Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
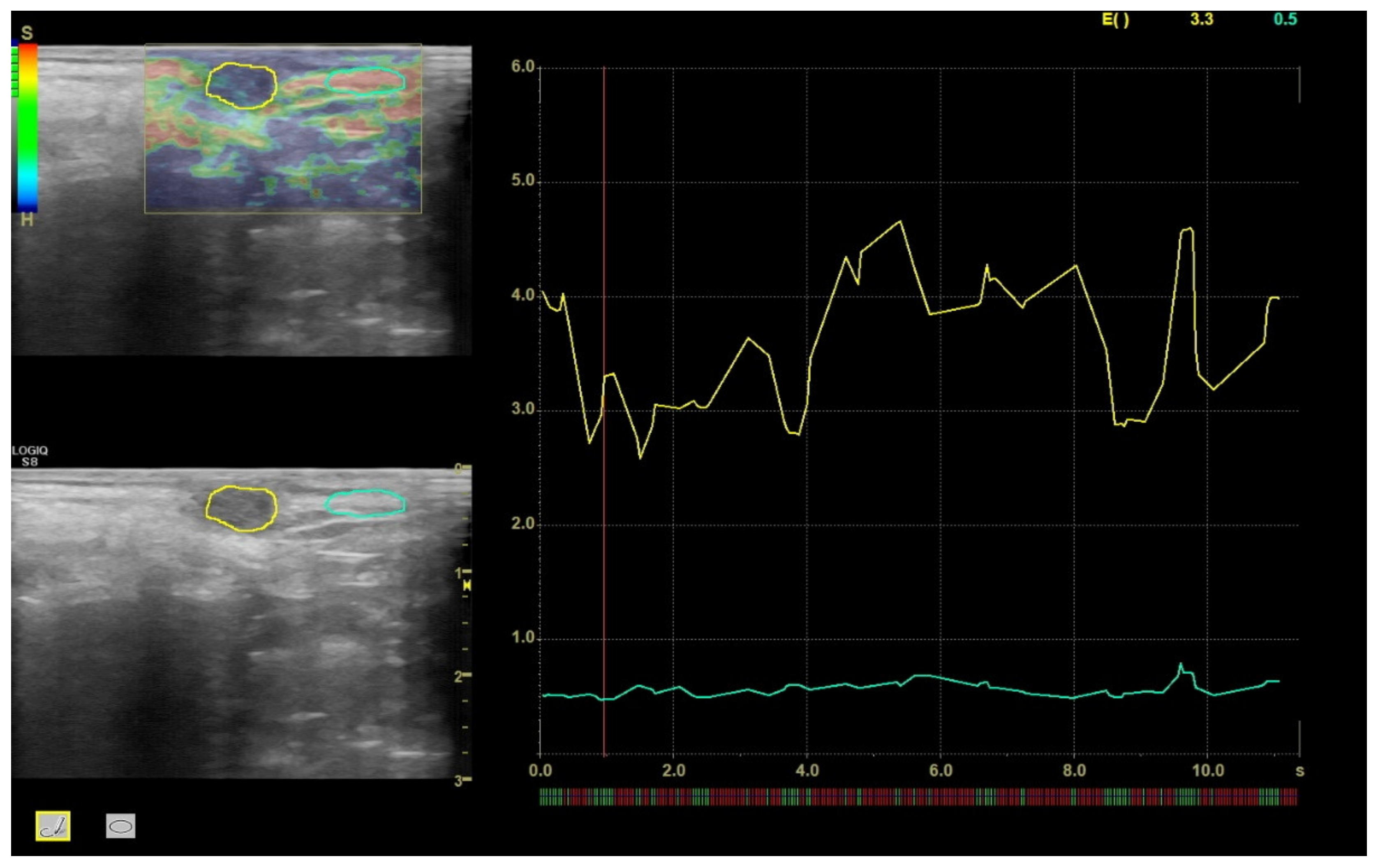
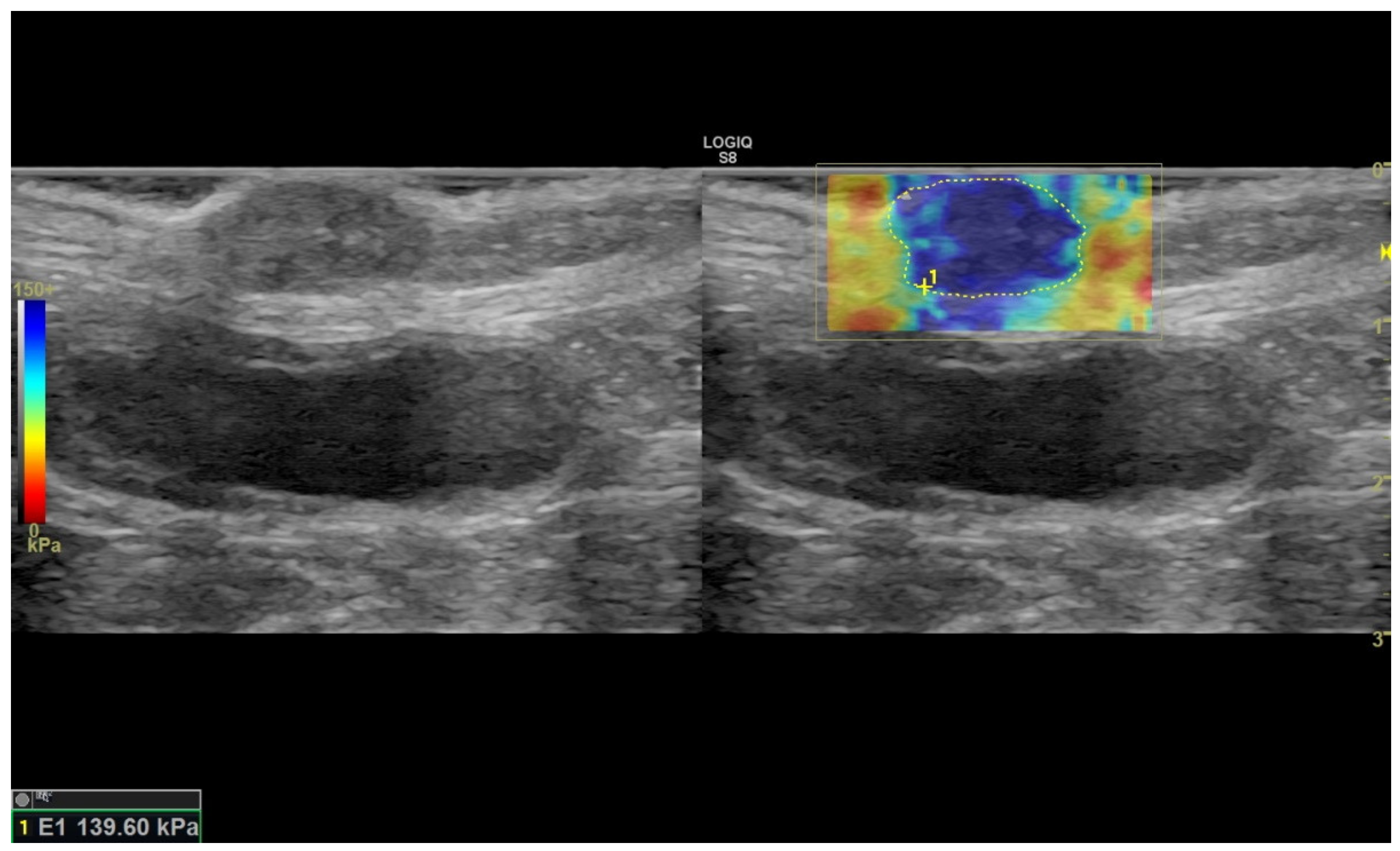
2.2. Ultrasound Sonoelastography
2.3. Surgery of Canine Mammary Nodular Lesions
2.4. Histopathological Evaluation of Canine Mammary Nodular Lesions
2.5. Statistical Design
3. Results
3.1. Sonoelastography and Correlation between Elastographic Parameters
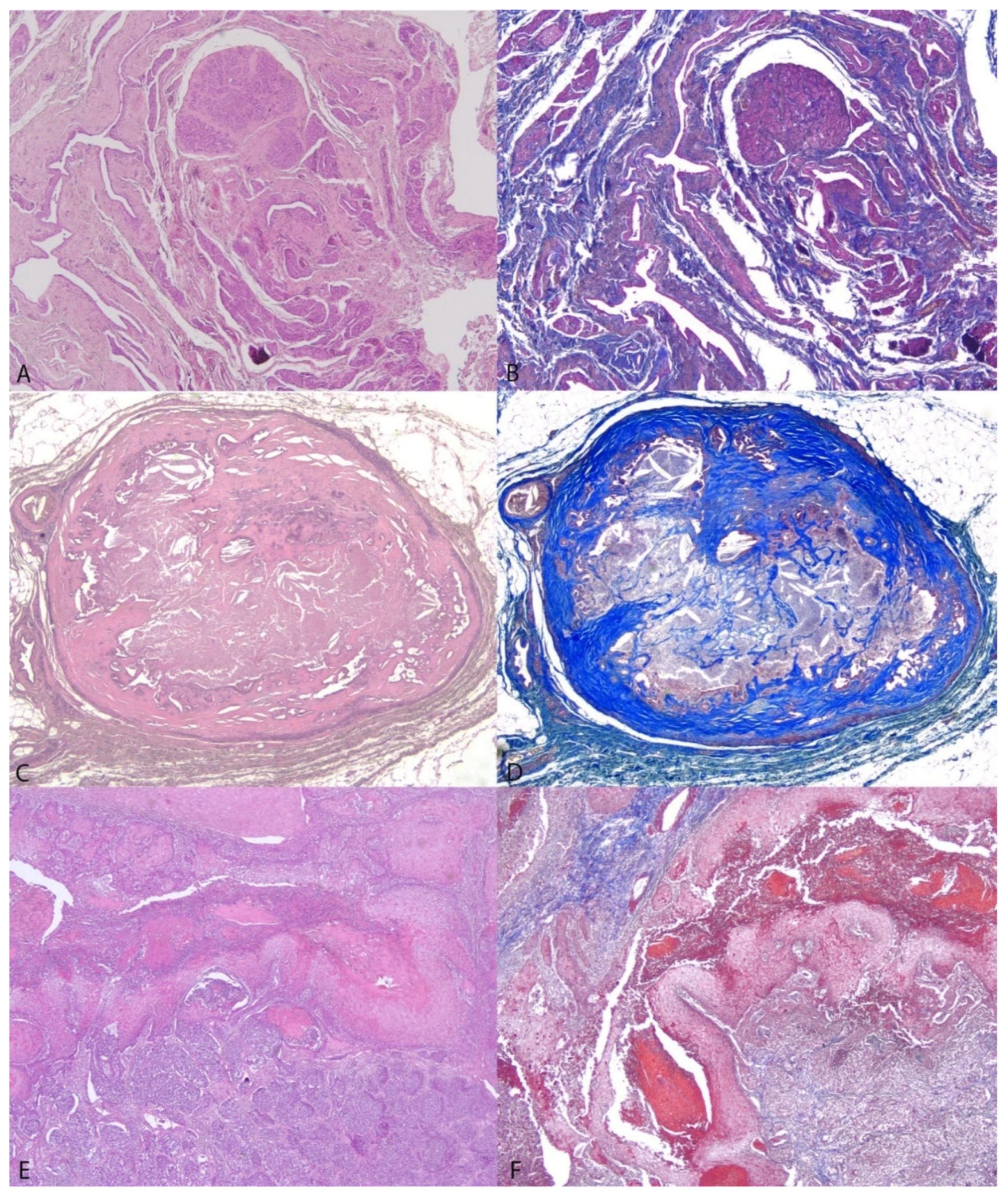
3.2. Histological Evaluations
3.3. Correlations between Elastographic Parameters and Histological Features of Canine Mammary Nodular Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N.N. Canine Neoplasia in the UK: Estimates of Incidence Rates from a Population of Insured Dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer Incidence in Pet Dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of Mammary Tumors in the Canine Population Living in the Veneto Region (Northeastern Italy): Risk Factors and Similarities to Human Breast Cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Meehan, J.; Martínez-Pérez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.N.; Fan, Z.Y.; Kang, S.; Liu, Y.J.; Zhang, Y.X.; Wang, X.M. Determination of the Elasticity of Breast Tissue during the Menstrual Cycle Using Real-Time Shear Wave Elastography. Ultrasound Med. Biol. 2015, 41, 3140–3147. [Google Scholar] [CrossRef]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In Vivo Models in Breast Cancer Research: Progress, Challenges and Future Directions. Dis. Model. Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and Grading of Canine Mammary Tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Liu, D.; Xiong, H.; Ellis, A.E.; Northrup, N.C.; Rodriguez, C.O.; O’Regan, R.M.; Dalton, S.; Zhao, S. Molecular Homology and Difference between Spontaneous Canine Mammary Cancer and Human Breast Cancer. Cancer Res. 2014, 74, 5045–5056. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez De Bulnes, A.; Garcia Fernandez, P.; Mayenco Aguirre, A.M.; Sanchez De La Muela, M. Ultrasonographic Imaging of Canine Mammary Tumours. Vet. Rec. 1998, 143, 687–689. [Google Scholar]
- Paulinelli, R.R.; Freitas-Junior, R.; de Lucena, C.Ê.M.; Moreira, M.A.R.; de Moraes, V.A.; Bernardes-Júnior, J.R.M.; da Silva Rocha Vidal, C.; Ruiz, A.N.; Lucato, M.T.; da Costa, N.G.S.; et al. Sonobreast: Predicting Individualized Probabilities of Malignancy in Solid Breast Masses with Echographic Expression. Breast J. 2011, 17, 152–159. [Google Scholar] [CrossRef]
- Vannozzi, I.; Tesi, M.; Zangheri, M.; Innocenti, V.M.; Rota, A.; Citi, S.; Poli, A. B-Mode Ultrasound Examination of Canine Mammary Gland Neoplastic Lesions of Small Size (Diameter <2 Cm). Vet. Res. Commun. 2018, 42, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Jordan, L.; Baker, L.; Thompson, A. Quantitative Shear Wave Ultrasound Elastography: Initial Experience in Solid Breast Masses. Breast Cancer Res. 2010, 12, R104. [Google Scholar] [CrossRef]
- Golatta, M.; Schweitzer-Martin, M.; Harcos, A.; Schott, S.; Gomez, C.; Stieber, A.; Rauch, G.; Domschke, C.; Rom, J.; Schütz, F.; et al. Evaluation of Virtual Touch Tissue Imaging Quantification, a New Shear Wave Velocity Imaging Method, for Breast Lesion Assessment by Ultrasound. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Ueno, E.; Tohno, E.; Kamma, H.; Takahashi, H.; Shiina, T.; Yamakawa, M.; Matsumura, T. Breast Disease: Clinical Application of US Elastography for Diagnosis. Radiology 2006, 239, 341–350. [Google Scholar] [CrossRef]
- Balleyguier, C.; Ciolovan, L.; Ammari, S.; Canale, S.; Sethom, S.; Al Rouhbane, R.; Vielh, P.; Dromain, C. Breast Elastography: The Technical Process and Its Applications. Diagn. Interv. Imaging 2013, 94, 503–513. [Google Scholar] [CrossRef]
- Pesce, K.; Binder, F.; Chico, M.J.; Swiecicki, M.P.; Galindo, D.H.; Terrasa, S. Diagnostic Performance of Shear Wave Elastography in Discriminating Malignant and Benign Breast Lesions: Our Experience with QelaXtoTM Software. J. Ultrasound 2020, 23, 575–583. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Zhou, C.; Zhan, W.W.; Jia, X.H.; Dong, Y.J.; Yang, Z.F. Elastography Ultrasound for Breast Lesions: Fat-to-Lesion Strain Ratio vs Gland-to-Lesion Strain Ratio. Eur. Radiol. 2014, 24, 3171–3177. [Google Scholar] [CrossRef]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.; McAleavey, S.; Trahey, G. Shear-Wave Generation Using Acoustic Radiation Force: In Vivo and Ex Vivo Results. Ultrasound Med. Biol. 2003, 29, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-Q.; Li, X.-R.; Zhou, H.-L.; Chen, J.-X.; Huang, X.; Dai, H.-X.; Li, J.-W.; Chen, X.-D.; Xu, X.-H. Acoustic Radiation Force Impulse Elastography of Breast Imaging Reporting and Data System Category 4 Breast Lesions. Clin. Breast Cancer 2012, 12, 420–427. [Google Scholar] [CrossRef]
- Xue, Y.; Yao, S.; Li, X.; Zhang, H. Value of Shear Wave Elastography in Discriminating Malignant and Benign Breast Lesions. Medicine (Baltimore) 2017, 96, e7412. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Jung, H.K.; Lee, J.T.; Ko, K.H. Shear-Wave Elastography in the Diagnosis of Solid Breast Masses: What Leads to False-Negative or False-Positive Results? Eur. Radiol. 2013, 23, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis Suppressor Gene KiSS-1 Encodes Peptide Ligand of a G-Protein-Coupled Receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Moon, W.K.; Cho, N.; Yi, A.; Koo, H.R.; Han, W.; Noh, D.-Y.; Moon, H.-G.; Kim, S.J. Clinical Application of Shear Wave Elastography (SWE) in the Diagnosis of Benign and Malignant Breast Diseases. Breast Cancer Res. Treat. 2011, 129, 89–97. [Google Scholar] [CrossRef]
- Athanasiou, A.; Tardivon, A.; Tanter, M.; Sigal-Zafrani, B.; Bercoff, J.; Deffieux, T.; Gennisson, J.-L.; Fink, M.; Neuenschwander, S. Breast Lesions: Quantitative Elastography with Supersonic Shear Imaging—Preliminary Results. Radiology 2010, 256, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.R.; Vicente, W.R.R.R.; Silva, M.A.M.M. Conventional and Doppler Ultrasound for the Differentiation of Benign and Malignant Canine Mammary Tumours. J. Small Anim. Pract. 2012, 53, 332–337. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography Methods for Predicting Malignancy in Canine Mammary Tumors. PLoS ONE 2017, 12, e0178143. [Google Scholar] [CrossRef]
- Pastor, N.; Espadas, L.; Santella, M.; Ezquerra, L.J.; Tarazona, R.; Durán, M.E. Comparison between Histological Features and Strain Elastographic Characteristics in Canine Mammary Carcinomas. Vet. Sci. 2022, 9, 9. [Google Scholar] [CrossRef]
- Zappulli, V.; Pena, L.; Rasotto, R.; Goldschmidt, M.; Gama, A.; Scruggs, J.; Kiupel, M. Mammary Tumors. Surgical Pathology of Tumors of Domestic Animals; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019; Volume 2. [Google Scholar]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic Value of Histological Grading in Noninflammatory Canine Mammary Carcinomas in a Prospective Study with Two-Year Follow-Up: Relationship With Clinical and Histological Characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Haeberle, L.; Insilla, A.C.; Kapp, A.C.; Steiger, K.; Schlitter, A.M.; Konukiewitz, B.; Demir, I.E.; Friess, H.; Esposito, I. Stroma Composition and Proliferative Activity Are Related to Therapy Response in Neoadjuvant Treated Pancreatic Ductal Adenocarcinoma. Histol. Histopathol. 2021, 36, 733–742. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorenmo, K.U.; Shofer, F.S.; Goldschmidt, M.H. Effect of Spaying and Timing of Spaying on Survival of Dogs with Mammary Carcinoma. J. Vet. Intern. Med. 2000, 14, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.R.; Taylor, D.O.N.; Schneider, R.; Hibbard, H.H.; Klauber, M.R. Survey of Animal Neoplasms in Alameda and Contra Costa Counties, California. Ii. Cancer Morbidity in Dogs and Cats from Alameda County. J. Natl. Cancer Inst. 1968, 40, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Sleeckx, N.; de Rooster, H.; Veldhuis Kroeze, E.; Van Ginneken, C.; Van Brantegem, L. Canine Mammary Tumours, an Overview. Reprod. Domest. Anim. 2011, 46, 1112–1131. [Google Scholar] [CrossRef]
- Woo, J.H.; Ko, E.Y.; Han, B.K. Comparison of 2 Shear Wave Elastography Systems in Reproducibility and Accuracy Using an Elasticity Phantom. Medicine 2021, 100, e24921. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.M.; Kim, B.; La Yun, B.; Jang, M.; Ko, Y.; Lee, S.H.; Jeong, H.; Chang, J.M.; Cho, N. Comparison of Strain and Shear Wave Elastography for Qualitative and Quantitative Assessment of Breast Masses in the Same Population. Sci. Rep. 2018, 8, 6197. [Google Scholar] [CrossRef]
- Seo, M.; Ahn, H.S.; Park, S.H.; Lee, J.B.; Choi, B.I.; Sohn, Y.M.; Shin, S.Y. Comparison and Combination of Strain and Shear Wave Elastography of Breast Masses for Differentiation of Benign and Malignant Lesions by Quantitative Assessment: Preliminary Study. J. Ultrasound Med. 2018, 37, 99–109. [Google Scholar] [CrossRef]
- Youk, J.H.; Son, E.J.; Gweon, H.M.; Kim, H.; Park, Y.J.; Kim, J.A. Comparison of Strain and Shear Wave Elastography for the Differentiation of Benign from Malignant Breast Lesions, Combined with b-Mode Ultrasonography: Qualitative and Quantitative Assessments. Ultrasound Med. Biol. 2014, 40, 2336–2344. [Google Scholar] [CrossRef]
- Barr, R.G. Sonographic Breast Elastography: A Primer. J. Ultrasound Med. 2012, 31, 773–783. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.R.; Maronezi, M.C.; Pavan, L.; Castanheira, T.L.; Simões, A.P.R.R.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R.R. ARFI Elastography as a Complementary Diagnostic Method for Mammary Neoplasia in Female Dogs– Preliminary Results. J. Small Anim. Pract. 2014, 55, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Ezquerra, L.J.; Santella, M.; Caballé, N.C.; Tarazona, R.; Durán, M.E. Prognostic Significance of Immunohistochemical Markers and Histological Classification in Malignant Canine Mammary Tumours. Vet. Comp. Oncol. 2020, 18, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Ramirez, R.A.U.; Maronezi, M.C.; Maciel, G.S.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; Gasser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of Four Ultrasonography Techniques in Predicting Histopathological Classification of Canine Mammary Carcinomas. Vet. Radiol. Ultrasound 2018, 59, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Guibal, A.; Renosi, G.; Rode, A.; Scoazec, J.Y.; Guillaud, O.; Chardon, L.; Munteanu, M.; Dumortier, J.; Collin, F.; Lefort, T. Shear Wave Elastography: An Accurate Technique to Stage Liver Fibrosis in Chronic Liver Diseases. Diagn. Interv. Imaging 2016, 97, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Menzilcioglu, M.S.; Duymus, M.; Citil, S.; Avcu, S.; Gungor, G.; Sahin, T.; Boysan, S.N.; Altunoren, O.; Sarica, A. Strain Wave Elastography for Evaluation of Renal Parenchyma in Chronic Kidney Disease. Br. J. Radiol. 2015, 88, 20140714. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Palmeri, M.L.; Guy, C.D.; Yang, L.; Hedlund, L.W.; Diehl, A.M.; Nightingale, K.R. In Vivo Quantification of Liver Stiffness in a Rat Model of Hepatic Fibrosis with Acoustic Radiation Force. Ultrasound Med. Biol. 2009, 35, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kitago, M.; Abe, T.; Itano, O.; Shinoda, M.; Abe, Y.; Yagi, H.; Hibi, T.; Ishii, M.; Nakano, Y.; et al. Evaluation of Pancreatic Fibrosis with Acoustic Radiation Force Impulse Imaging and Automated Quantification of Pancreatic Tissue Components. Pancreas 2018, 47, 1277–1282. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic Density and Breast Cancer Risk: Current Understanding and Future Prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef]
- Case, A.; Brisson, B.K.; Durham, A.C.; Rosen, S.; Monslow, J.; Buza, E.; Salah, P.; Gillem, J.; Ruthel, G.; Veluvolu, S.; et al. Identification of Prognostic Collagen Signatures and Potential Therapeutic Stromal Targets in Canine Mammary Gland Carcinoma. PLoS ONE 2017, 12, e0180448. [Google Scholar] [CrossRef]
- Maller, O.; Hansen, K.C.; Lyons, T.R.; Acerbi, I.; Weaver, V.M.; Prekeris, R.; Tan, A.C.; Schedin, P. Collagen Architecture in Pregnancy-Induced Protection from Breast Cancer. J. Cell Sci. 2013, 126, 4108–4120. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Benign | Malignant | ||||||
|---|---|---|---|---|---|---|---|
| Hyperplastic | Neoplastic | Total | Grade I | Grade II | Grade III | Total | |
| (n = 3) | (n = 2) | (n = 19) | (n = 11) | (n = 2) | |||
| Age (y) | 8.9 ± 0.8 | 10.7 ± 2.7 | 9.6 ± 1 | 9.7 ± 0.5 | 9 ± 0.7 | 9.4 ± 2.6 | 9.4 ± 0.4 |
| Fibrosis (%) | 37.06 ± 15.59 | 70.50 ± 0.79 | 50.44 ± 11.84 | 32.35 ± 4.43 | 34.98 ± 6.13 | 34.52 ± 16.46 | 32.95 ± 3.32 |
| SWE-S (kPA) | 100.8 ± 15.8 | 137.4 ± 4.8 | 115.4 ± 12.6 | 114.9 ± 4.9 | 120.1 ± 6.7 | 88.7 ± 56 | 115.5 ± 4.5 |
| STE-I | 3.5 ± 0.3 | 4.7 ± 0.3 | 4 ± 0.3 | 4 ± 0.2 | 3.7 ± 0.2 | 3 ± 0.5 | 3.8 ± 0.1 |
| STE-R | 6.74 ± 1.82 | 7.64 ± 1.76 | 7.1 ± 1.16 | 5.69 ± 0.54 | 5.53 ± 0.6 | 4.46 ± 2.54 | 5.6 ± 0.38 |
| N° | Histological Classification | Grade | Fibrosis (%) | Vimentin-Positive Cells (%) | Necrosis (%) | Chondroid (%) | Bone (%) | Cystic Spaces/Tubular Secretion (%) | ECM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mixed carcinoma | II | 12.2 | 36.7 | 0.5 | 0.15 | 4.9 | ++ | |
| 2a | Carcinoma arising in benign mixed tumour | I | 25.7 | 29.1 | 0.09 | 12.10 | 5.5 | +++ | |
| 2b | Tubular carcinoma | I | 20.4 | 22.5 | 0.2 | 14.5 | + | ||
| 2c | Carcinoma arising in complex adenoma | I | 10.8 | 28.3 | 20 | 1 | +++ | ||
| 3 | Comedocarcinoma | II | 76.0 | 11.6 | 24 | 0.5 | ++ | ||
| 4 | Mixed carcinoma | I | 61.6 | 25.4 | 1.13 | 12.83 | 0.41 | 3.29 | +++ |
| 5a | Intraductal papillary carcinoma | II | 44.9 | 28.7 | 5.42 | 38.02 | N.A. | ||
| 5b | Complex carcinoma | I | 6.2 | 50.0 | 0.85 | +++ | |||
| 6 | Intraductal papillary carcinoma | I | 21.0 | 34.7 | 6.6 | +++ | |||
| 7a | Complex carcinoma | II | 3.8 | 51.0 | 0.57 | 1.02 | ++ | ||
| 7b | Adenosquamous carcinoma | III | 18.1 | 23.3 | 3.91 | 0.26 | N.A. | ||
| 8a | Mixed carcinoma | I | 11.4 | 27.2 | 11.43 | 9.75 | N.A. | ||
| 8b | Lobular hyperplasia with fibrosis | N.A. | 67.4 | 10.1 | 0.23 | N.A. | |||
| 9 | Intraductal papillary carcinoma | II | 27.3 | 18.4 | 4.98 | 51.93 | ++ | ||
| 10 | Complex carcinoma | I | 27.8 | 34.3 | 0.33 | 20.33 | +++ | ||
| 11 | Lobular hyperplasia with atypia | N.A. | 28.0 | 9.0 | 0.27 | ++ | |||
| 12a | Lobular hyperplasia with atypia | N.A. | 15.8 | 12.4 | 18.98 | ++ | |||
| 12b | Simple adenoma, sclerosing | N.A. | 71.3 | 20.0 | 9.33 | 3.04 | ++ | ||
| 12c | Intraductal papillary carcinoma | I | 38.0 | 36.9 | 5.52 | 19.21 | +++ | ||
| 12d | Carcinoma arising in benign mixed tumour | I | 57.3 | 28.1 | 0.06 | 36.93 | 4.18 | 0.1 | + |
| 13a | Carcinoma arising in complex adenoma | I | 35.2 | 27.1 | 0.45 | 26.08 | +++ | ||
| 13b | Carcinoma arising in benign mixed tumour | I | 22.2 | 43.5 | 0.57 | 9.93 | 1.6 | +++ | |
| 13c | Ductal carcinoma | I | 33.5 | 63.1 | 0.43 | 2.46 | ++ | ||
| 14a | Intraductal papillary carcinoma | II | 44.9 | 32.8 | 1.43 | 27.97 | +++ | ||
| 14b | Intraductal papillary carcinoma | II | 38.4 | 21.4 | 0.5 | 4.9 | +++ | ||
| 15 | Ductal carcinoma | I | 30.8 | 12.4 | 0.04 | 3.25 | +++ | ||
| 16 | Ductal carcinoma | I | 55.4 | 17.0 | 4.45 | +++ | |||
| 17 | Mixed carcinoma | I | 22.8 | 24.2 | 0.12 | 0.44 | 37.42 | ||
| 18a | Intraductal papillary carcinoma | I | 21.0 | 25.0 | 0.8 | 3.25 | 25.96 | ++ | |
| 18b | Carcinoma and- malignant myoepithelioma | III | 51.0 | 34.6 | 13.52 | 1.02 | ++ | ||
| 18c | Intraductal papillary carcinoma | II | 13.6 | 24.6 | 20 | 13.00 | + | ||
| 19 | Complex carcinoma | II | 35.0 | 16.1 | 1.29 | +++ | |||
| 20 | Carcinosarcoma | N.A. | 18.9 | 27.2 | 2.05 | 26.25 | 13.18 | 3.41 | +++ |
| 21 | Multinodular: Lobular hyperplasia/simple adenoma/intraductal papillary adenoma–carcinoma/tubular–solid carcinoma | II | 49.9 | 19.8 | 2.3 | 15.0 | + | ||
| 22a | Complex carcinoma | II | 39.0 | 70.9 | 6.1 | 10.0 | + | ||
| 22b | Intraductal papillary carcinoma | I | 32.4 | 41.3 | 0.07 | 13.8 | + | ||
| 22c | Multinodular: Lobular hyperplasia with secretory activity –with atypia/benign mixed tumour/simple adenoma/complex adenoma/intraductal papillary adenoma | N.A. | 69.8 | 54.0 | 0.8 | 0.1 | 12.8 | ++ | |
| 22d | Carcinoma arising in complex adenoma | I | 81.3 | 50.0 | 0.22 | 2.22 | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massimini, M.; Gloria, A.; Romanucci, M.; Della Salda, L.; Di Francesco, L.; Contri, A. Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Vet. Sci. 2022, 9, 506. https://doi.org/10.3390/vetsci9090506
Massimini M, Gloria A, Romanucci M, Della Salda L, Di Francesco L, Contri A. Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Veterinary Sciences. 2022; 9(9):506. https://doi.org/10.3390/vetsci9090506
Chicago/Turabian StyleMassimini, Marcella, Alessia Gloria, Mariarita Romanucci, Leonardo Della Salda, Lucia Di Francesco, and Alberto Contri. 2022. "Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions" Veterinary Sciences 9, no. 9: 506. https://doi.org/10.3390/vetsci9090506
APA StyleMassimini, M., Gloria, A., Romanucci, M., Della Salda, L., Di Francesco, L., & Contri, A. (2022). Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Veterinary Sciences, 9(9), 506. https://doi.org/10.3390/vetsci9090506






