Abstract
The concept of polypharmacy encompasses adverse drug reactions and non-adherence factors in elderly individuals. It also leads to the increased use of healthcare services and negative health outcomes. The problem is further alleviated by the odds of potentially inappropriate medications (PIM), which lead to the development of drug-related problems. Since polypharmacy is more commonly observed in the elderly population, urgency is required to introduce operative protocols for preventing and managing this problem. The family medicine model of care can be associated with favorable illness outcomes regarding satisfaction with consultation, treatment adherence, self-management behaviors, adherence to medical advice, and healthcare utilization. Hence, interventions built on family medicine models can provide significant support in improving the outcomes of the older population and their quality of life. In this regard, the authors have taken up the task of explaining the accessible resources which can be availed to improve the application of health care services in the field of geriatric medicine.
1. Introduction
Polypharmacy is the term given when multiple drugs are prescribed to treat diseases and improve other health conditions [1,2,3]. The WHO defines polypharmacy as the concurrent use of five or more medications by a patient which may include over-the-counter, prescription, and/or traditional and complementary medicines [4]. This process represents a major concern for older adults. This population suffers from multiple chronic conditions, e.g., depression, arthritis, asthma, coronary heart disease, diabetes, hypertension, vision and hearing impairment, lower lean body mass, mobility, and chronic obstructive pulmonary disease [5,6,7,8]. Major adverse outcomes associated with polypharmacy includes increased length of stay in hospital, mortality, falls and readmission to hospital soon after discharge [9,10]. Moreover, the probability of these undesirable effects rises when the medications increase in number. Hence, inappropriate polypharmacy (excessive or unnecessary usage of several medications) elevates the risk of adverse drug effects which can lead to drug–disease interactions and cognitive impairment, which worsens another disease or eventually leads to the occurrence of new one [11].
The complications of polypharmacy can be reduced with the intervention of a professionally trained health care team dedicated to the care of geriatrics. In the context of Saudi Arabia, a continuous growth in the life expectancy of the general population has been observed in the last decade. The elderly population, accounting for 2.96% in 2011 to 3.65% in 2021, are projected to constitute 25% of the population by 2050. However, the proportion of people aged 80 years and above may reach 4% by the same year, and this population is considered to be growing faster, as reflected in the significant growth noticed in the healthcare and management sector of Saudi Arabia. On the other hand, it raises drug related issues, e.g., adverse drug reaction, adverse drug events, and drug interactions [4]. Most of the identified geriatric cases are prescribed with anticholinergics, sedatives, and hypnotics, cardiovascular agents, analgesics, and antidiabetic agents, which carry potential risk in the case of polypharmacy. Such cases can be reviewed with the assigned tools. If needed, a de-prescribing protocol must be developed and implemented in selected cases to avoid complications of adverse drug reactions, avoid high risk medications, and reduce the inapplicable medications, if prescribed [12].
Continuous educational activities are required that include education of the elderly population in relation to the medication intake, summarizing the strategies to reduce polypharmacy, and development of inter-professional team relations and attitude to reduce the effects of polypharmacy to a degree of 40% in the case of untoward reactions [4], regarding which budding healthcare professionals may lack knowledge and encounter difficulties. Hence, in this regard, the authors have taken up the task of compiling the modern methods of trouble shooting the issues related to the polypharmacy so that the information can be used by the readers such that better health care can be provided in relation to the management of polypharmacy related issues in the case of geriatrics.
2. Complications Leading to Polypharmacy
The risk factors for polypharmacy can occur either (or both) at the level of the health care system and patient [13,14]. For example, maintenance of poor medical record leads to polypharmacy when such ceased medications are automatically prescribed by a physician. However, at the patient level, polypharmacy is mainly recognized in the elderly group as they are more vulnerable to suffer from one or more chronic conditions which subsequently leaves them with a long list of medication. Moreover, the elderly population with no primary care physician or multiple subspecialist physicians are also at great risk of polypharmacy [15,16].
Polypharmacy in the young population occurs due to heart diseases, diabetes, cancer, stroke, chronic pain such as fibromyalgia, developmental disabilities, and chronic medical disorders as they involve multiple treatments and modalities [17]. Polypharmacy becomes more severe in the case of mentally challenged candidates. Such individuals stay on psychotropic medications with adverse effects and numerous medications are prescribed to reduce their side effects. Patients suffering from chronic neurological disorders and similar medical complications also need multiple medications for preventing the progress of disease [18]. The occurrence of adverse drug events (ADEs) is associated with increased morbidity and mortality, prolonged hospitalizations, and higher cost of care. Moreover, Stevenson and coworkers have recently observed the association of frailty with medication-related harm in a prospective observational cohort study through an integrated health and social care approach, to reduce inappropriate polypharmacy [19].
3. Mode of Assessment for Potentially Inappropriate Medication
Several assessment tools have been used earlier (Figure 1) to trace the potentially inappropriate medication (PIM). They include the Medication Appropriateness Index (MAI), Beers, screening tool of older people’s prescriptions (STOPP), and screening tool to alert for right treatment (START). Explicit tools, such as STOPP and START, assist with easy and quick decision-making criteria. These tools help in comparing patient’s medication list with PIM which leads to disease interactions, medication and medication duplication [20,21]. Beers criteria further classify PIM by disease state and class of drug [22]. START and STOPP scale are applied in combination for identifying medications which are considered unsuitable (STOPP) followed by the application of alternative medications for treating the disease (START) [20]. MAI represents an implicit assessment tool as it considers patient complexity. It is more time-consuming and patient-centered, involving physician judgment in contrast to the set guidelines of the assessment tools [23]. The attitude, experience, and knowledge of a physician limit this assessment method inherently, and hence it is less reliable than explicit mode of assessment in providing meaningful insight to a clinical problem. MAI poses ten questions, including consideration of medication duplications; medication requirement; medication and disease interactions; dosage suitability, drug formulation and treatment duration; and directions for use, and optimal therapy for diseases and conditions. It should be noted that MAI takes appreciable time to prescribe medication even though these questions seem clear and straightforward [24,25,26].
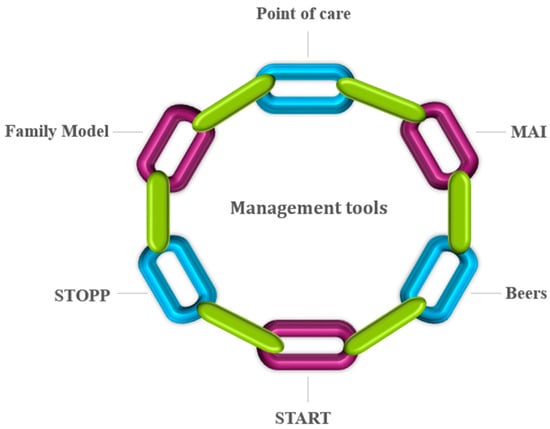
Figure 1.
Assessment tools for tracing potentially inappropriate medications.
It should be noted that none of the PIMs tools exhibit complete efficacy in improving patient-related outcomes. Hence, these procedures can lead to unnecessary polypharmacy risks [27]. One of the prominent strategies used to reduce pill burden involves deprescribing unnecessary medications by evaluating the patient’s active medication lists. It helps in reducing the negative impact of ADE and financial hardship. Hence, deprescribing offered an initial therapeutic intervention plan and management protocol, simultaneously with the introduction of clinically appropriate therapy [28,29,30]. Point-of-care tools also help the patient to understand initially the urgency to cut down the unnecessary medication, thereby reducing the risks of polypharmacy. This step leads to priorities and preferences required to implement the prescription of new drugs for slowing down the progression of disease and to address the symptoms for improving the health of an individual [31,32].
4. Deprescribing Approaches
Deprescribing is a patient-centered process of medication withdrawal intended to achieve improved health outcomes through discontinuation of one or more medications that are either potentially harmful or no longer required [33]. It is a five-step, patient-centered systematic approach (Figure 2) used for evaluating and discontinuing medications in patients where potential harms exceed potential benefits in relation to individual existing functioning capability, care goals, preferences, values and life expectancy. Hence, this process suspends medication, changes medication, and decreases medication dosages for obtaining the best clinical outcomes [34]. This is because deprescribing approaches are patient-specific and focused and follow interventions with appreciable variability in medications used and patient characteristics [35]. The process of deprescription should be initiated as a “therapeutic intervention” simultaneously by introducing clinically appropriate therapy. Hence, it becomes imperative for a physician to examine patient approach on goals of therapy. It also takes into consideration the basic parameters of chronic conditions and medications and affords prime concern regarding prescription to slow down the disease progression along with minimizing the health decline [36,37]. It has been observed that only a limited percentage of the elderly community debate health-related decision-making priorities with their primary care physicians. Hence, in order to optimize disease control, the physician should examine specific therapeutic goals at every patient visit. Additionally, health systems and practices need to adopt rationalized and updated methods for tracking and medication reconciliation as up-to-date medication lists form the basis of identifying potential medications for deprescribing, which could minimize the burden of staff, patient, and physician [38,39,40]. Sawan et al. (2020) have recently summarized the common deprescribing interventions, including enablers and barriers to implement deprescribing across settings (e.g., primary, secondary, residential care facilities) and current deprescribing polices in place internationally [41]. Several deprescribing tools have been used recently, e.g., mobilizing community pharmacists [42], creating evidence-based deprescribing guidelines [43], geriatric pharmacoeconomics [44], and engaging primary care providers in deprescribing trials [45,46]. The dedicated team involved in the management of health-related issues with the elderly patients shall be advised to follow the process of deprescribing medication qualitatively (focused groups) and quantitatively by Delphi’s criteria [12]. Further, Bruyere deprescribing guidelines could prove beneficial in case of managing the deprescription in relation to the elderly patients in case of antihypertensive medications, psychotic medications, proton pump inhibitors, antihistamines, anti-hyperglycemic agents, sedatives and hypnotics, anticholinergics, and cholinesterase inhibitors; and OncPal deprescribing guidelines in the case of oncological and palliative care medications [47].
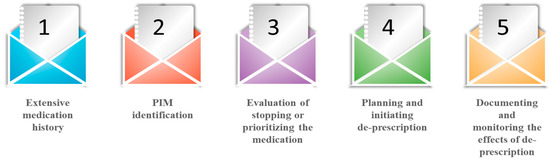
Figure 2.
Deprescription process.
5. General Medications That Cause Problems in Elderly Population
Polypharmacy is an area of concern for elderly because such individuals are at a greater risk for adverse drug reactions (ADR) which lead to metabolic changes and reduced drug clearance [48,49,50]. This risk is furthermore exacerbated by the increasing number of drugs used. Polypharmacy may sometimes lead to “prescribing cascades.” A prescribing cascade arises when signs and symptoms (multiple and nonspecific) of an ADR are misinterpreted as a disease and a new treatment/drug therapy is further added to the earlier prescribed treatment to treat the condition. This increases the potential to develop further side-effects, leading to a prescribing cascade [51]. It has been proposed earlier by several groups of researchers that the use of multiple medications comes with an increased risk for negative health outcomes, such as higher healthcare costs, ADEs, drug–drug interactions, medication non-adherence, decreased functional status, and geriatric syndromes [52,53,54,55]. The medications listed in Table 1 cause problems in elderly individuals, when prescribed in combination or even alone.

Table 1.
Adverse drug events of some medications in older population.
6. Management Plan
Appropriate polypharmacy deals with the prescription of multiple medications in an optimized way to an individual suffering from complex conditions or for multiple conditions according to best evidence. The management plan for polypharmacy involves medication assessment and home assessment. It also incorporates balance, gait, and strength assessment (Figure 3). These are achieved partly or as a whole by eliminating duplicate medications, conducting medication reconciliations at care transition, assessing drug–drug interactions, and by reviewing drug dosages to reduce the incidence of polypharmacy [70,71,72,73,74]. It also ensures patient safety and reduces hospitalization, thereby decreasing the associated costs. It has been well documented that various tools and treatment approaches are used in parallel as there exist no ideal tools to manage polypharmacy in the elderly population [75]. It depends on the application of medications and time suitability which enables the users to employ one of existing interventions and/or tools to obtain the optimized results. Several researchers concluded that the utilization of a drug based upon the explicit assessment tools offered the best practical approach [76,77,78,79,80,81].
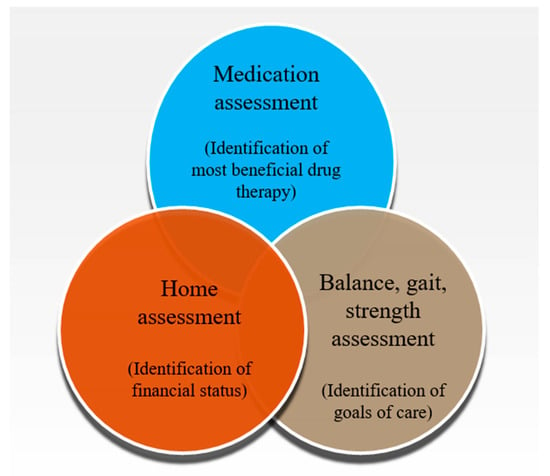
Figure 3.
Management plans for polypharmacy.
The World Health Organization has identified five sets of factors that affect patients’ adherence to therapy. They include socio-economic, health care team-, and system-, condition-, therapy-, and patient-related factors. These factors including health care team and system-related factors are mostly dependent on patient characteristics and experiences. For example, a good patient–provider relationship can improve adherence [82]. However, in particular, patients’ satisfaction with therapy has been linked to adherence [83,84]. Cognitive impairment in older adults has a variety of possible causes, including medication side effects; metabolic and/or endocrine derangements; delirium due to illness (such as a urinary tract or related infection); depression; and dementia, with Alzheimer’s dementia being most common. Some causes, e.g., medication side effects and depression, can be reversed or improved with treatment. Others, such as Alzheimer’s, cannot be reversed, but symptoms can be treated for a period of time, and importantly, families can be prepared for predictable changes and address safety concerns [85].
It has been documented that a multidisciplinary team involving nurses may facilitate future treatment and rehabilitation in geriatric patients with cardiovascular disorders [86]. Dedicated centralized team leaders are best suited in implementing the guidelines for residential aged care facility residents requiring specialized geriatric care via telehealth and counselling [87]. It has also been observed that the enthusiastic involvement of pharmacists in deprescription has proved beneficial in the case of elderly patients in all types of resource settings [88]. The medication management goals followed by the suggested guidelines may ensure that older patients may have a realistic understanding of their medical conditions. This pre-intervention workup, combined with engagement with family members and interdisciplinary teams, may improve post-interventional outcomes [89]. Additionally, artificial intelligence applications and computational tools could serve as a potential tool in developing management plans for individualized geriatric management of healthy aging [90].
Comprehensive geriatric assessment (CGA) involves a multidisciplinary diagnostic and treatment process for identifying medical, psychosocial, and functional limitations of a frail older person in order to develop a coordinated plan to maximize overall health with aging [91]. CGA assessment tools can be in the form of a pre-visit questionnaire which can serve as a timesaving method to gather a large amount of information. Major components of CGA that should be evaluated include functional capacity, fall risk, cognition, mood, polypharmacy, nutrition/weight change, urinary incontinence, vision/hearing, dentition, living situation, social support, financial concerns, goals of care, spirituality, and advance care preferences. Incorporating CGA for evaluating the functional outcomes in transition care using a suite of assessment tools was feasible and enabled a holistic assessment [92,93,94].
7. Conclusions
Here, it has been overviewed that polypharmacy is linked with duplicated therapy and contraindicated drug combinations. The symptoms caused by polypharmacy vary from tiredness, sleepiness, decreased alertness, constipation, diarrhea or incontinence, loss of appetite, confusion, falls, depression or lack of interest in usual activities, to weakness, tremors, visual or auditory hallucinations, anxiety or excitability, and/or dizziness. In this regard, communication among elderly individuals and physicians needs to be improved in applying a proper explicit or implicit tool to minimize adverse consequences of polypharmacy by reducing the pill burden. The process of deprescription should be initiated as a therapeutic intervention by introducing clinically appropriate therapy. Additionally, health systems need to adopt rationalized and updated methods for tracking and medication reconciliation as up-to-date medication lists form the basis of identifying potential medications for deprescribing.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Acknowledgments
Shakeel Ahmed Ansari (School of Medicine, Batterjee Medical College for Sciences and Technology, Jeddah, Saudi Arabia) is gratefully acknowledged for sharing his views while preparing this article. I would like to thank reviewers as well as the academic editor of Geriatrics journal for their valuable feedback on this article.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
ADEs; adverse drug events, START; Screening tool to alert for right treatment, PIM; potentially inappropriate medications, STOPP; Screening tool of older people’s prescriptions, MAI; Medication Appropriateness Index, POC; Point of care.
References
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Ger. 2017, 10, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.A. The epidemiology of polypharmacy. Clin. Med. 2016, 16, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Dagli, R.J.; Sharma, A. Polypharmacy: A global risk factor for elderly people. J. Int. Oral Health 2014, 6, i–ii. [Google Scholar] [PubMed]
- World Health Organization. Medication Safety in Polypharmacy; WHO/UHC/SDS/2019.11; World Health Organization: Geneva, Switzerland, 2019; Licence: CC BY-NC-SA 3.0 IGO.
- Karlamangla, A.; Tinetti, M.; Guralnik, J.; Studenski, S.; Wetle, T.; Reuben, D. Comorbidity in older adults: Nosology of impairment, diseases and conditions. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 296–300. [Google Scholar] [CrossRef]
- Kim, J.; Parish, A.L. Polypharmacy and medication management in older adults. Nurs. Clin. N. Am. 2017, 52, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Belrose, J.C.; Noppens, R.R. Anesthesiology and cognitive impairment: A narrative review of current clinical literature. BMC Anesthesiol. 2019, 19, 241–252. [Google Scholar] [CrossRef]
- Novak, J.; Goldberg, A.; Dharmarajan, K.; Amini, A.; Maggiore, R.J.; Presley, C.J.; Nightingale, G. Polypharmacy in older adults with cancer undergoing radiotherapy: A review. J. Geriatr. Oncol. 2022, 25, 1879–1899. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, K.; Parekh, N.; Stevenson, J.M.; Davies, J.G.; Bremner, S.; Rajkumar, C. Characterising older adult’s risk of harm from blood-pressure lowering medications: A sub-analysis from the PRIME study. Age Ageing 2022, 51, 45–53. [Google Scholar]
- Lertkovit, S.; Siriussawakul, A.; Suraarunsumrit, P.; Lertpipopmetha, W.; Manomaiwong, N.; Wivatdechakul, W.; Srinonprasert, V. Polypharmacy in older adults undergoing major surgery: Prevalence, association with postoperative cognitive dysfunction and potential associated anesthetic agents. Front. Med. 2022, 9, 811954. [Google Scholar] [CrossRef]
- Rochon, P.A.; Petrovic, M.; Cherubini, A.; Onder, G.; O’Mahony, D.; Sternberg, S.A.; Stall, N.M.; Gurwitz, J.H. Polypharmacy, inappropriate prescribing, and deprescribing in older people: Through a sex and gender lens. Lancet 2021, 2, 290–300. [Google Scholar] [CrossRef]
- Farrell, B.; Thompson, W.; Black, C.D.; Archibald, D.; Raman-Wilms, L.; Grassau, P.; Patel, T.; Weaver, L.; Eid, K.; Winslade, N. Health care providers’ roles and responsibilities in management of polypharmacy: Results of a modified Delphi. Can. Pharm. J. 2018, 11, 395–407. [Google Scholar] [CrossRef]
- Dahal, R.; Bista, S. Strategies to Reduce Polypharmacy in the Elderly; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/34662064/) (accessed on 8 September 2022).
- UN Population Division. United Nations World Population Prospects; UN Population Division: New York, NY, USA, 2012; Available online: https://www.un.org/en/development/desa/publications/world-population-prospects-the-2012revision.html#:~:text=The%20current%20world%20population%20of,more%20than%20half%20in%20Africa (accessed on 8 September 2022).
- Arnoldo, L.; Cattani, G.; Cojutti, P.; Pea, F.; Brusaferro, S. Monitoring polypharmacy in healthcare systems through a multi-setting survey: Should we put more attention on long term care facilities? J. Pub. Health Res. 2016, 5, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Alhindi, Y.Z.; Falemban, A.H.; Alshanberi, A.M.; Ayoub, N.A.; Alsanosi, S.M. Patients perspectives on polypharmacy: A survey-based cross-sectional study. J. Res. Med. Den. Sci. 2022, 10, 87–93. [Google Scholar]
- Eshel, N.; Raz, R.; Chodick, G.; Guindy, M. Characteristics of the elderly who do not visit primary care physicians. Isr. J. Health Policy Res. 2013, 2, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Edge, D.S.; Tranmer, J.E.; Wei, X.; Van, E.G. A population profile of older adults with prescription encounters with nurse practitioners and family physicians in Ontario: A descriptive retrospective cohort study. Can. Med. Assoc. Open Access J. 2019, 7, E323–E332. [Google Scholar]
- Menditto, E.; Gimeno Miguel, A.; Moreno Juste, A.; Poblador Plou, B.; Aza Pascual-Salcedo, M.; Orlando, V.; González Rubio, F.; Prados Torres, A. Patterns of multimorbidity and polypharmacy in young and adult population: Systematic associations among chronic diseases and drugs using factor analysis. PLoS ONE 2019, 14, e0210701. [Google Scholar] [CrossRef]
- Sohn, M.; Burgess, M.; Bazzi, M. Antipsychotic polypharmacy among children and young adults in office-based or hospital outpatient department settings. Pharmacy 2017, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.M.; Parekh, N.; Chua, K.C.; Davies, J.G.; Schiff, R.; Rajkumar, C.; Ali, K. A multi-centre cohort study on healthcare use due to medication-related harm: The role of frailty and polypharmacy. Age Ageing 2022, 51, afac054. [Google Scholar]
- O’Mahony, D. STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: Origin and progress. Exp. Rev. Clin. Pharmacol. 2019, 13, 15–22. [Google Scholar] [CrossRef]
- O’Connor, J.; Adabavazeh, B.; Choi, H.; Khan, A.; Shah, S.; Shah, S. Use of the STOPP and START criteria to address polypharmacy for elderly patients in University Hospital Lewisham Clinical Decisions Unit. Hong Kong J. Emerg. Med. 2019, 28, 79–84. [Google Scholar] [CrossRef]
- Alturki, A.; Aama, T.A.; Alomran, Y.; Al-Jedai, A.; Almudaiheem, H.; Watfa, G. Potentially inappropriate medications in older patients based on Beers criteria: A cross-sectional study of a family medicine practice in Saudi Arabia. BJGP Open 2020, 4, bjgpopen20X101009. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, J.T.; Schmader, K.E. The Medication Appropriateness Index at 20: Where it Started, Where it has been and Where it May be Going. Drugs Aging 2013, 30, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, J.T.; Schmader, K.E. The Medication Appropriateness Index: A clinimetric measure. Psychother. Psychosom. 2022, 91, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, K.M.; Mecca, M.C.; Fried, T.R. Medication appropriateness criteria for older adults: A narrative review of criteria and supporting studies. Herapeutic Adv. Drug Saf. 2019, 10, 204–210. [Google Scholar] [CrossRef]
- Silva-Almodóvar, A.; Nahata, M.C. Clinical utility of medication-based risk scores to reduce polypharmacy and potentially avoidable healthcare utilization. Pharmaceuticals 2022, 15, 681. [Google Scholar] [CrossRef]
- Cura-Gonzalez, I.; Lopez-Rodríguez, J.A.; Leiva-Fernández, F.; Gimeno-Miguel, A.; Poblador-Plou, B.; Lopez-Verde, F.; Lozano-Hernandez, C.; Pico-Soler, V.; Bujalance-Zafra, M.J.; Gimeno-Feliu, L.A.; et al. How to improve healthcare for patients with multimorbidity and polypharmacy in primary care: A Pragmatic cluster-randomized clinical trial of the MULTIPAP intervention. J. Pers. Med. 2022, 12, 752. [Google Scholar] [CrossRef]
- Coe, A.; Kaylor-Hughes, C.; Fletcher, S.; Murray, E.; Gunn, J. Deprescribing intervention activities mapped to guiding principles for use in general practice: A scoping review. BMJ Open. 2021, 11, e052547. [Google Scholar] [CrossRef]
- Zermansky, A.G. Deprescribing: Fashion Accessory or Fig Leaf? Pharmacy 2019, 7, 49. [Google Scholar] [CrossRef]
- Sadowski, C.A. Deprescribing-A few steps further. Pharmacy 2018, 6, 112. [Google Scholar] [CrossRef]
- Baysari, M.T.; Duong, M.H.; Hooper, P.; Stockey-Bridge, M.; Awad, S.; Zheng, W.Y.; Hilmer, S.N. Supporting deprescribing in hospitalised patients: Formative usability testing of a computerised decision support tool. BMC Med. Inform. Dec. Mak. 2021, 21, 116–123. [Google Scholar] [CrossRef]
- Perpetuo, C.; Placido, A.I.; Rodrigues, D.; Aperta, J.; Pineiro-Lamas, M.; Figueiras, A.; Herdeiro, M.T.; Roque, F. Prescription of potentially inappropriate medication in older inpatients of an internal medicine ward: Concordance and overlap among the EU(7)-PIM list and Beers and STOPP Criteria. Front. Pharm. 2021, 12, 676020. [Google Scholar] [CrossRef] [PubMed]
- Page, A.T.; Clifford, R.; Potter, K.; Etherton-Beer, C. A concept analysis of deprescribing medications in older people. J. Pharm. Prac. Res. 2018, 48, 132–148. [Google Scholar] [CrossRef]
- Junius-Walker, U.; Viniol, A.; Michiels-Corsten, M.; Gerlach, N.; Donner-Banzhoff, N.; Schleef, T. MediQuit, an electronic deprescribing tool for patients on polypharmacy: Results of a feasibility study in German general practice. Drug. Aging 2021, 38, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.L.; Schmalbach, J.E.; Gonzalez, C.V.; Rodriguez, D.; Figueras, A. Development of a stepwise tool to aide primary health care professionals in the process of deprescribing in older persons. Pharm. Pract. Granada 2021, 18, 213–220. [Google Scholar]
- Aharaz, A.; Rasmussen, J.H.; McNulty, H.B.Ø.; Cyron, A.; Fabricius, P.K.; Bengaard, A.K.; Sejberg, H.R.C.; Simonsen, R.R.L.; Treldal, C.; Houlind, M.B. A collaborative deprescribing intervention in a subacute medical outpatient clinic: A pilot randomized controlled trial. Metabolites 2021, 11, 204. [Google Scholar] [CrossRef]
- Dalin, D.A.; Frandsen, S.; Madsen, G.K.; Vermehren, C. Exploration of symptom scale as an outcome for deprescribing: A medication review study in nursing homes. Pharmaceuticals 2022, 15, 505. [Google Scholar] [CrossRef]
- Buzancic, I.; Hadziabdic, M.O. Development and Validation of Comprehensive Healthcare Providers Opinions, Preferences, and Attitudes towards Deprescribing (CHOPPED Questionnaire). Pharmacy 2022, 10, 76. [Google Scholar] [CrossRef]
- Flood, B. Deprescribing of psychotropic medications in the adult population with intellectual disabilities: A commentary. Pharmacy 2018, 6, 28. [Google Scholar] [CrossRef]
- Corrick, F.; Conroy, S.; Sammons, H.; Choonara, I. Paediatric rational prescribing: A systematic review of assessment tools. Int. J. Environ. Res. Public Health 2020, 17, 1473. [Google Scholar] [CrossRef]
- Sawan, M.; Reeve, E.; Turner, J.; Todd, A.; Steinman, M.A.; Petrovic, M.; Gnjidic, D. A systems approach to identifying the challenges of implementing deprescribing in older adults across different health-care settings and countries: A narrative review. Exp. Rev. Clin. Pharm. 2020, 13, 233–245. [Google Scholar] [CrossRef]
- Mobilizing Community Pharmacists as Catalysts for Deprescribing. Available online: https://deprescribing.org/news/mobilizing-community-pharmacists-catalysts-deprescribing-deprescribing-catalyst-project/ (accessed on 25 August 2022).
- Creating Evidence-Based Deprescribing Guidelines. Available online: https://deprescribing.org/news/creating-evidence-based-deprescribing-guidelines/ (accessed on 25 August 2022).
- Geriatric Pharmacoeconomics: Cost Savings from Deprescribing and Appropriate Prescribing. Available online: https://deprescribing.org/news/geriatric-pharmacoeconomics-cost-savings-from-deprescribing/ (accessed on 25 August 2022).
- Farrell, B.; Black, C.; Thompson, W.; McCarthy, L.; Rojas-Fernandez, C.; Lochnan, H. Deprescribing anti-hyperglycemic agents in older persons. Evidence-based clinical practice guideline. Can. Fam. Phys. 2017, 63, 832–843. [Google Scholar]
- Engaging Primary Care Providers in Deprescribing Trials. Available online: https://deprescribing.org/news/the-canadian-primary-care-sentinel-surveillance-network-seniors-deprescribing-trial/ (accessed on 25 August 2022).
- D-PRESCRIBE Trial—Harnessing the Power of the Physician, Pharmacist and Patient Triad. Available online: https://deprescribing.org/news/d-prescribe-trial-harnessing-the-power-of-the-physician-pharmacist-and-patient-triad/ (accessed on 25 August 2022).
- Khanderparkar, A.; Rataboli, P.V. A study of harmful drug-drug interactions due to polypharmacy in hospitalized patients of Goa Medical College. Perspect. Clin. Res. 2017, 8, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.V.; Wallace, B.K.; Chase, H.S. Prevalence of potentially harmful multidrug interactions on medication lists of elderly ambulatory patients. BMC Ger. 2021, 21, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Chaddad, A.; Hanna, J.; Matta, J.; Daoud, Z. Understanding the epidemiology of multidrug resistant gram negative Bacilli in the Middle East using a one health approach. Front. Microbiol. 2019, 10, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.A.; Guy-Alfandary, S.; Rochon, P.A. Prescribing cascades in older adults. Can. Med. Assoc. J. 2021, 193, E215–E220. [Google Scholar] [CrossRef]
- Saraf, A.A.; Peterson, A.W.; Simmons, S.F.; Schnelle, J.F.; Bell, S.P.; Kriplani, S.; Myers, A.P.; Mixon, A.S.; Long, E.A.; Jacobsen, J.M.L.; et al. Medications associated with geriatric syndrome (MAGS) and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J. Hosp. Med. 2017, 11, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Kamara, R.F.; Saunders, M.J.; Sahr, P.F.; Losa-Garcia, J.E.; Foray, L.; Davies, P.G.; Wingfield, T. Social and health factors associated with adverse treatment outcomes among people with multidrug resistant tuberculosis in Sierra Leone: A national, retrospective cohort study. Lancet 2022, 10, 543–554. [Google Scholar] [CrossRef]
- Wohlgemuth, A.; Michalowsky, B.; Wucherer, D.; Eichler, T.; Thyrian, J.R.; Zwingmann, I.; Radke, A.; Hoffmann, W. Drug related problems increase healthcare costs for people living with dementia. J. Alzh. Dis. 2020, 73, 791–799. [Google Scholar] [CrossRef]
- Jungo, K.T.; Streit, S.; Lauffenburge, J. Utilization and spending on potentially inappropriate medications by US older adults with multiple chronic conditions using multiple medications. Arch. Gerontol. Geriat. 2021, 93, 104326–104332. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, N.K.; Lee, J.; Seong, J.M.; Park, M.J.; Lee, S.H.; Park, B.J. Risk of ischemic stroke associated with the use of antipsychotic drugs in elderly patients: A retrospective cohort study in Korea. PLoS ONE 2015, 10, e0119931. [Google Scholar] [CrossRef]
- Rojas-Fernandez, C.H.; Chen, Y. Use of ultra-low-dose (≤6 mg) doxepin for treatment of insomnia in older people. Can. Pharm. J. 2014, 147, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Leppik, I.E. Contemporary Diagnosis and Management of the Patient with Epilepsy, 5th ed.; Handbooks in Health Care: Newtown, PA, USA, 2001. [Google Scholar]
- Passmore, A.P.; Johnston, G.D. Digoxin Toxicity in the Aged. Drugs Aging 1991, 1, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Mallet, L.; Kuyumjian, J. Indomethacin-induced behavioral changes in an elderly patient with dementia. Ann. Pharmacother. 1998, 32, 201–203. [Google Scholar] [CrossRef]
- Roberts, C.G.; Ladenson, P.W. Hypothyroidism. Lancet 2004, 363, 793–803. [Google Scholar] [CrossRef]
- ISeifert, C.F.; Kennedy, S. Meperidine is alive and well in the new millennium: Evaluation of meperidine usage patterns and frequency of adverse drug reactions. Pharmacotherapy 2004, 24, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Khalil, Y.A. Methyldopa. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551671/ (accessed on 8 September 2022).
- Spence, M.M.; Shin, P.J.; Lee, E.A.; Gibbs, N.E. Risk of injury associated with skeletal muscle relaxant use in older adults. Ann. Pharmacother. 2013, 47, 993–998. [Google Scholar] [CrossRef]
- Tinetti, M.E. Clinical practice. Preventing falls in elderly persons. N. Engl. J. Med. 2003, 348, 42–49. [Google Scholar] [CrossRef]
- Fang, M.C.; Chang, Y.C.; Hylek, E.M.; Rosand, J.; Greenberg, S.M.; Go, A.S.; Singer, D.E. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann. Intern. Med. 2004, 141, 745–752. [Google Scholar] [CrossRef]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef]
- Ohta, K.; Fukuchi, Y.; Grouse, L.; Mizutani, R.; Rabe, K.F.; Rennard, S.I.; Zhong, N.S. A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir. Med. 2004, 98, 1016–1024. [Google Scholar] [CrossRef][Green Version]
- Mian, P.; Allegaert, K.; Spriet, I.; Tibboel, D.; Petrovic, M. Paracetamol in older people: Towards evidence-based dosing? Drugs Aging 2018, 35, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Magid, S.; Forrer, C.; Shaha, S. Duplicate orders: An unintended consequence of computerized provider/physician order entry (CPOE) implementation: Analysis and mitigation strategies. Appl. Clin. Inform. 2012, 3, 377–391. [Google Scholar] [PubMed]
- Liang, Y.; Wang, K.H.; Huang, M.H.; Shia, B.C.; Chan, S.Y.; Ho, C.W.; Liu, C.K.; Chen, M. Reducing medication problems among minority individuals with low socioeconomic status through pharmacist home visits. Int. J. Environ. Res. Public Health 2022, 19, 4234. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Cahir, C.; Malo, S.; Aguilar-Palacio, I.; Almada, M.; Costa, E.; Giardini, A.; Peinado, M.G.; Mesquida, M.M.; Mucherino, S.; et al. Persistence as a robust indicator of medication adherence-related quality and performance. Int. J. Environ. Res. Public Health 2021, 18, 4872. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Kamkar, N.; Pieruccini-Faria, F.; Osman, A.; Sarquis-Adamson, Y.; Close, J.; Hogan, D.B.; Hunter, S.W.; Kenny, R.A.; Lipsitz, L.A.; et al. Task force on global guidelines for falls in older adults: Evaluation of clinical practice guidelines on fall prevention and management for older adults: A systematic review. JAMA Net. Open 2021, 1, e2138911. [Google Scholar] [CrossRef]
- Beuscart, J.B.; Pelayo, S.; Robert, L.; Thevelin, S.; Marien, S.; Dalleur, O. Medication review and reconciliation in older adults. Eur. Geriat. Med. 2021, 12, 499–507. [Google Scholar] [CrossRef]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef]
- Curtin, D.; Gallagher, P.F.; O’Mahony, D. Explicit criteria as clinical tools to minimize inappropriate medication use and its consequences. Ther. Adv. Drug Saf. 2019, 13, 431–440. [Google Scholar] [CrossRef]
- Bahat, G.; Ilhan, B.; Bay, I.; Kilic, C.; Kucukdagli, P.; Oren, M.M.; Karan, M.A. Explicit versus implicit evaluation to detect inappropriate medication use in geriatric outpatients. Aging Male 2020, 23, 179–184. [Google Scholar] [CrossRef]
- Kurczewska-Michalak, M.; Lewek, P.; Jankowska-Polanska, B.; Giardini, A.; Granata, N.; Maffoni, M.; Costa, E.; Midao, L.; Kardas, P. Polypharmacy management in the older adults: A scoping review of available interventions. Front. Pharmacol. 2021, 26, 734–745. [Google Scholar] [CrossRef]
- Gutierrez-Valencia, M.; Martinez-Velilla, N.; Vilches-Moraga, A. Polypharmacy in older people: Time to take action. Eur. Geriat. Med. 2019, 10, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Monteiro-Soares, M.; Matos, C.; Ribeiro-Vaz, I.; Teixeira, A.; Martins, C. Inappropriate prescriptions in older people-Translation and adaptation to Portuguese of the STOPP/START screening tool. Int. J. Environ. Res. Public Health 2022, 19, 6896. [Google Scholar] [CrossRef] [PubMed]
- Alshanberi, A.; Tallant, C.; Huddleston, P.; Imam, A.; Glidan, A.; Passmore, C.; van Zuilen, M.H. Advance directives in patients over 60 years old: Assessment of perceived value and need for education in the outpatient setting. Arch. Med. 2018, 10, 1–5. [Google Scholar]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003.
- Weaver, M.; Patrick, D.L.; Markson, L.E.; Martin, D.; Frederic, I.; Berger, M. Issues in the measurement of satisfaction with treatment. Am. J. Manag. Care 1997, 3, 579–594. [Google Scholar] [PubMed]
- Zhang, C.; Xiang, C.; Tian, X.; Xue, J.; He, G.; Wu, X.; Mei, Z.; Li, T. Roles of nursing in the management of geriatric cardiovascular diseases. Front. Med. 2021, 8, 682218. [Google Scholar] [CrossRef] [PubMed]
- Haydon, H.M.; Caffery, L.J.; Snoswell, C.L.; Thomas, E.E.; Taylor, M.; Budge, M.; Probert, J.; Smith, A.C. Optimizing specialist geriatric medicine services by telehealth. J. Telemed. Telecare 2021, 27, 674–679. [Google Scholar] [CrossRef]
- Byrne, A.; Byrne, S.; Dalton, K. A pharmacist’s unique opportunity within a multidisciplinary team to reduce drug-related problems for older adults in an intermediate care setting. Res. Soc. Admin. Pharm. 2022, 18, 2625–2633. [Google Scholar] [CrossRef]
- Zietlow, K.E.; Wong, S.; Heflin, M.T.; McDonald, S.R.; Sickeler, R.; Devinney, M.; Blitz, J.; Lagoo-Deenadayalan, S.; Berger, M. Geriatric preoperative optimization: A review. Am. J. Med. 2022, 135, 39–48. [Google Scholar] [CrossRef]
- Louka, A.M.; Tsagkaris, C.; Christoforou, P.; Khan, A.; Alexiou, F.; Simou, P.; Haranas, I.; Gkigkitzis, I.; Zouganelis, G.; Jha, N.K.; et al. Current Trends of Computational Tools in Geriatric Medicine and Frailty Management. Front. Biosci. 2022, 29, 232–240. [Google Scholar] [CrossRef]
- Nduaguba, S.O.; Soremekun, R.O.; Olugbake, O.A.; Barner, J.C. The relationship between patient-related factors and medication adherence among Nigerian patients taking highly active anti-retroviral therapy. Afr. Health Sci. 2017, 17, 738–745. [Google Scholar] [CrossRef][Green Version]
- Assessing Cognitive Impairment in Older Patients. Available online: https://www.nia.nih.gov/health/assessing-cognitive-impairment-older-patients (accessed on 8 September 2022).
- Ward, K.T.; Reuben, D.B. Comprehensive Geriatric Assessment. Edited by KE Schmader. Available online: https://www.uptodate.com/contents/comprehensive-geriatric-assessment#disclaimerContent (accessed on 8 September 2022).
- Wong, Y.G.; Hang, J.A.; Francis-Coad, J.; Hill, A.M. Using comprehensive geriatric assessment for older adults undertaking a facility-based transition care program to evaluate functional outcomes: A feasibility study. BMC Geriatr. 2022, 22, 598. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).