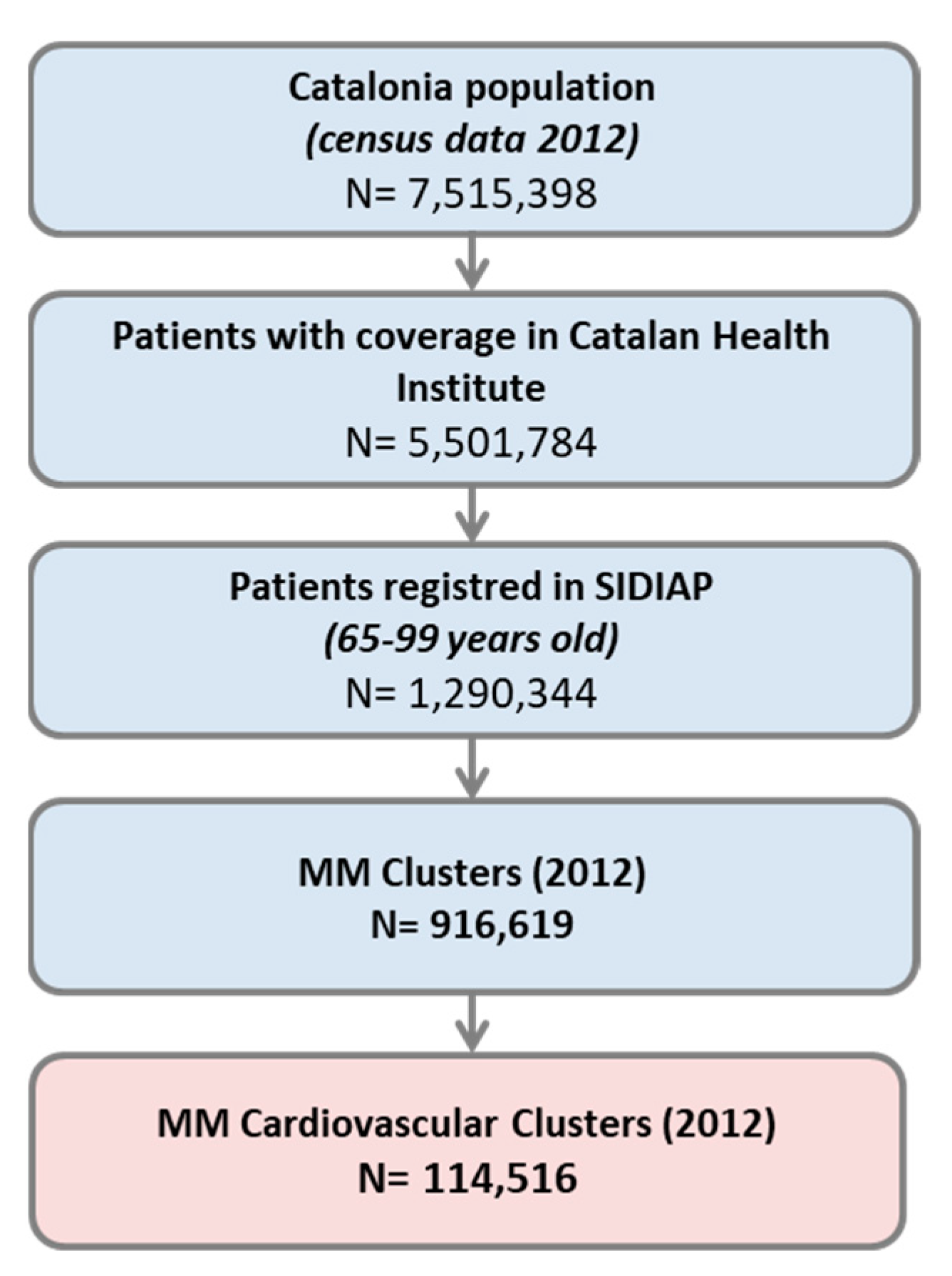
2.1. Design, Setting, and Population
2.2. Data Source
2.3. Variables
2.3.1. Chronic Diseases and Multimorbidity
2.3.2. Drugs and Classification
2.3.3. Drug Groups and Chronic Disease Mapping
2.3.4. Analytical Variables
2.3.5. Demographic and Socioeconomic Variables
2.4. Statistical Analysis
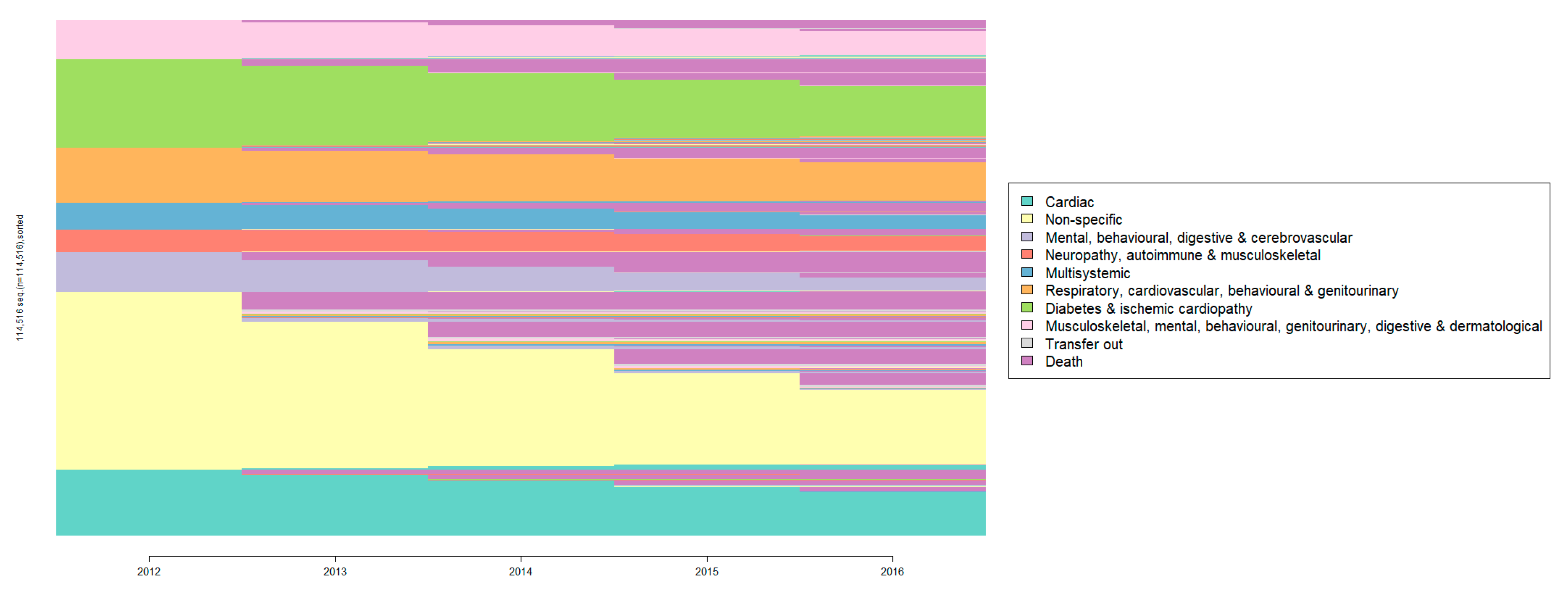
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 15 September 2022).
- Khan, T. WHO Health Topics: Cardiovascular Diseases; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 15 September 2022).
- World Health Organization. The Global Health Observatory: Global Health Estimates: Leading Causes of Death; World Health Organization: Geneva, Switzerland, 2022; pp. 1–4. [Google Scholar]
- Bots, S.H.; Peters, S.A.E.; Woodward, M. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2017, 2, e000298. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- World Health Organization. Medication Safety in Polypharmacy; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Hoel, R.W.; Connolly, R.M.G.; Takahashi, P.Y. Polypharmacy management in older patients. Mayo Clin. Proc. 2021, 96, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Mariño, A.; Roso-Llorach, A.; López-Jiménez, T.; Villen, N.; Amado-Guirado, E.; Fernández-Bertolin, S.; Carrasco-Ribelles, L.A.; Borras, J.M.; Violán, C.; Vetrano, L. Medication-related problems in older people with multimorbidity in catalonia: A real-world data study with 5 years’ follow-up. J. Clin. Med. 2021, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.T.; Park, H.; Kim, D.W.; Jeon, E.K.; Rhee, C.M.; Kalantar-Zadeh, K.; Kang, E.W.; Kang, S.-W.; Han, S.H. Polypharmacy, hospitalization, and mortality risk: A nationwide cohort study. Sci. Rep. 2020, 10, 18964. [Google Scholar] [CrossRef]
- Tamargo, J.; Kjeldsen, K.P.; Delpón, E.; Semb, A.G.; Cerbai, E.; Dobrev, D.; Savarese, G.; Sulzgruber, P.; Rosano, G.; Borghi, C.; et al. Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European society of cardiology working group on cardiovascular pharmacotherapy. Eur. Heart J. Cardiovasc. Pharm. 2022, 8, 406–419. [Google Scholar] [CrossRef]
- Leelakanok, N.; Holcombe, A.L.; Lund, B.C.; Gu, X.; Schweizer, M.L. Association between polypharmacy and death: A systematic review and meta-analysis. J. Am. Pharm. Assoc. 2017, 57, 729.e10–738.e10. [Google Scholar] [CrossRef]
- Corsonello, A.; Pedone, C.; Incalzi, R. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr. Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef]
- Poulose, N.; Raju, R. Aging and injury: Alterations in cellular energetics and organ function. Aging Dis. 2014, 5, 101–108. [Google Scholar] [CrossRef]
- Mair, A.; Fernandez-Llimos, F.; Alonso, A.; Harrison, C.; Hurding, S.; Kempen, T.; Kinnear, M.; Michael, N.; McIntosh, J.; Wilson, M. Polypharmacy a Patient Safety by 2030: Management Challenge; SIMPATHY Consortium: New Delhi, India, 2017. [Google Scholar]
- Violan, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.M. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity patterns: A systematic review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Rajoo, S.S.; Wee, Z.J.; Lee, P.S.S.; Wong, F.Y.; Lee, E.S. A systematic review of the patterns of associative multimorbidity in Asia. Biomed. Res. Int. 2021, 2021, 6621785. [Google Scholar] [CrossRef]
- Busija, L.; Lim, K.; Szoeke, C.; Sanders, K.M.; McCabe, M.P. Do replicable profiles of multimorbidity exist? Systematic review and synthesis. Eur. J. Epidemiol. 2019, 34, 1025–1053. [Google Scholar] [CrossRef]
- Villén, N.; Guisado-Clavero, M.; Guisado-Clavero, M.; Fernández-Bertolín, S.; Fernández-Bertolín, S.; Troncoso-Mariño, A.; Foguet-Boreu, Q.; Foguet-Boreu, Q.; Foguet-Boreu, Q.; Amado, E.; et al. Multimorbidity patterns, polypharmacy and their association with liver and kidney abnormalities in people over 65 years of age: A longitudinal study. BMC Geriatr. 2020, 20, 206. [Google Scholar] [CrossRef]
- Marengoni, A.; Roso-Llorach, A.; Vetrano, D.L.; Fernández-Bertolín, S.; Guisado-Clavero, M.; Violán, C.; Calderón-Larrañaga, A. Patterns of multimorbidity in a population-based cohort of older people: Sociodemographic, lifestyle, clinical, and functional differences. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 798–805. [Google Scholar] [CrossRef]
- Ho, I.S.S.; Azcoaga-Lorenzo, A.; Akbari, A.; Black, C.; Davies, J.; Hodgins, P.; Khunti, K.; Kadam, U.; Lyons, R.A.; McCowan, C.; et al. Examining variation in the measurement of multimorbidity in research: A systematic review of 566 studies. Lancet Public Health 2021, 6, e587–e597. [Google Scholar] [CrossRef]
- Ng, S.K.; Tawiah, R.; Sawyer, M.; Scuffham, P. Patterns of multimorbid health conditions: A systematic review of analytical methods and comparison analysis. Int. J. Epidemiol. 2018, 47, 1687–1704. [Google Scholar] [CrossRef]
- Asogwa, O.A.; Boateng, D.; Marzà-Florensa, A.; Peters, S.; Levitt, N.; van Olmen, J.; Klipstein-Grobusch, K. Multimorbidity of non-communicable diseases in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 2022, 12, 49133. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Elmore, J.G.; Cannon, C.P.; Givens, J.; Parikh, N. Overview of Established Risk Factors for Cardiovascular Disease; UpToDate: Waltham, MA, USA; Available online: https://www.uptodate.com/contents/overview-of-established-risk-factors-for-cardiovascular-disease?search=Overview of Risk Factors for Cardiovascular Disease&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 15 September 2022).
- Scottish Government Polypharmacy Model of Care Group. Polypharmacy Guidance, Realistic Prescribing; Scottish Government: Edinburgh, Scotland, 2018. [Google Scholar]
- Caruana, E.J.; Roman, M.; Hernández-Sánchez, J.; Solli, P. Longitudinal studies. J. Thorac. Dis. 2015, 7, E537–E540. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Vetrano, D.L.; Palmer, K.; Trevisan, C.; Amato, L.; Berti, F.; Campomori, A.; Catalano, L.; Corsonello, A.; Kruger, P.; et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin. Exp. Res. 2022, 34, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Statistical Institute of Catalonia. Idescat. Statistical Yearbook of Catalonia. Available online: https://www.idescat.cat/pub/?id=aec&lang=en (accessed on 15 September 2022).
- Institut Català de la Salut. Memòria d’activitats 2012. Available online: http://ics.gencat.cat/ca/detall/publicacio/memoria_2012-00007 (accessed on 17 October 2022).
- García-Gil, M.M.; Hermosilla, E.; Prieto-Alhambra, D.; Fina, F.; Rosell, M.; Ramos, R.; Rodriguez, J.; Williams, T.; van Staa, T.; Bolíbar, B. Construction and validation of a scoring syst.tem for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform. Prim. Care 2011, 19, 135–145. [Google Scholar]
- World Health Organization Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. 2022. Available online: https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/ (accessed on 17 October 2022).
- Stafford, G.; Villén, N.; Roso-Llorach, A.; Troncoso-Mariño, A.; Monteagudo, M.; Violán, C. Combined multimorbidity and polypharmacy patterns in the elderly: A cross-sectional study in primary health care. Int. J. Environ. Res. Public Health 2021, 18, 9216. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Berjón, M.F.; Borrell, C.; Cano-Serral, G.; Esnaola, S.; Nolasco, A.; Pasarín, M.I.; Ramis, R.; Saurina, C.; Escolar-Pujolar, A. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas (proyecto MEDEA). Gac Sanit 2008, 22, 179–187. [Google Scholar] [CrossRef]
- Chavent, M.; Kuentz-Simonet, V.; Labenne, A.; Saracco, J. Multivariate analysis of mixed data: The R package PCAmixdata. arXiv 2017, arXiv:1411.4911. [Google Scholar]
- Karlis, D.; Saporta, G.; Spinakis, A. A simple rule for the selection of principal components. Commun. Stat. Theory Methods 2003, 32, 643–666. [Google Scholar] [CrossRef]
- Bezdek, J.C. Pattern Recognition with Fuzzy Objective Function Algorithms, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Rabinier, L.R. A tutorial on hidden markov models and selected applications in speech recognition. Proc. IEEE 1989, 77, 257–286. [Google Scholar] [CrossRef]
- Arbelo, E.; Bax, J.J.; Blomströ m-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; Fauchier, L.; Filippatos, G.; Kalman, J.M.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Nunes, B.P.; Flores, T.R.; Mielke, G.I.; Thumé, E.; Facchini, L.A. Multimorbidity and mortality in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2016, 67, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, W.S.; Akalu, T.Y.; Aynalem, Y.A. Risk factors for anemia in patients with chronic renal failure: A systematic review and meta-analysis. Ethiop. J. Health Sci. 2020, 30, 829–842. [Google Scholar] [CrossRef]
- Kung, W.-M.; Yuan, S.-P.; Lin, M.-S.; Wu, C.-C.; Atique, S.; Touray, M.; Huang, C.-Y.; Wang, Y.-C. Anemia and the risk of cognitive impairment: An updated systematic review and meta-analysis. Brain Sci. 2021, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Greaves, S.; van den Brink, W.; Antoniou, T.; Mamdani, M.M.; Paterson, J.M.; Martins, D.; Juurlink, D.N. Pregabalin and the risk for opioid-related death: A nested case-control study. Ann. Intern. Med. 2018, 169, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Mehta, Z.; Ahmed, E. Prevention and Management of Side Effects in Patients Receiving Opioids for Chronic Pain; UpToDate: Waltham, MA, USA; Available online: www.uptodate.com (accessed on 15 September 2022).
- Oliveira, M.C.; Vullings, J.; van de Loo, F.A.J. Osteoporosis and osteoarthritis are two sides of the same coin paid for obesity. Nutrition 2020, 70, 110486. [Google Scholar] [CrossRef]
- Suh, S.; Park, M.K. Glucocorticoid-induced diabetes mellitus: An important but overlooked problem. Endocrinol. Metab. 2017, 32, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.J.; Cosentino, F.; Marx, N. Diabetes and coronary artery disease: Not just a risk factor. Heart 2020, 106, 1357–1364. [Google Scholar] [CrossRef]
- BMJ Publishing Group. Chronic obstructive pulmonary disease (COPD). In BMJ Best Practice; BMJ Publishing Group: London, UK, 2022. [Google Scholar]
- Harężlak, T.; Religioni, U.; Szymański, F.M.; Hering, D.; Barańska, A.; Neumann-Podczaska, A.; Allan, M.; Merks, P. Drug interactions affecting kidney function: Beware of health threats from triple whammy. Adv. Ther. 2022, 39, 140–147. [Google Scholar] [CrossRef]
- Nguyen, H.; Manolova, G.; Daskalopoulou, C.; Vitoratou, S.; Prince, M.; Prina, A.M. Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies. J. Comorb. 2019, 9, 2235042X19870934. [Google Scholar] [CrossRef]
- Guisado-Clavero, M.; Roso-Llorach, A.; López-Jimenez, T.; Pons-Vigués, M.; Foguet-Boreu, Q.; Angel Muñoz, M.; Violán, C. Multimorbidity patterns in the elderly: A prospective cohort study with cluster analysis. BMC Geriatr. 2019, 18, 16. [Google Scholar] [CrossRef]
- Violán, C.; Foguet-Boreu, Q.; Fernández-Bertolín, S.; Guisado-Clavero, M.; Cabrera-Bean, M.; Formiga, F.; Valderas, J.M.; Roso-Llorach, A. Soft clustering using real-world data for the identification of multimorbidity patterns in an elderly population: Cross-sectional study in a mediterranean population. BMJ Open 2019, 9, e029594. [Google Scholar] [CrossRef]
- Gimeno-Miguel, A.; Gracia Gutiérrez, A.; Poblador-Plou, B.; Coscollar-Santaliestra, C.; Pérez-Calvo, I.; Divo, M.J.; Calderón-Larrañaga, A.; Prados-Torres, A.; Ruiz-Laiglesia, F.J. Multimorbidity patterns in patients with heart failure: An observational spanish study based on electronic health records. BMJ Open 2019, 9, e033174. [Google Scholar] [CrossRef]
- Recalde, M.; Manzano-Salgado, C.B.; Díaz, Y.; Puente, D.; Garcia-Gil, M.D.M.; Marcos-Gragera, R.; Ribes-Puig, J.; Galceran, J.; Posso, M.; Macià, F.; et al. Validation of cancer diagnoses in electronic health records: Results from the information system for research in primary care (SIDIAP) in Northeast Spain. Clin. Epidemiol. 2019, 11, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Larrañaga, A.; Vetrano, D.L.; Onder, G.; Gimeno-Feliu, L.A.; Coscollar-Santaliestra, C.; Carfí, A.; Pisciotta, M.S.; Angleman, S.; Melis, R.J.F.; Santoni, G.; et al. Assessing and measuring chronic multimorbidity in the older population: A proposal for its operationalization. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2017, 72, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Amador, M.; Matias-Guiu, X.; Sancho-Pardo, G.; Contreras Martinez, J.; de la Torre-Montero, J.C.; Peñuelas Saiz, A.; Garrido, P.; García-Sanz, R.; Rodríguez-Lescure; Paz-Ares, L. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO Open 2021, 6, 100157. [Google Scholar] [CrossRef] [PubMed]
- Mora, N.; Fina, F.; Méndez-Boo, L.; Cantenys, R.; Benítez, M.; Moreno, N.; Balló, E.; Hermosilla, E.; Fàbregas, M.; Guiriguet, C.C.X.; et al. Decline and uneven recovery from 7 common long-term conditions managed in the catalan primary care after two pandemic years: An observational retrospective population-based study using primary care electronic health records. Res. Sq. Preprint 2022. [Google Scholar] [CrossRef]
- Duffy, E.; Chilazi, M.; Cainzos-Achirica, M.; Michos, E.D. Cardiovascular disease prevention during the COVID-19 pandemic: Lessons learned and future opportunities. Methodist DeBakey Cardiovasc. J. 2021, 17, 68–78. [Google Scholar] [CrossRef]
- Feachem, R.G.A.; Sekhri, N.K.; White, K.L. Papers getting more for their dollar: A comparison of the NHS with California’s kaiser permanente. BMJ 2002, 324, 135–143. [Google Scholar] [CrossRef]

| Variables | Cardiac | Non-Specific | Mental, Behavioural, Digestive and Cerebrovascular | Neuropathy, Autoimmune and Musculoskeletal | Multisystemic | Respiratory, Cardiovascular, Behavioural and Genitourinary | Diabetes and Ischemic Cardiopathy | Musculoskeletal, Mental, Behavioural, Genitourinary, Digestive and Dermatological | Overall |
|---|---|---|---|---|---|---|---|---|---|
| N2012 = 14,533 (12.7%)–N2016 = 11,145 (15.5%) | N2012 = 39,530 (34.5%)–N2016 = 17,749 (24.6%) | N2012 = 8937 (7.8%)–N2016 = 4757 (6.6%) | N2012 = 5002 (4.4%)–N2016 = 4393 (6.1%) | N2012 = 5929 (5.2%)–N2016 = 5031 (7.0%) | N2012 = 12,270 (10.7%)–N2016 = 10,024 (13.9%) | N2012 = 19,601 (17.1%)–N2016 = 12,122 (16.8%) | N2012 = 8714 (7.6%)–N2016 = 6870 (9.5%) | N2012 = 114,516–N2016 = 72,091 | |
| Sex: | |||||||||
| Female | 7116 (49.0%)–5524 (49.6%) | 16,751 (42.4%)–6917 (39.0%) | 5574 (62.4%)–2925 (61.5%) | 3054 (61.1%)–2548 (58.0%) | 1914 (32.3%)–1554 (30.9%) | 95 (0.77%)–96 (0.96%) | 6644 (33.9%)–3637 (30.0%) | 5108 (58.6%)–3896 (56.7%) | 46,256 (40.4%)–27,097 (37.6%) |
| Male | 7417 (51.0%)–5621 (50.4%) | 22,779 (57.6%)–10,832 (61.0%) | 3363 (37.6%)–1832 (38.5%) | 1948 (38.9%)–1845 (42.0%) | 4015 (67.7%)–3477 (69.1%) | 12,175 (99.2%)–9928 (99.0%) | 12,957 (66.1%)–8485 (70.0%) | 3606 (41.4%)–2974 (43.3%) | 68,260 (59.6%)–44,994 (62.4%) |
| Patients with Multimorbidity: | 14,533 (100%)–11,145 (100%) | 39,524 (100.0%)–17,734 (99.9%) | 8937 (100%)–4757 (100%) | 5002 (100%)–4393 (100%) | 5929 (100%)–5031 (100%) | 122,70 (100%)–10,024 (100%) | 19,601 (100%)–12,122 (100%) | 8714 (100%)–6870 (100%) | 114,510 (100.0%)–72,076 (100.0%) |
| Chronic diseases number (median, IQR): | 8.00 [7.00; 10.0]–10.0 [8.00; 12.0] | 7.00 [6.00; 9.00]–9.00 [7.00; 10.0] | 10.0 [8.00; 11.0]–11.0 [9.00; 13.0] | 11.0 [9.00; 13.0]–13.0 [11.0; 15.0] | 11.0 [9.00; 13.0]–13.0 [11.0; 15.0] | 9.00 [7.00; 11.0]–11.0 [9.00; 13.0] | 9.00 [8.00; 11.0]–11.0 [9.00; 12.0] | 11.0 [9.00; 12.0]–12.0 [10.0; 14.0] | 9.00 [7.00; 11.0]–10.0 [9.00; 13.0] |
| Patients with Polypharmacy: | 12,961 (89.2%)–10,003 (89.8%) | 25,588 (64.7%)–11,785 (66.4%) | 8546 (95.6%)–4516 (94.9%) | 4877 (97.5%)–4284 (97.5%) | 5646 (95.2%)–4848 (96.4%) | 10,840 (88.3%)–9043 (90.2%) | 19,013 (97.0%)–11,812 (97.4%) | 8117 (93.1%)–6425 (93.5%) | 95,588 (83.5%)–62,716 (87.0%) |
| Drugs number (median, IQR): | 8.00 [6.00; 10.0]–8.00 [6.00; 10.0] | 6.00 [3.00; 8.00]–6.00 [4.00; 8.00] | 10.0 [7.00; 12.0]–9.00 [7.00; 11.0] | 11.0 [8.00; 13.0]–11.0 [9.00; 13.0] | 10.0 [8.00; 13.0]–10.0 [8.00; 13.0] | 8.00 [6.00; 11.0]–9.00 [6.00; 11.0] | 9.00 [7.00; 12.0]–10.0 [8.00; 12.0] | 9.00 [7.00; 12.0]–9.00 [7.00; 12.0] | 8.00 [6.00; 11.0]–8.00 [6.00; 11.0] |
| Age (Mean, SD): | 76.3 (6.4)–75.2 (6.0) | 80.6 (7.5)–78.4 (7.2) | 84.3 (6.1)–82.1 (5.8) | 76.0 (6.2)–75.1 (5.9) | 80.6 (6.4)–78.8 (6.1) | 74.5 (6.5)–73.5 (6.0) | 77.0 (6.8)–75.6 (6.36) | 79.2 (6.7)–77.8 (6.4) | 78.8 (7.4)–76.8 (6.8) |
| Age (n, %): | |||||||||
| [65,70) | 2678 (18.4%)–2417 (21.7%) | 3931 (9.94%)–2615 (14.7%) | 150 (1.68%)–127 (2.67%) | 935 (18.7%)–983 (22.4%) | 352 (5.94%)– 429 (8.53%) | 3408 (27.8%)–3165 (31.6%) | 3304 (16.9%)–2571 (21.2%) | 859 (9.86%)–845 (12.3%) | 15,617 (13.6%)–13,152 (18.2%) |
| [70,80) | 7123 (49.0%)–5945 (53.3%) | 12,539 (31.7%)–6947 (39.1%) | 1714 (19.2%)–1382 (29.1%) | 2540 (50.8%)–2319 (52.8%) | 2138 (36.1%)–2237 (44.5%) | 6000 (48.9%)–5097 (50.8%) | 9046 (46.2%)–6113 (50.4%) | 3464 (39.8%)–3171 (46.2%) | 44,564 (38.9%)–33,211 (46.1%) |
| [80,90) | 4498 (31.0%)–2710 (24.3%) | 18,578 (47.0%)–7212 (40.6%) | 5356 (59.9%)–2830 (59.5%) | 1480 (29.6%)–1073 (24.4%) | 3011 (50.8%)–2195 (43.6%) | 2685 (21.9%)–1713 (17.1%) | 6690 (34.1%)–3289 (27.1%) | 3897 (44.7%)–2671 (38.9%) | 46,195 (40.3%)–23,693 (32.9%) |
| [90,99] | 234 (1.61%)–73 (0.66%) | 4482 (11.3%)–975 (5.49%) | 1717 (19.2%)–418 (8.79%) | 47 (0.94%)–18 (0.41%) | 428 (7.22%)–170 (3.38%) | 177 (1.44%)–49 (0.49%) | 561 (2.86%)–149 (1.23%) | 494 (5.67%)–183 (2.66%) | 8140 (7.11%)–2035 (2.82%) |
| MEDEA *: | |||||||||
| R | 2961 (22.6%)–2228 (20.6%) | 7996 (23.0%)–3406 (19.9%) | 1955 (27.3%)– 980 (21.6%) | 764 (17.0%)–636 (15.0%) | 1141 (22.7%)–946 (19.3%) | 1866 (16.9%)–1461 (15.1%) | 3368 (19.1%)–2035 (17.3%) | 1507 (19.7%)–1164 (17.5%) | 21,558 (21.4%)–12,856 (18.4%) |
| U1 | 1974 (15.1%)–1706 (15.8%) | 5718 (16.5%)–2786 (16.3%) | 1255 (17.5%)–824 (18.2%) | 489 (10.9%)–490 (11.5%) | 722 (14.4%)–744 (15.2%) | 1558 (14.1%)–1409 (14.5%) | 2487 (14.1%)–1690 (14.3%) | 1188 (15.5%)–1066 (16.1%) | 15,391 (15.3%)–10,715 (15.4%) |
| U2 | 2100 (16.0%)–1797 (16.6%) | 5412 (15.6%)–2793 (16.3%) | 1093 (15.3%)–710 (15.7%) | 656 (14.6%)–633 (14.9%) | 701 (13.9%)–761 (15.6%) | 1714 (15.5%)–1565 (16.1%) | 2638 (15.0%)–1811 (15.4%) | 1176 (15.4%)–1089 (16.4%) | 15,490 (15.4%)–11,159 (16.0%) |
| U3 | 1983 (15.1%)–1668 (15.4%) | 5638 (16.2%)–2879 (16.8%) | 1048 (14.6%)–727 (16.1%) | 748 (16.7%)–738 (17.4%) | 761 (15.1%)–818 (16.7%) | 1797 (16.3%)–1609 (16.6%) | 2953 (16.8%)–2009 (17.0%) | 1225 (16.0%)–1092 (16.5%) | 16,153 (16.0%)– 11,540 (16.6%) |
| U4 | 2087 (15.9%)–1772 (16.4%) | 5203 (15.0%)–2774 (16.2%) | 948 (13.2%)–657 (14.5%) | 846 (18.8%)–797 (18.8%) | 833 (16.6%)–801 (16.4%) | 1978 (17.9%)–1783 (18.4%) | 3050 (17.3%)–2113 (17.9%) | 1248 (16.3%)–1105 (16.6%) | 16,193 (16.1%)–11,802 (16.9%) |
| U5 | 1999 (15.3%)–1631 (15.1%) | 4751 (13.7%)–2471 (14.4%) | 866 (12.1%)–631 (13.9%) | 989 (22.0%)–955 (22.5%) | 871 (17.3%)–823 (16.8%) | 2117 (19.2%)–1871 (19.3%) | 3107 (17.7%)–2133 (18.1%) | 1311 (17.1%)–1121 (16.9%) | 16,011 (15.9%)–11,636 (16.7%) |
| N. of visits (median, IQR): | 25.0 [15.0; 35.0]–27.0 [18.0; 36.0] | 13.0 [7.00; 23.0]–13.0 [7.00; 23.0] | 17.0 [10.0; 29.0]–17.0 [8.00; 29.0] | 21.0 [13.0; 33.0]–22.0 [13.0; 35.0] | 24.0 [14.0; 37.0]–25.0 [14.0; 39.0] | 14.0 [9.00; 22.0]–15.0 [9.00; 23.0] | 16.0 [10.0; 26.0]–16.0 [10.0; 26.0] | 19.0 [11.0; 31.8]–20.0 [12.0; 33.0] | 17.0 [9.00; 28.0]–18.0 [10.0; 30.0] |
| Year of Follow-up | Analytical Variables | Cardiac (N = 14,533) | Non-Specific (N = 39,530) | Mental, Behavioural, Digestive and Cerebrovascular (N = 8937) | Neuropathy, Autoimmune and Musculoskeletal (N = 5002) | Multisystemic (N = 5929) | Respiratory, Cardiovascular, Behavioural and Genitourinary (N = 12,270) | Diabetes and Ischemic Cardiopathy (N = 19,601) | Musculoskeletal, Mental, Behavioural, Genitourinary, Digestive and Dermatological (N = 8714) | All Population (N = 114,516) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | Patients with Determination (%) | Patients with ≥1 Altered Determination (N, %) | ||
| 2012 | ALB_ser | 11.3% | 549 (3.78%) | 10.9% | 1288 (3.26%) | 19.3% | 340 (3.80%) | 12.5% | 222 (4.44%) | 16.1% | 295 (4.98%) | 10.3% | 511 (4.16%) | 12.4% | 856 (4.37%) | 12.0% | 315 (3.61%) | 12.2% | 4376 (3.82%) |
| TCHOL | 70.8% | 841 (5.79%) | 64.8% | 2267 (5.73%) | 74.2% | 582 (6.51%) | 81.1% | 381 (7.62%) | 74.6% | 315 (5.31%) | 73.0% | 568 (4.63%) | 80.4% | 972 (4.96%) | 73.0% | 711 (8.16%) | 71.7% | 6637 (5.80%) | |
| CREAT | 71.7% | 1557 (10.7%) | 66.0% | 3848 (9.73%) | 75.3% | 1562 (17.5%) | 81.3% | 699 (14.0%) | 76.9% | 1268 (21.4%) | 73.7% | 1046 (8.52%) | 80.5% | 3331 (17.0%) | 74.1% | 833 (9.56%) | 72.6% | 14,144 (12.4%) | |
| ALP | 26.2% | 1034 (7.11%) | 21.1% | 1963 (4.97%) | 24.3% | 582 (6.51%) | 26.7% | 300 (6.00%) | 29.0% | 499 (8.42%) | 24.1% | 558 (4.55%) | 24.4% | 1124 (5.73%) | 25.5% | 508 (5.83%) | 23.9% | 6568 (5.74%) | |
| GGT | 47.5% | 2070 (14.2%) | 43.3% | 3053 (7.72%) | 49.2% | 699 (7.82%) | 51.4% | 550 (11.0%) | 51.9% | 887 (15.0%) | 49.2% | 1174 (9.57%) | 51.0% | 1839 (9.38%) | 49.6% | 782 (8.97%) | 47.5% | 11,054 (9.65%) | |
| GLYC | 72.0% | 6000 (41.3%) | 66.5% | 13,501 (34.2%) | 75.9% | 3718 (41.6%) | 82.6% | 3023 (60.4%) | 76.9% | 2711 (45.7%) | 74.6% | 5501 (44.8%) | 82.0% | 12,696 (64.8%) | 74.3% | 2953 (33.9%) | 73.3% | 50,103 (43.8%) | |
| AST (or SGOT) | 27.1% | 323 (2.22%) | 23.6% | 599 (1.52%) | 26.1% | 152 (1.70%) | 29.1% | 79 (1.58%) | 30.2% | 157 (2.65%) | 27.0% | 248 (2.02%) | 27.7% | 381 (1.94%) | 27.2% | 121 (1.39%) | 26.2% | 2060 (1.80%) | |
| ALT (or SGPT) | 63.9% | 631 (4.34%) | 59.3% | 1254 (3.17%) | 67.9% | 235 (2.63%) | 72.3% | 219 (4.38%) | 68.6% | 294 (4.96%) | 67.4% | 634 (5.17%) | 70.8% | 951 (4.85%) | 67.6% | 261 (3.00%) | 65.1% | 4479 (3.91%) | |
| HbA1c | 33.5% | 2745 (18.9%) | 29.1% | 6298 (15.9%) | 42.6% | 2304 (25.8%) | 59.6% | 2200 (44.0%) | 40.9% | 1422 (24.0%) | 39.2% | 2936 (23.9%) | 64.8% | 9714 (49.6%) | 28.8% | 1104 (12.7%) | 39.8% | 28,723 (25.1%) | |
| eGFR | 58.7% | 3536 (24.3%) | 52.9% | 8358 (21.1%) | 64.0% | 3530 (39.5%) | 69.5% | 1613 (32.2%) | 64.8% | 2377 (40.1%) | 62.2% | 1874 (15.3%) | 68.1% | 7042 (35.9%) | 62.3% | 2077 (23.8%) | 60.1% | 30,407 (26.6%) | |
| 2016 | ALB_ser | 18.6% | 713 (6.40%) | 15.7% | 776 (4.37%) | 25.9% | 214 (4.50%) | 23.3% | 323 (7.35%) | 24.2% | 349 (6.94%) | 19.4% | 708 (7.06%) | 21.3% | 815 (6.72%) | 19.4% | 395 (5.75%) | 19.7% | 4293 (5.95%) |
| TCHOL | 75.0% | 451 (4.05%) | 68.7% | 821 (4.63%) | 73.8% | 261 (5.49%) | 82.7% | 227 (5.17%) | 77.1% | 194 (3.86%) | 77.0% | 321 (3.20%) | 81.0% | 393 (3.24%) | 75.9% | 412 (6.00%) | 75.4% | 3080 (4.27%) | |
| CREAT | 77.7% | 1562 (14.0%) | 71.3% | 1897 (10.7%) | 77.0% | 830 (17.4%) | 84.5% | 823 (18.7%) | 80.5% | 1305 (25.9%) | 79.7% | 1245 (12.4%) | 82.8% | 2724 (22.5%) | 78.9% | 792 (11.5%) | 77.9% | 11,178 (15.5%) | |
| ALP | 30.0% | 861 (7.73%) | 23.2% | 879 (4.95%) | 23.8% | 316 (6.64%) | 32.6% | 372 (8.47%) | 33.9% | 537 (10.7%) | 29.9% | 582 (5.81%) | 29.5% | 845 (6.97%) | 28.1% | 428 (6.23%) | 28.1% | 4820 (6.69%) | |
| GGT | 53.6% | 1883 (16.9%) | 47.1% | 1524 (8.59%) | 49.2% | 435 (9.14%) | 56.8% | 613 (14.0%) | 56.9% | 905 (18.0%) | 56.3% | 1116 (11.1%) | 55.0% | 1298 (10.7%) | 53.1% | 805 (11.7%) | 52.7% | 8579 (11.9%) | |
| GLYC | 77.4% | 4818 (43.2%) | 71.3% | 6527 (36.8%) | 77.0% | 1990 (41.8%) | 84.8% | 2714 (61.8%) | 80.1% | 2493 (49.6%) | 80.1% | 4975 (49.6%) | 83.5% | 8089 (66.7%) | 78.6% | 2618 (38.1%) | 78.0% | 34,224 (47.5%) | |
| AST (or SGOT) | 26.8% | 294 (2.64%) | 22.5% | 297 (1.67%) | 24.0% | 85 (1.79%) | 28.8% | 112 (2.55%) | 30.5% | 153 (3.04%) | 28.6% | 252 (2.51%) | 28.5% | 273 (2.25%) | 25.8% | 120 (1.75%) | 26.4% | 1586 (2.20%) | |
| ALT (or SGPT) | 69.1% | 367 (3.29%) | 63.9% | 430 (2.42%) | 68.4% | 117 (2.46%) | 74.8% | 158 (3.60%) | 72.3% | 186 (3.70%) | 72.3% | 410 (4.09%) | 72.9% | 460 (3.79%) | 71.1% | 166 (2.42%) | 69.6% | 2294 (3.18%) | |
| HbA1c | 36.3% | 1956 (17.6%) | 30.4% | 2568 (14.5%) | 42.1% | 1156 (24.3%) | 60.1% | 1742 (39.7%) | 42.8% | 1183 (23.5%) | 43.5% | 2509 (25.0%) | 66.7% | 5698 (47.0%) | 30.5% | 870 (12.7%) | 42.7% | 17,682 (24.5%) | |
| eGFR | 71.9% | 3951 (35.5%) | 65.3% | 5288 (29.8%) | 72.5% | 2301 (48.4%) | 80.3% | 1975 (45.0%) | 76.3% | 2706 (53.8%) | 74.0% | 2635 (26.3%) | 78.4% | 5888 (48.6%) | 73.3% | 2346 (34.1%) | 72.6% | 27,090 (37.6%) | |
| Baseline (2012) | End of Follow-Up (2016) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pattern | Disease or Disease-Drug Group | Prev C1 | OE C1 | Exc C1 | Pattern | Disease or Disease-Drug Group | Prev C1 | OE C1 | Exc C1 |
| Mental, behavioural, digestive and cerebrovascular | Dementia | 48.96 | 8.67 | 67.69 | Mental, behavioural, digestive and cerebrovascular | Dementia | 57.70 | 8.02 | 52.95 |
| Depression mood | 48.20 | 3.54 | 27.64 | Other digestive (D) | 17.87 | 4.10 | 27.07 | ||
| Other digestive (D) | 10.43 | 3.42 | 26.66 | Depression mood | 49.91 | 3.12 | 20.61 | ||
| Other psychiatric and behavioural | 14.05 | 2.71 | 21.17 | Other psychiatric and behavioural | 24.24 | 2.73 | 18.03 | ||
| Neurotic, stress and somatoform | 28.43 | 2.64 | 20.61 | Neurotic, stress and somatoform | 29.58 | 2.07 | 13.66 | ||
| Anaemia | 33.21 | 2.54 | 19.85 | Anaemia | 30.29 | 2.07 | 13.65 | ||
| Cerebrovascular | 30.18 | 2.04 | 15.92 | Cerebrovascular | 27.50 | 2.00 | 13.18 | ||
| Colitis related diseases | 30.97 | 1.83 | 14.31 | Chronic pancreas, biliary-gallbladder (D) | 8.66 | 1.54 | 10.16 | ||
| Chronic pancreas, biliary-gallbladder (D) | 6.99 | 1.83 | 14.25 | Colitis related diseases | 23.50 | 1.32 | 8.71 | ||
| Autoimmune | 3.70 | 1.66 | 12.98 | Chronic kidney | 48.48 | 1.27 | 8.37 | ||
| Chronic kidney | 39.45 | 1.51 | 11.79 | Glaucoma | 10.51 | 1.26 | 8.30 | ||
| Sleep | 10.53 | 1.45 | 11.30 | Autoimmune | 3.51 | 1.17 | 7.71 | ||
| Glaucoma | 10.09 | 1.37 | 10.68 | Sleep | 14.23 | 1.13 | 7.45 | ||
| Diabetes | 39.52 | 1.19 | 9.25 | Diabetes | 38.28 | 1.09 | 7.21 | ||
| Osteoarthritis, degenerative joint | 23.93 | 1.08 | 8.42 | Thyroid | 7.21 | 1.03 | 6.83 | ||
| Hypertension | 80.13 | 1.08 | 8.40 | Hypertension | 79.40 | 1.01 | 6.68 | ||
| Osteoporosis | 7.65 | 1.07 | 8.36 | Osteoarthritis, degenerative joint | 27.10 | 1.01 | 6.67 | ||
| Thyroid | 5.96 | 1.06 | 8.25 | Solid neoplasms (D) | 23.10 | 0.97 | 6.39 | ||
| Solid neoplasms (D) | 19.66 | 1.04 | 8.09 | Osteoporosis | 4.88 | 0.93 | 6.15 | ||
| Cataract lens (D) | 25.09 | 1.02 | 7.93 | Cataract lens (D) | 29.68 | 0.92 | 6.09 | ||
| Multisystemic | Chronic pancreas, biliary-gallbladder (D) | 27.74 | 7.24 | 37.50 | Multisystemic | Chronic pancreas, biliary-gallbladder (D) | 31.01 | 5.51 | 38.45 |
| Inflammatory arthropathies | 25.48 | 6.71 | 34.75 | Inflammatory arthropathies | 27.83 | 4.94 | 34.47 | ||
| Other respiratory (D) | 11.01 | 4.76 | 24.63 | Other respiratory (D) | 13.52 | 3.37 | 23.51 | ||
| Other cardiovascular diseases (D) | 17.22 | 3.94 | 20.39 | Autoimmune | 8.75 | 2.91 | 20.31 | ||
| Autoimmune | 8.58 | 3.85 | 19.95 | Other cardiovascular diseases (D) | 17.21 | 2.89 | 20.17 | ||
| Other digestive (D) | 9.36 | 3.07 | 15.88 | Other digestive (D) | 12.03 | 2.76 | 19.27 | ||
| Bradycardias conduction (D) | 31.57 | 2.69 | 13.95 | Bradycardias conduction (D) | 38.54 | 2.33 | 16.27 | ||
| Peripheral neuropathy | 10.58 | 2.19 | 11.32 | Dorsopathies | 23.63 | 1.79 | 12.47 | ||
| Dorsopathies | 19.67 | 2.12 | 10.98 | Osteoarthritis, degenerative joint | 46.95 | 1.75 | 12.22 | ||
| Other genitourinary | 6.53 | 2.03 | 10.48 | Peripheral neuropathy | 12.72 | 1.74 | 12.16 | ||
| Osteoarthritis, degenerative joint | 43.11 | 1.94 | 10.06 | Other genitourinary | 6.98 | 1.73 | 12.05 | ||
| COPD, emphysema, chronic bronchitis | 38.66 | 1.86 | 9.64 | COPD, emphysema, chronic bronchitis | 38.96 | 1.65 | 11.53 | ||
| Other musculoskeletal joint | 15.37 | 1.85 | 9.59 | Heart failure | 68.79 | 1.63 | 11.35 | ||
| Chronic kidney | 47.06 | 1.80 | 9.33 | Anaemia | 23.79 | 1.62 | 11.34 | ||
| Allergy | 2.78 | 1.76 | 9.13 | Other musculoskeletal joint | 18.64 | 1.62 | 11.31 | ||
| Anaemia | 22.43 | 1.72 | 8.89 | Allergy | 4.75 | 1.61 | 11.22 | ||
| Heart failure | 62.35 | 1.71 | 8.83 | Chronic kidney | 61.30 | 1.60 | 11.20 | ||
| Other skin (D) | 3.17 | 1.61 | 8.33 | Sleep | 18.58 | 1.47 | 10.28 | ||
| Sleep | 11.62 | 1.60 | 8.28 | Colitis related diseases | 26.24 | 1.47 | 10.28 | ||
| Colitis related diseases | 25.22 | 1.49 | 7.73 | Atrial fibrillation | 47.31 | 1.34 | 9.37 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villén, N.; Roso-Llorach, A.; Gallego-Moll, C.; Danes-Castells, M.; Fernández-Bertolin, S.; Troncoso-Mariño, A.; Monteagudo, M.; Amado, E.; Violán, C. Polypharmacy Patterns in Multimorbid Older People with Cardiovascular Disease: Longitudinal Study. Geriatrics 2022, 7, 141. https://doi.org/10.3390/geriatrics7060141
Villén N, Roso-Llorach A, Gallego-Moll C, Danes-Castells M, Fernández-Bertolin S, Troncoso-Mariño A, Monteagudo M, Amado E, Violán C. Polypharmacy Patterns in Multimorbid Older People with Cardiovascular Disease: Longitudinal Study. Geriatrics. 2022; 7(6):141. https://doi.org/10.3390/geriatrics7060141
Chicago/Turabian StyleVillén, Noemí, Albert Roso-Llorach, Carlos Gallego-Moll, Marc Danes-Castells, Sergio Fernández-Bertolin, Amelia Troncoso-Mariño, Monica Monteagudo, Ester Amado, and Concepción Violán. 2022. "Polypharmacy Patterns in Multimorbid Older People with Cardiovascular Disease: Longitudinal Study" Geriatrics 7, no. 6: 141. https://doi.org/10.3390/geriatrics7060141
APA StyleVillén, N., Roso-Llorach, A., Gallego-Moll, C., Danes-Castells, M., Fernández-Bertolin, S., Troncoso-Mariño, A., Monteagudo, M., Amado, E., & Violán, C. (2022). Polypharmacy Patterns in Multimorbid Older People with Cardiovascular Disease: Longitudinal Study. Geriatrics, 7(6), 141. https://doi.org/10.3390/geriatrics7060141






