Cognitive Plasticity in Young-Old Adults and Old-Old Adults and Its Relationship with Successful Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedures
2.4. Statistical Analysis
3. Results
3.1. Performance in the AVLT-LP Trials, Delayed Recall (A7) and Gain Score as a Function of Age Range, Gender and Cognitive Status
3.2. Distribution of the Participants According to Their Age Range, Cognitive Status, Gender and Cognitive Plasticity Status
3.3. Performance in the AVLT-LP: Comparing the Young-Old Adults and the Old-Old Adults with and without Cognitive Impairment
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat. Population Structure and Ageing; Eurostat Statistics Explained: Luxembourg, 2017. [Google Scholar]
- Fowles, D.G.; Greenberg, S. A Profile of Older Americans: 2008; Administration on Aging U.S. Department of Health and Human Services: Washington, DC, USA, 2008.
- Sales-Galán, A.; Meléndez-Moral, J.C.; Mayordomo-Rodríguez, T. Using a cognitive plasticity measure to detect mild cognitive impairment. Arch. Clin. Neuropsychol. 2013, 28, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Buczylowska, D.; Petermann, F. Age-related differences and heterogeneity in executive functions: Analysis of NAB executive functions module scores. Arch. Clin. Neuropsychol. 2016, 31, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lövdén, M.; Bäckman, L.; Lindenberger, U.; Schaefer, S.; Schmiedek, F. A theoretical framework for the study o adult cognitive plasticity. Psychol. Bull. 2010, 136, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Rönnlund, M.; Nyberg, L.; Bäckman, L.; Nilsson, L.G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychol. Aging 2005, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- DeFrias, C.M.; Lövden, M.; Lindenberger, U.; Nilsson, L.G. Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence 2007, 35, 381–392. [Google Scholar] [CrossRef]
- Lindenberger, U.; Ghisletta, P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychol. Aging 2009, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baltes, P.B. Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Dev. Psychol. 1987, 23, 611–626. [Google Scholar] [CrossRef]
- Verhaeghen, P. The interplay of growth and decline: Theoretical and empirical aspects of plasticity of intellectual and memory performance in normal old age. In Cognitive Rehabilitation in Old Age; Hill, R.D., Bäckman, L., Stigsdotter, A., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 3–22. ISBN 0-19-511985-1. [Google Scholar]
- Wilkinson, A.J.; Yang, L. Inhibition plasticity in older adults: Practice and transfer effects using a multiple task approach. Neural Plast. 2016. [Google Scholar] [CrossRef] [PubMed]
- Baltes, P.B.; Willis, S. Plasticity and enhancement of intellectual function in old age. Pennstage’s adult development and enrichment proyect (ADEPT). In Aging and Cognitive Processes; Craik, F.I.M., Treudse, S.E., Eds.; Plenum Press: New York, NY, USA, 1982; pp. 353–389. ISBN 978-1-4684-4178-9. [Google Scholar]
- Raykov, T.; Baltes, M.M.; Neher, K.M.; Sowarka, D. A comparative study of two psychometric approaches to detect risk status for dementia. Gerontology 2002, 48, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Nyber, L.; Sandblom, J.; Stigsdotter-Neely, A.; Ingvar, M.; Petersson, K.M.; Bäckman, L. Cognitive and neural plasticity in aging: General and task-specific limitation. Neurosci. Biobehav. R. 2006, 30, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Satz, P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychol 1993, 7, 273–295. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballesteros, R.; Zamarrón, M.D.; Calero, M.D.; Tárraga, L. Cognitive plasticity and cognitive impairment. In GeroPsychology. European Perspectives for an Ageing World; Fernández-Ballesteros, R., Ed.; Hogrefe and Huber: Göttingen, UK, 2007; pp. 145–164. ISBN 9780889373402. [Google Scholar]
- Dahlin, E.; Stigsdotter, N.; Larsson, A.; Bäckman, L.; Nyberg, L. Transfer of learning after updating training mediated by the striatum. Science 2008, 320, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Sandblom, J.; Stigsdotter, N.; Petersson, J.M.; Igvar, M.; Bäckman, L. Neural correlates of training-ralated memory improvement in adulthood and aging. Proc. Natl. Acad. Sci. USA 2003, 100, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballesteros, R.; Zamarrón, M.D.; Tárraga, L. Learning potential: A new method for assessing cognitive impairment. Int. Psychogeriatr. 2005, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kliegl, R.; Smith, J.; Baltes, P.B. Testing-the limits and the study of adult age differences in cognitive plasticity of a mnemonic skill. Dev. Psychol. 1989, 25, 247–256. [Google Scholar] [CrossRef]
- Zamarrón, M.D.; Tárraga, L.; Fernández-Ballesteros, R. Plasticidad cognitiva en personas con enfermedad de Alzheimer que reciben programas de estimulación cognitiva [Cognitive plasticity in people with Alzheimer’s disease who receive cognitive stimulation programs]. Psicothema 2008, 20, 432–437. [Google Scholar]
- Swansons, H.L.; Lussier, C.M. A selective synthesis of the experimental literature on dynamic assessment. Rev. Educ. Res. 2001, 71, 321–363. [Google Scholar] [CrossRef]
- Navarro, E. La evaluación del potencial de aprendizaje en la vejez. [Learning potential assessment in the elderly]. In Evaluación del Potencial de Aprendizaje: Fundamentos y Aplicaciones; Learning Potential Assessment: Basis and Applications; Calero, M.D., Ed.; EOS: Madrid, Spain, 2012; pp. 207–238. ISBN 9788497274456. [Google Scholar]
- Baltes, M.; Raykow, T. Prospective validity of cognitive plasticity in the diagnosis of mental status: A structural equation model. Neuropsychology 1996, 10, 549–556. [Google Scholar] [CrossRef]
- Calero, M.D.; Navarro, E. Relation between plasticity, mild cognitive impairment and cognitive decline. Arch. Clin. Neuropsychol. 2004, 19, 623–660. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.M.; Parasuraman, R. Neuronal and Cognitive Plasticity: A Neurocognitive Framework for Ameliorating Cognitive Aging. Front. Aging Neurosci. 2010, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Linderberger, U. Research on human plasticity in adulthood: A lifespan agenda. In Handbook of the Psychology of Aging 2006, 8th ed.; Schaie, K.W., Willis, S.L., Eds.; Elsevier: London, UK, 2006; pp. 105–123. ISBN 9780124115231. [Google Scholar]
- Yang, L.; Krampe, R.T.; Baltes, P.B. Basic forms of cognitive plasticity extended into the oldest-old: Retest learning, age, and cognitive functioning. Psychol. Aging 2006, 21, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Krampe, R.T. Long-term maintenance of retest learning in young old and oldest old adults. J. Gerontol. B Sci. Soc. Sci. 2009, 64, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Baltes, P.B.; Smith, J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology 2003, 49, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Halpert, B.P.; Zimmerman, M.K. The health status of the “old-old”: A reconsideration. Soc. Sci. Med. 1986, 22, 893–899. [Google Scholar] [CrossRef]
- Martin, A.S.; Palmer, B.W.; Rock, D.; Gelston, C.V.; Jesten, D.V. Associations of self-perceived successful aging in young-old versus old-old adults. Int. Psychogeriatr. 2015, 27, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Carnes, B.; Olsharsky, S. A realist view of aging, mortality and future longevity. Popul. Dev. Rev. 2007, 33, 367–381. [Google Scholar] [CrossRef]
- Salthouse, T.A. Theoretical Perspectives on Cognitive Aging; University of Illinois Press: New York, NY, USA, 2016; ISBN 9781317717294. [Google Scholar]
- Bäckman, L.; Small, B.J.; Wahlin, A.; Larsson, M. Cognitive functioning in very old age. In Handbook of Cognitive Aging, 2nd ed.; Craik, F.I.M., Salthouse, T.A., Eds.; Psychology Press Taylor and Francis Group: New York, NY, USA, 2000; pp. 499–558. ISBN 978-0-8058-5990-4. [Google Scholar]
- Bendayan, R.; Piccinin, A.M.; Hofer, S.M.; Cadar, D.; Johansson, B.; Muniz-Terrera, G. Decline in memory, visuospatial ability and crystallized cognitive abilities in older adults: Normative aging or terminal decline? J. Aging Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Snitz, B.E.; Unverzagt, F.W.; Chang, C.C.; Bilt, J.; Gao, S.; Saxton, J.; Hall, K.S.; Ganguli, M. Effects of age, gender, education and race on two tests of language ability in community-based older adults. Int. Psychogeriatr. 2009, 21, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Wiedl, K.; Wienobst, J.; Schöttke, H. Interindividual differences in cognitive remediation research with schizophrenic patients—Indicators of rehabilitation potential? Int. J. Rehabil. Res. 1999, 22, 1–5. [Google Scholar] [CrossRef]
- Calero, M.D.; Navarro, E. La Plasticidad Cognitiva en la Vejez: Técnicas de Evaluación e Intervención; Cognitive Plasticity in Old Age: Assessment Techniques and Intervention; Octaedro: Barcelona, Spain, 2006; ISBN 9788480638050. [Google Scholar]
- Rey, A. L’examin Clinique en Psychologie; Clinical Examination in Psychology; Presses Universitaires de France: Paris, France, 1958; ISBN 10:2853851788. [Google Scholar]
- Wiedl, K.H.; Schöttke, H.; Calero, M.D. Dynamic assessment of cognitive rehabilitation potential in schizophrenic persons and in old people with and without dementia. Eur. J. Psychol. Assess. 2001, 17, 112–119. [Google Scholar] [CrossRef]
- Navarro, E.; Calero, M.D.; Becerra, D. Trayectorias de envejecimiento en una muestra de personas mayores: Un estudio longitudinal. Trajectories of aging in a simple of elderly people: A longitudinal study. Rev. Esp. Geriatr. Gerontol. 2015, 50, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballesteros, R.; Botella, J.; Zamarrón, M.D.; Molina, M.A.; Cabras, E.; Schettini, R.; Tárraga, L. Cognitive plasticity in normal and pathological aging. Clin. Interv. Aging 2012, 7, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Ezquerra, J.; Gómez, F.; Sala, J.M.; Seva-Diaz, A. El Mini-Examen Cognoscitivo. Un test sencillo y práctico para detectar alteraciones intelectuales en pacientes médicos. The Mini-Examen-Cognoscitivo. An easy and practical test to detect intelectual alterations in medical patients. Actas Luso-Esp. Neurol. 1979, 7, 189–201. [Google Scholar]
- Folstein, M.; Folstein, S.; McHugh, P. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Vinyoles, E.; Vila, J.; Argimon, J.M.; Espinàs, J.; Abos, T.; Limón, E. Concordance among Mini-Examen Cognoscitivo and Mini-Mental State Examination in cognitive impairment screening. Aten. Primaria 2002, 30, 5–15. [Google Scholar] [CrossRef]
- Manubens, J.M.; Martínez-Lage, P.; Martínez-Lage, J.M.; Larrumbe, R.; Murazabal, J.; Martínez, M.A. Variación de las puntuaciones en el Mini-Mental-State con la edad y el nivel educativo. Datos normalizados en la población mayor de 70 años de Pamplona. [Variations in the Mini-Mental-State scores with age and level of education. Normative data for Pamplona’s population older than 70 years old]. Neurología 1998, 13, 111–119. [Google Scholar] [PubMed]
- Calero, M.D.; Navarro, E.; Robles, P.; García-Berbén, T. Estudio de validez del Mini-Examen-Cognitivo de Lobo et al. para la detección del deterioro cognitivo asociado a demencias. Validity study of Lobo’s Mini-Examen-Cognitivo for the detection of cognitive impairment associated with dementia. Neurología 2000, 15, 337–342. [Google Scholar] [PubMed]
- Schöttke, H.; Bartram, M.; Wiedl, K. Psychometric implications of learning potential assessment: A typological approach. In Learning Potential Assessment: Theoretical, Methodological and Practical Issues; Hamers, J., Sijtsma, K., Ruijssemaars, A.J.J., Eds.; Swets & Zetilinger: Lisse, The Neetherlands, 1993; pp. 153–173. ISBN 90-265-1238-4. [Google Scholar]
- IBM Corp. Released IBM SPSS Statistics for Windows, v. 23; IBM Corp.: Armonk, NY, USA, 2014. [Google Scholar]
- Willis, S.L.; Schaie, K.W. Cognitive training and plasticity: Theoretical perspective and methodological consequences. Restor. Neurol. Neurosci. 2009, 27, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Lindenberger, U.; Baltes, P.B. Plasticity of memory for new learning in very old age: A story of major loss? Psychol. Aging 2003, 306–317. [Google Scholar] [CrossRef]
- Beinhoff, U.; Tumani, H.; Brettschneider, J.; Bittner, D.; Riepe, M.W. Gender-specificities in Alzheimer’s disease and mild cognitive impairment. J. Neurol. 2008, 255, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lamar, M.; Resnick, S.M.; Zonderman, A.B. Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology 2003, 60, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Messinis, L.; Tsakona, I.; Malefaki, S.; Papathanasopoulos, P. Normative data and discriminant validity of Rey’s Verbal Learning Test for the Greek adults population. Arch. Clin. Neuropsychol. 2007, 22, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, M.L.; Bolla-Wilson, K.; Agnew, J.; Meyers, D.A. Age-related sex differences in verbal memory. J. Clin. Psychol. 1988, 44, 403–411. [Google Scholar] [CrossRef]
- Speer, P.; Wersching, H.; Bruchmann, S.; Bracht, D.; Stehling, C.; Thielsch, M.; Knecht, S.; Lohmann, H. Age—and gender—Adjusted normative data for the German version of Rey’s Auditory Verbal Learning Test from healthy subjects aged between 50 and 70 years. J. Clin. Exp. Neuropsychol. 2014, 36, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Faille, L. Performance on a Brain Plasticity Based Memory Training Computer Program for the Elderly as Influenced by Cognitive Functioning and Gender; California School of Professional Psychology: San Francisco, CA, USA, 2006; ISBN 9781109940763. [Google Scholar]
- Duff, K.; Beglinger, L.J.; Schultz, S.K.; Moser, D.J.; McCaffrey, R.J.; Haase, R.F.; Westervelt, H.J.; LangBehn, D.R.; Paulsen, J.S. Practice effects in the prediction of long-term cognitive outcome in three patient samples: A novel prognostic index. Arch. Clin. Neurospychol. 2007, 22, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.D.; Navarro, E. Cognitive plasticity as a modulating variable on the effects of memory training in elderly persons. Arch. Clin. Neuropsychol. 2007, 22, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Fernaeus, S.E.; Östberg, P.; Wahlund, L.O.; Hellström, A. Memory factors in Rey AVLT: Implications for early staging of cognitive decline. Scand. J. Psychol. 2014, 55, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Calero, D.; Navarro, E. Differences in cognitive performance, level of dependency and quality of life (QoL), related to age and cognitive status in a sample of Spanish adults under and over 80 years of age. Arch. Gerontol. Geriatr. 2011, 53, 292–294. [Google Scholar] [CrossRef] [PubMed]
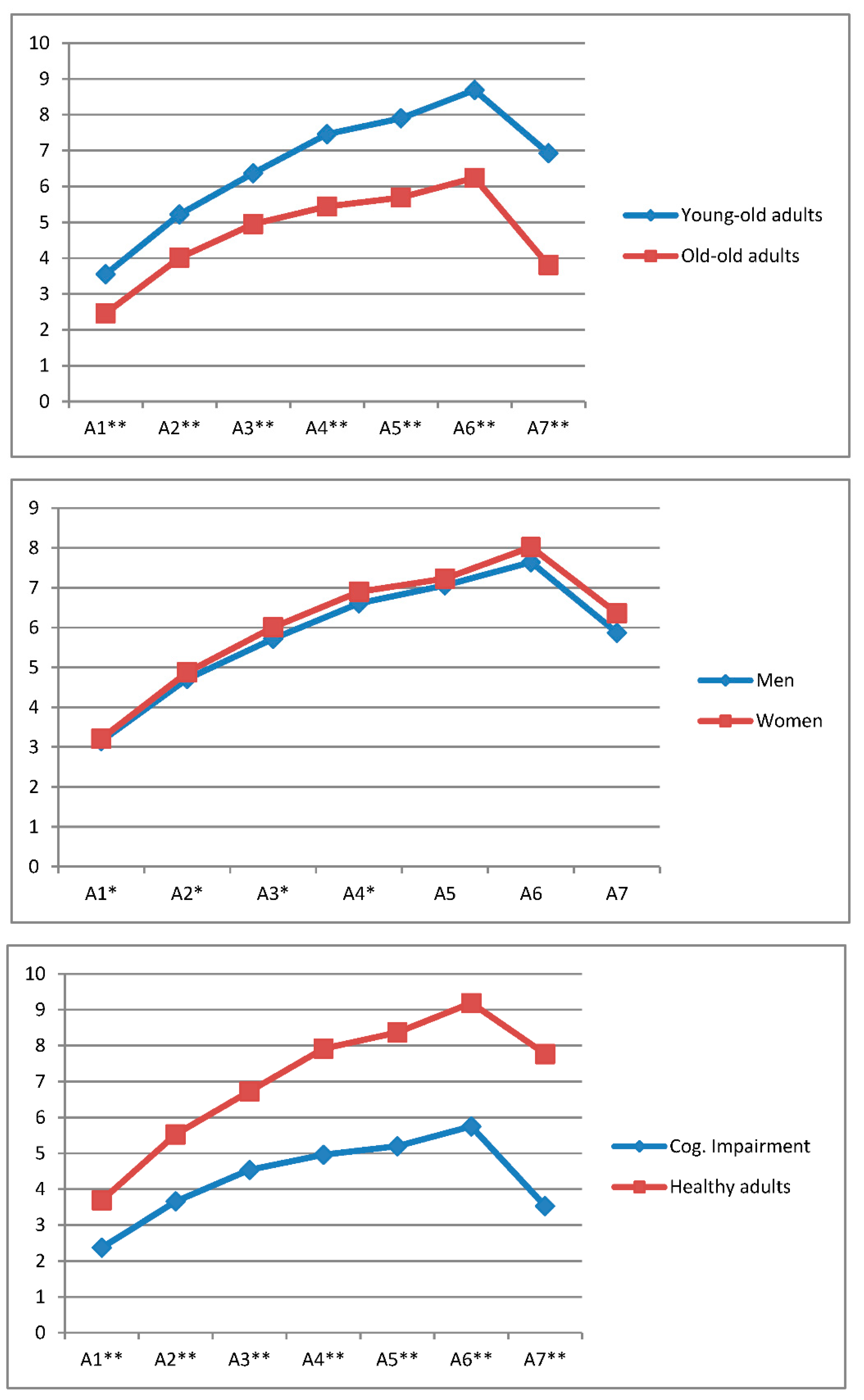
| AVLT-LP TRIALS | A1 | A2 | A3 | A4 | A5 | A6 | A7 | Gain Score |
|---|---|---|---|---|---|---|---|---|
| AGE RANGE | ||||||||
| 60–80 (n = 379) | 3.55 | 5.22 | 6.37 | 7.46 | 7.90 | 8.69 | 6.93 | 3.89 |
| 81+ (n = 223) | 2.46 | 4.01 | 4.95 | 5.44 | 5.69 | 6.24 | 3.81 | 2.74 |
| F(1/568) | 62.980 ** | 48.988 ** | 45.578 ** | 67.126 ** | 66.811 ** | 72.870 ** | 45.695 ** | 35.801 ** |
| ŋ2 | 0.100 | 0.080 | 0.074 | 0.106 | 0.105 | 0.114 | 0.117 | 0.059 |
| observed power | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| GENDER | ||||||||
| Men (n = 219) | 3.15 | 4.71 | 5.72 | 6.61 | 7.06 | 7.64 | 5.87 | 3.42 |
| Women (n = 350) | 3.21 | 4.88 | 6.01 | 6.90 | 7.23 | 8.02 | 6.36 | 3.57 |
| F(1/568) | 5.448 * | 5.837 * | 6.546 * | 3.882 * | 2.616 | 2.429 | 1.438 | 0.731 |
| ŋ2 | 0.000 | 0.002 | 0.003 | 0.002 | 0.001 | 0.003 | 0.004 | 0.001 |
| observed power | 0.847 | 0.872 | 0.908 | 0.701 | 0.521 | 0.489 | 0.307 | 0.100 |
| COGNITIVE STATUS | ||||||||
| Cognitive impairment (n = 217) | 2.37 | 3.66 | 4.54 | 4.96 | 5.20 | 5.75 | 3.53 | 2.47 |
| Healthy adults (n = 352) | 3.69 | 5.53 | 6.73 | 7.92 | 8.37 | 9.19 | 7.77 | 4.15 |
| F(1/568) | 53.511 ** | 72.748 ** | 66.728 ** | 90.434 ** | 88.355 ** | 93.312 ** | 66.101 ** | 48.189 ** |
| ŋ2 | 0.155 | 0.200 | 0.188 | 0.240 | 0.231 | 0.238 | 0.274 | 0.131 |
| observed power | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Plasticity Status | Age range | Gender | Cognitive Status | |||
|---|---|---|---|---|---|---|
| 60–80 | 81+ | Men | Women | Cognitive Impairment | Healthy Adults | |
| Low plasticity | 157 | 121 | 111 | 167 | 147 | 70 |
| High plasticity | 222 | 69 | 108 | 183 | 131 | 221 |
| χ2 = 26.027 p < 0.0001 | χ2 = 0.476 p > 0.05 | χ2 = 50.063 p < 0.0001 | ||||
| Old-Old Adults | Young-Old Adults | Cognitive Status | Age Range | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVLT-LP TRIALS | Group | M | SD | M | SD | F(1,4) | η2 partial | O.P. | F(1,4) | η2 partial | O.P. | F(1,4) | η2 partial | O.P. |
| A1 | Cog. Im | 2.04 | 1.31 | 2.65 | 1.44 | 60.66 ** | 0.003 | 0.164 | 33.42 ** | 0.056 | 1.00 | 0.1776 | 0.003 | 0.265 |
| Healthy | 2.97 | 1.23 | 3.93 | 1.58 | ||||||||||
| A2 | Cog. Im | 3.36 | 1.58 | 3.90 | 1.58 | 92.96 ** | 0.001 | 0.102 | 21.57 ** | 0.037 | 0.996 | 1.873 | 0.003 | 0.277 |
| Healthy | 4.79 | 1.63 | 5.78 | 1.97 | ||||||||||
| A3 | Cog. Im | 4.29 | 1.60 | 4.75 | 1.95 | 81.05 ** | 0.001 | 0.127 | 18.84 ** | 0.033 | 0.991 | 0.3317 | 0.008 | 0.555 |
| Healthy | 5.77 | 2.43 | 7.06 | 2.48 | ||||||||||
| A4 | Cog. Im | 4.59 | 1.82 | 5.29 | 2.24 | 116.94 ** | 0.000 | 0.082 | 33.37 ** | 0.056 | 1.00 | 7.209 * | 0.013 | 0.764 |
| Healthy | 6.48 | 2.56 | 8.48 | 2.72 | ||||||||||
| A5 | Cog. Im | 4.84 | 2.12 | 5.46 | 2.46 | 112.78 ** | 0.001 | 0.142 | 33.42 ** | 0.056 | 1.00 | 10.719 ** | 0.019 | 0.905 |
| Healthy | 6.68 | 2.75 | 8.93 | 2.99 | ||||||||||
| A6 | Cog. Im | 5.27 | 2.43 | 6.10 | 2.75 | 122.38 ** | 0.004 | 0.318 | 38.77 ** | 0.065 | 1.00 | 9.402 * | 0.017 | 0.865 |
| Healty | 7.36 | 2.74 | 9.80 | 3.97 | ||||||||||
| A7 | Cog. Im | 3.08 | 2.08 | 4.09 | 2.61 | 7.59 * | 0.051 | 0.782 | 4.515 * | 0.03 | 0.560 | 0.350 | 0.002 | 0.090 |
| Healthy | 4.90 | 2.85 | 6.51 | 3.44 | ||||||||||
| A7 − A1 | Cog. Im | 1.35 | 2.33 | 1.43 | 2.38 | 3.76 * | 0.026 | 0.487 | 0.616 | 0.004 | 0.122 | 0.507 | 0.004 | 0.109 |
| Healthy | 2.30 | 2.45 | 3.07 | 2.79 | ||||||||||
| AVLT-LP gain score | Cog. Im | 2.36 | 1.79 | 2.53 | 1.94 | 59.37 ** | 0.096 | 1.000 | 16.826 ** | 0.148 | 0.984 | 9.915 * | 0.017 | 0.882 |
| Healty | 3.14 | 2.08 | 4.49 | 2.13 | ||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, E.; Calero, M.D. Cognitive Plasticity in Young-Old Adults and Old-Old Adults and Its Relationship with Successful Aging. Geriatrics 2018, 3, 76. https://doi.org/10.3390/geriatrics3040076
Navarro E, Calero MD. Cognitive Plasticity in Young-Old Adults and Old-Old Adults and Its Relationship with Successful Aging. Geriatrics. 2018; 3(4):76. https://doi.org/10.3390/geriatrics3040076
Chicago/Turabian StyleNavarro, Elena, and M. Dolores Calero. 2018. "Cognitive Plasticity in Young-Old Adults and Old-Old Adults and Its Relationship with Successful Aging" Geriatrics 3, no. 4: 76. https://doi.org/10.3390/geriatrics3040076
APA StyleNavarro, E., & Calero, M. D. (2018). Cognitive Plasticity in Young-Old Adults and Old-Old Adults and Its Relationship with Successful Aging. Geriatrics, 3(4), 76. https://doi.org/10.3390/geriatrics3040076





