Marginal Zinc Deficiency Aggravated Intestinal Barrier Dysfunction and Inflammation through ETEC Virulence Factors in a Mouse Model of Diarrhea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Samples
2.3. Intestinal Morphology
2.4. Inflammatory Cytokines
2.5. Intestinal Permeability
2.6. Real-Time RT-PCR
2.7. Stool ETEC Shedding and Tissue Burden
2.8. ETEC Growth Curve
2.9. Zinc Content
2.10. Virulence Factor Analysis
2.11. Data Analysis
3. Results
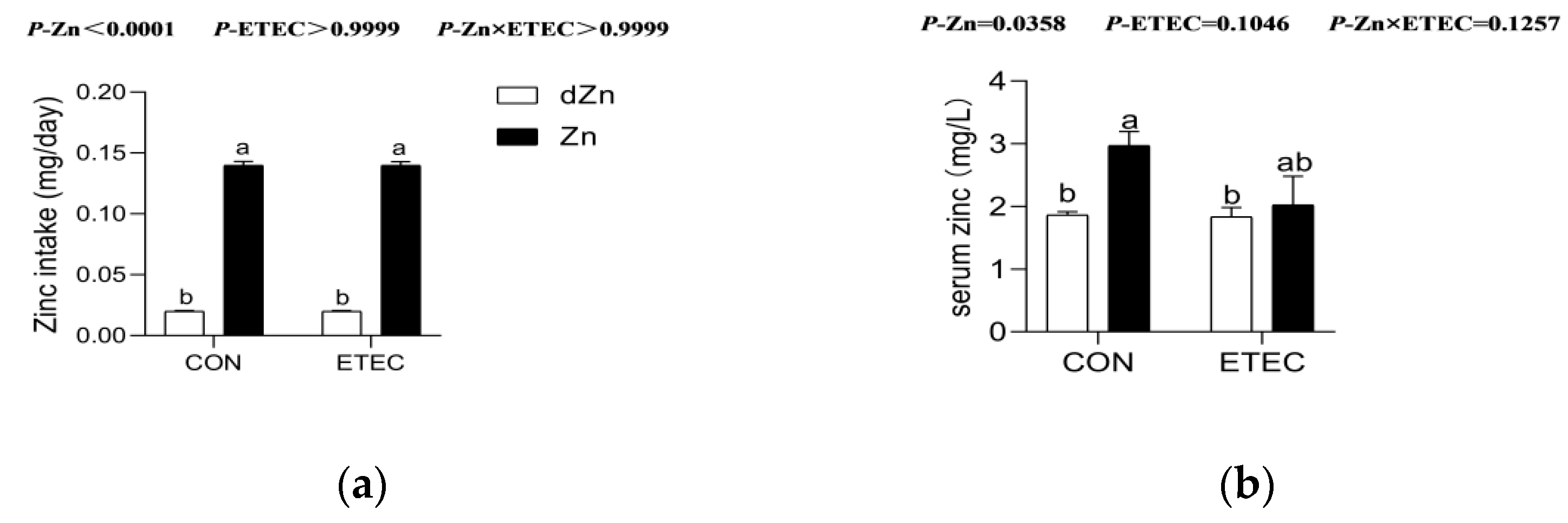
3.1. Effect of Marginal Zinc Deficiency on Serum Zinc Concentration of Mice with ETEC Challenge
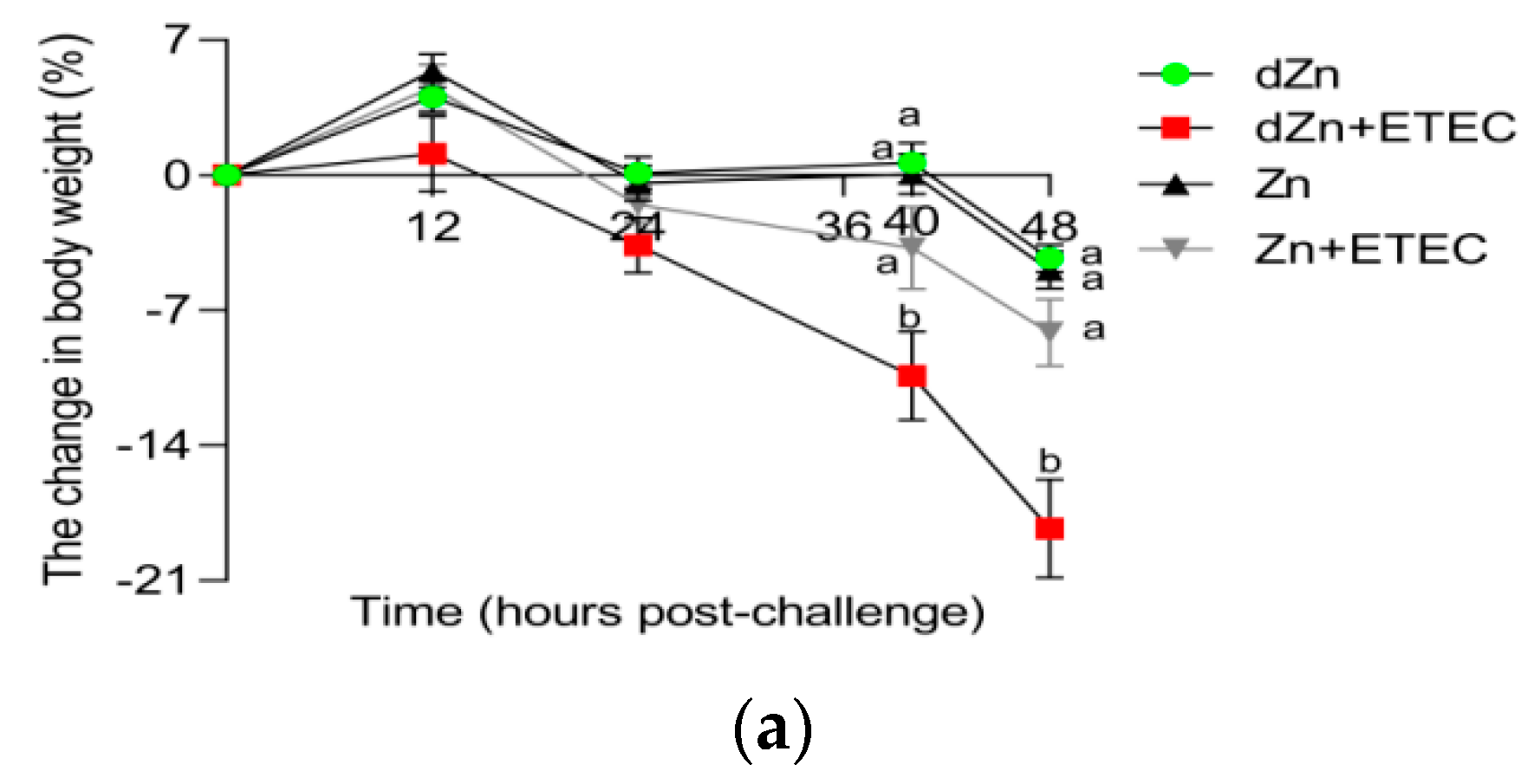
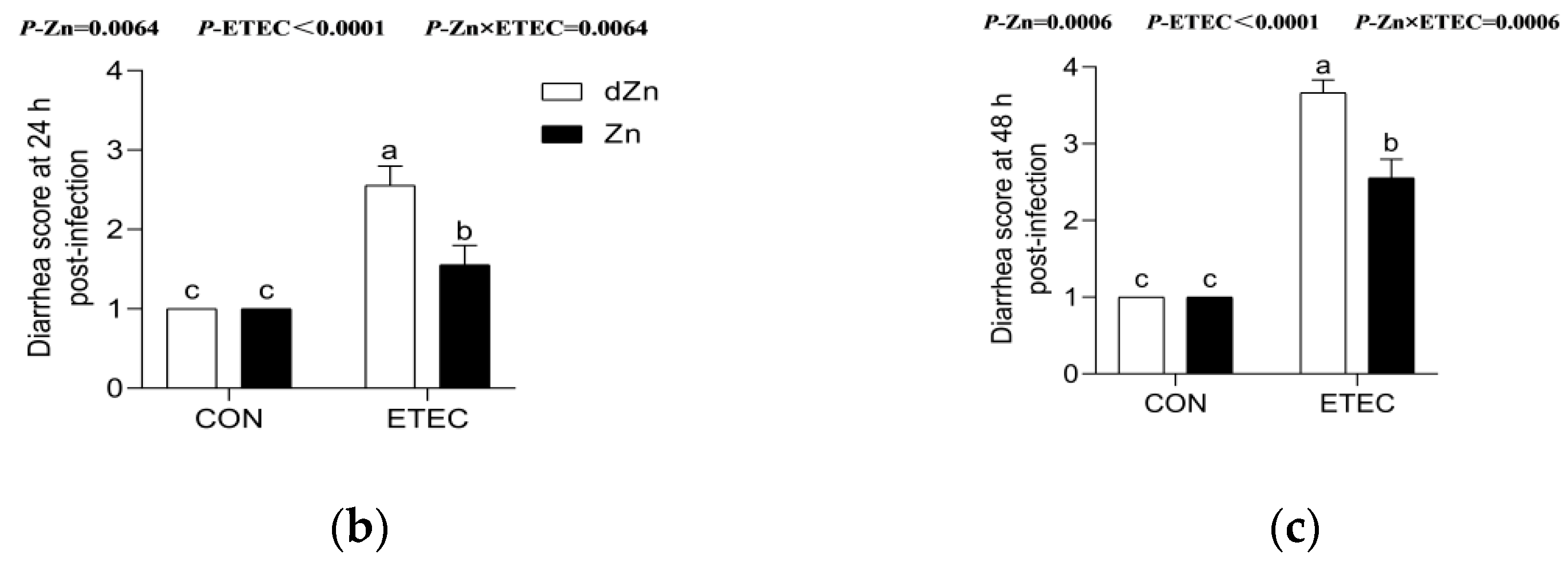
3.2. Marginal Zinc-Deficient Mice Have Greater Diarrhea Scores and Body Weight Losses after ETEC Challenge
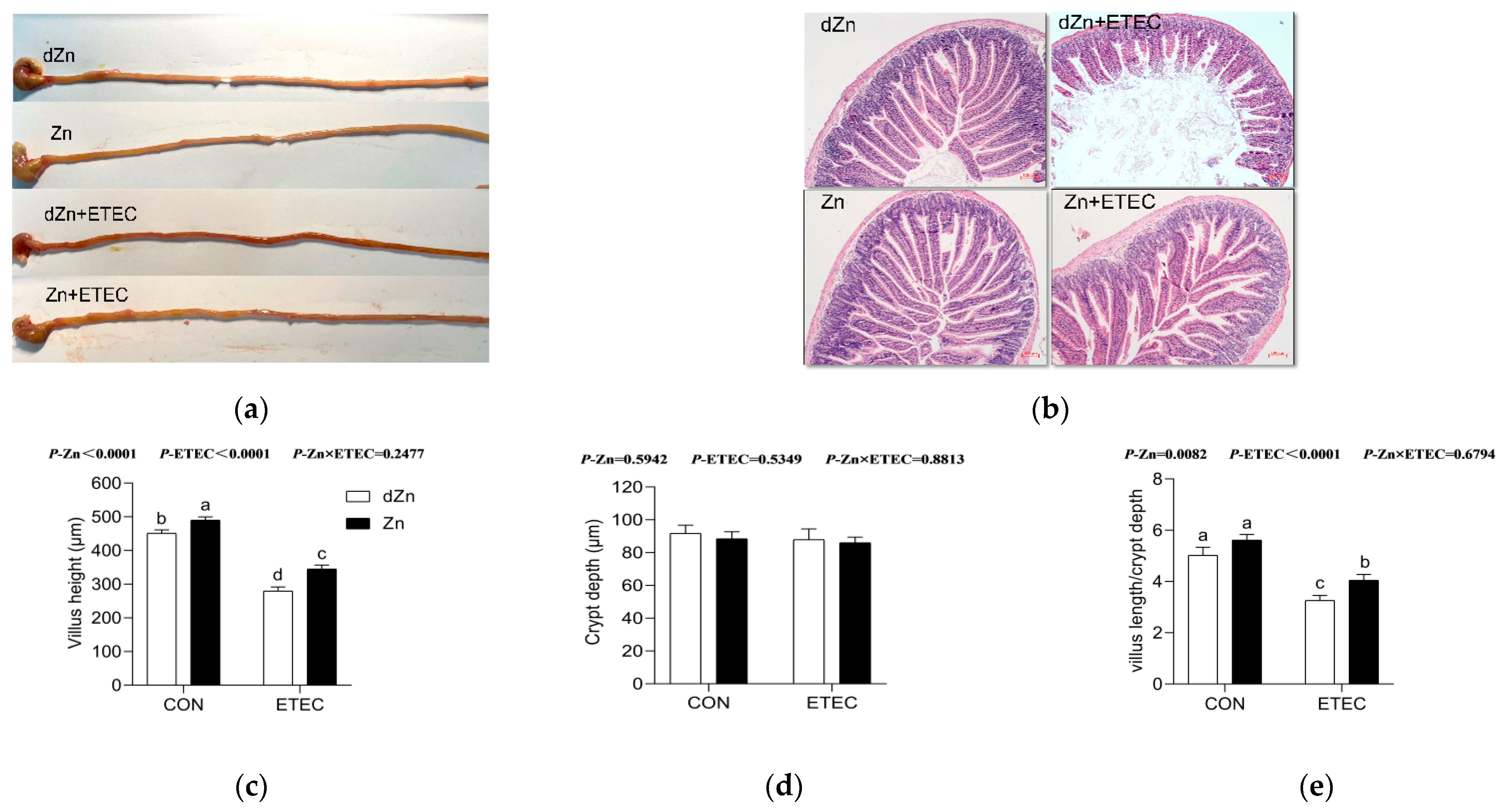
3.3. Intestinal Morphology Was Altered in Marginal Zinc-Deficient Mice with ETEC Challenge
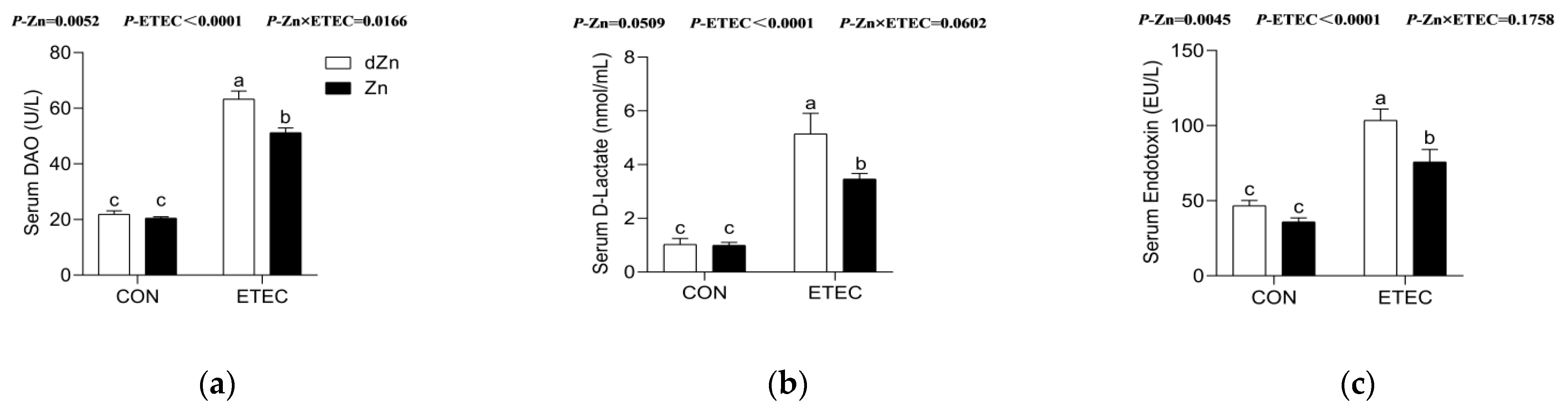
3.4. Marginal Zinc Deficiency Decreased Host Defense against ETEC Infection Induced Disruption of Intestinal Barrier and Permeability
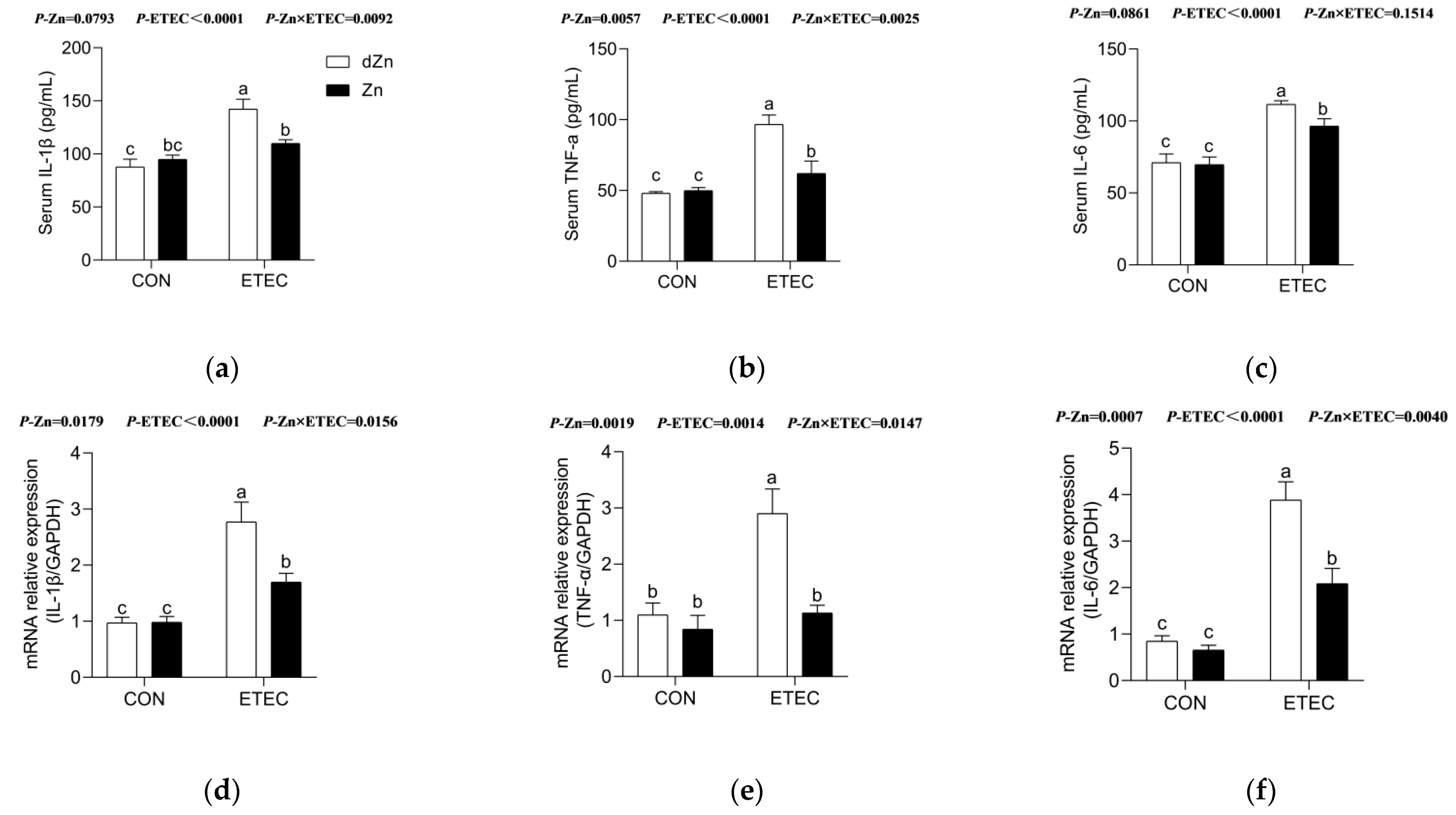
3.5. Marginal Zinc Deficiency Aggravated Intestinal Inflammation in Mice with ETEC Challenge
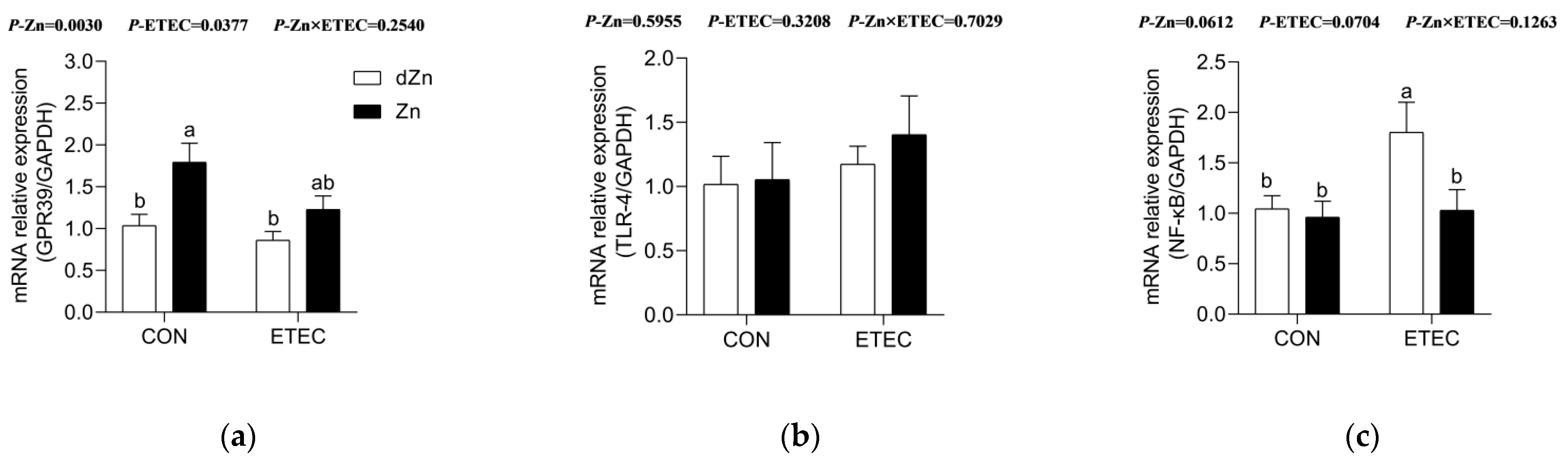
3.6. Marginal Zinc Deficiency Aggravated Intestinal Injury Was Associated with NF-κB
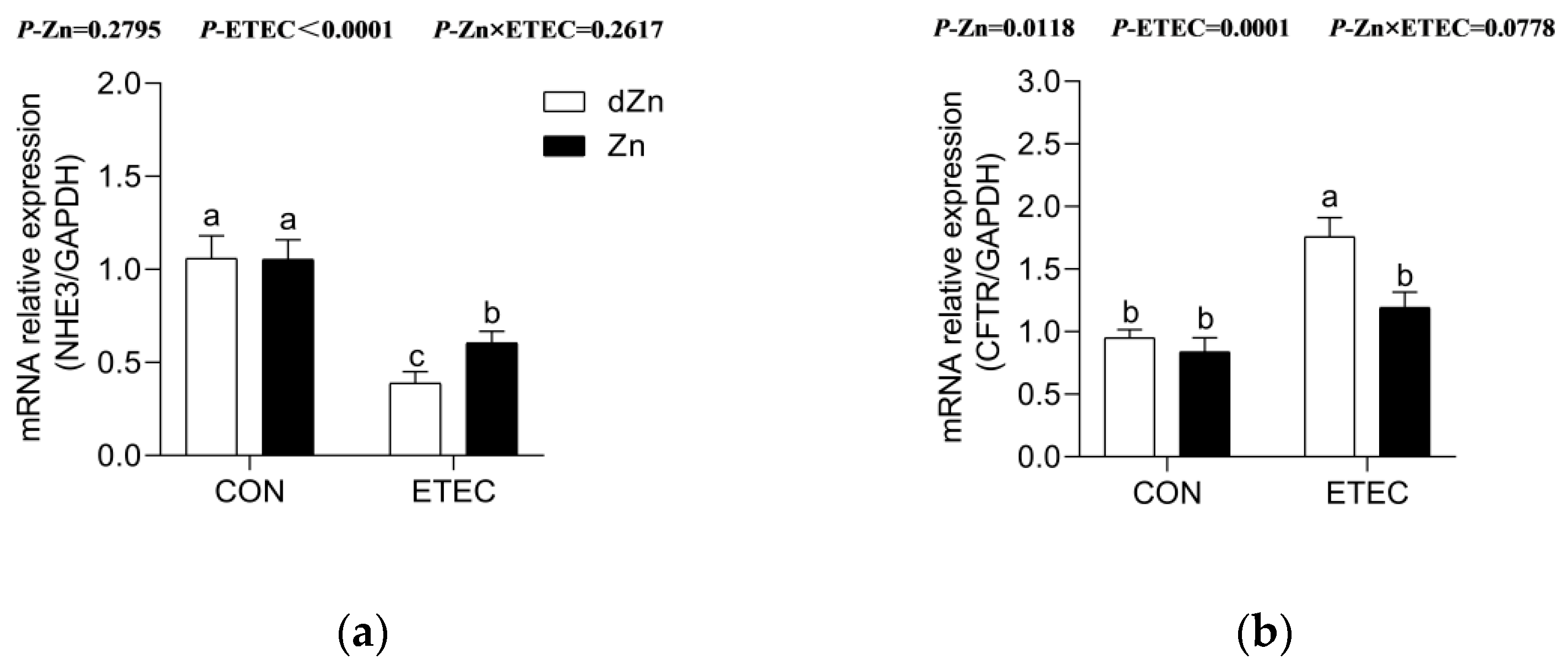
3.7. Marginal Zinc Deficiency Altered Anion Transporters in Mice with ETEC Challenge
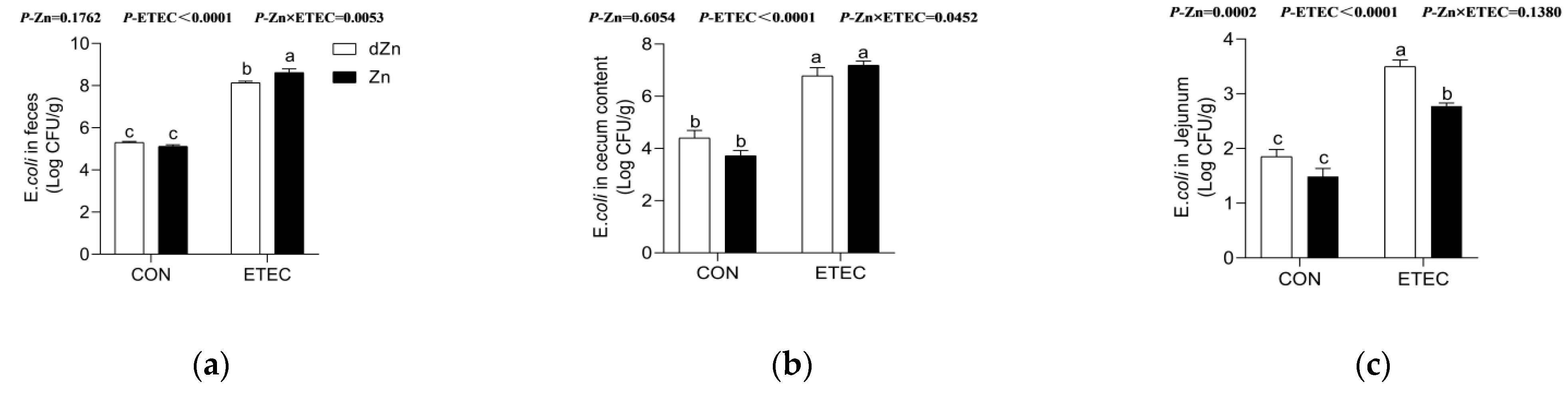
3.8. Marginal Zinc Deficiency Increased the ETEC Shedding in the Jejunum of Mice with ETEC Challenge
3.9. Marginal Zinc Deficiency Enhanced Virulence Factors in Mice with ETEC Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera-Sosa, L.; Ochoa, T.J. Escherichia coli Diarrhea. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 481–485. [Google Scholar]
- Black, R.; Fontaine, O.; Lamberti, L.; Bhan, M.; Huicho, L.; El Arifeen, S.; Masanja, H.; Walker, C.F.; Mengestu, T.K.; Pearson, L.; et al. Drivers of the reduction in childhood diarrhea mortality 1980–2015 and interventions to eliminate preventable diarrhea deaths by 2030. J. Glob. Health 2019, 9, 020801. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porcine Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef]
- Das, J.K.; Tripathi, A.; Ali, A.; Hassan, A.; Dojosoeandy, C.; Bhutta, Z.A. Vaccines for the prevention of diarrhea due to cholera, shigella, ETEC and rotavirus. BMC Public Health 2013, 13, S11. [Google Scholar] [CrossRef] [PubMed]
- Bolick, D.T.; Medeiros, P.; Ledwaba, S.E.; Lima, A.A.M.; Nataro, J.P.; Barry, E.M.; Guerrant, R.L. Critical Role of Zinc in a New Murine Model of Enterotoxigenic Escherichia coli Diarrhea. Infect. Immun. 2018, 86, e00183-18. [Google Scholar] [CrossRef]
- Xiao, K.; Yang, Y.; Zhang, Y.; Lv, Q.; Huang, F.; Wang, D.; Zhao, J.; Liu, Y. Long chain PUFA ameliorate ETEC-induced intestinal inflammation and cell injury by modulating pyroptosis and necroptosis signaling pathways in porcine intestinal epithelial cells. Br. J. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Gong, Y.; Jin, X.; Yuan, B.; Lv, Y.; Yan, G.; Liu, M.; Xie, C.; Liu, J.; Tang, Y.; Gao, H.; et al. G Protein-Coupled Receptor 109A Maintains the Intestinal Integrity and Protects Against ETEC Mucosal Infection by Promoting IgA Secretion. Front. Immunol. 2020, 11, 583652. [Google Scholar] [CrossRef]
- Vipin Madhavan, T.P.; Sakellaris, H. Chapter Five–Colonization Factors of Enterotoxigenic Escherichia coli. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 90, pp. 155–197. [Google Scholar]
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: Intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 2022, 14, 2055943. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Motyka, N.I.; Stewart, S.R.; Hollifield, I.E.; Kyllo, T.R.; Mansfield, J.A.; Norton, E.B.; Clements, J.D.; Bitoun, J.P. Elevated Extracellular cGMP Produced after Exposure to Enterotoxigenic Escherichia coli Heat-Stable Toxin Induces Epithelial IL-33 Release and Alters Intestinal Immunity. Infect. Immun. 2021, 89, 4e00707-20. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- HO/UNICEF. Clinical Management of Acute Diarrhea; Report No. WHO/FCH/CAH 04.7; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Dhingra, U.; Kisenge, R.; Sudfeld, C.R.; Dhingra, P.; Somji, S.; Dutta, A.; Bakari, M.; Deb, S.; Devi, P.; Liu, E.; et al. Lower-Dose Zinc for Childhood Diarrhea—A Randomized, Multicenter Trial. N. Engl. J. Med. 2020, 383, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Buccigrossi, V.; Passariello, A. Mechanisms of action of zinc in acute diarrhea. Curr. Opin. Gastroenterol. 2011, 27, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Pongkorpsakol, P.; Buasakdi, C.; Chantivas, T.; Chatsudthipong, V.; Muanprasat, C. An agonist of a zinc-sensing receptor GPR39 enhances tight junction assembly in intestinal epithelial cells via an AMPK-dependent mechanism. Eur. J. Pharmacol. 2019, 842, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Xia, T.; Wu, H.; Zhang, W.J.; Zhu, Y.H.; Xue, J.X.; He, D.T.; Zhang, L.Y. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim. Feed Sci. Technol. 2019, 258, 114312. [Google Scholar] [CrossRef]
- Quan, G.; Xia, P.; Lian, S.; Wu, Y.; Zhu, G. Zinc uptake system ZnuACB is essential for maintaining pathogenic phenotype of F4ac(+) enterotoxigenic E. coli (ETEC) under a zinc restricted environment. Vet. Res. 2020, 51, 127. [Google Scholar] [CrossRef]
- Bolick, D.T.; Kolling, G.L.; Moore, J.H.; de Oliveira, L.A.; Tung, K.; Philipson, C.; Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J.; Guerrant, R.L. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes 2014, 5, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Yin, J.; Duan, J.L.; Liu, G.; Zhu, X.P.; Chen, S.; Li, T.J.; Wang, S.P.; Tang, Y.L.; Hardwidge, P.R. Mouse intestinal innate immune responses altered by enterotoxigenic Escherichia coli (ETEC) infection. Microbes Infect. 2014, 16, 954–961. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Zeng, X.; Cai, S.; Wang, G.; Liu, L.; Huang, S.; Li, N.; Liu, H.; Ding, X. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020, 34, 1018–1037. [Google Scholar] [CrossRef]
- Lin, S.; Yang, X.; Yuan, P.; Yang, J.; Wang, P.; Zhong, H.; Zhang, X.; Che, L.; Feng, B.; Li, J.; et al. Undernutrition Shapes the Gut Microbiota and Bile Acid Profile in Association with Altered Gut-Liver FXR Signaling in Weaning Pigs. J. Agric. Food Chem. 2019, 67, 3691–3701. [Google Scholar] [CrossRef]
- Wang, P.; Zhong, H.; Song, Y.; Yuan, P.; Li, Y.; Lin, S.; Zhang, X.; Li, J.; Che, L.; Feng, B.; et al. Targeted metabolomics analysis of maternal-placental-fetal metabolism in pregnant swine reveals links in fetal bile acid homeostasis and sulfation capacity. Am. J. Physiol.-Cell Physiol. 2019, 317, G8–G16. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ren, W.; Fang, J.; Hu, C.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. L-Glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids 2017, 49, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, P.; Bolick, D.T.; Roche, J.K.; Noronha, F.; Pinheiro, C.; Kolling, G.L.; Lima, A.; Guerrant, R.L. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence 2013, 4, 624–633. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Gawenis, L.R.; Franklin, C.L.; Simpson, J.E.; Palmer, B.A.; Walker, N.M.; Wiggins, T.M.; Clarke, L.L. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 2003, 125, 1148–1163. [Google Scholar] [CrossRef]
- Yang, K.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef]
- Che, L.; Xu, Q.; Wu, C.; Luo, Y.; Huang, X.; Zhang, B.; Auclair, E.; Kiros, T.; Fang, Z.; Lin, Y.; et al. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2017, 118, 949–958. [Google Scholar] [CrossRef]
- Peng, C.; Cheng, Q.; Liu, Y.; Zhang, Z.; Wang, Z.; Ma, H.; Liu, D.; Wang, L.; Wang, C. Marginal Zinc Deficiency in Mice Increased the Number of Abnormal Sperm and Altered the Expression Level of Spermatogenesis-Related Genes. Biol. Trace Elem. Res. 2022, 200, 3738–3749. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Sarkar, P.; Saha, T.; Kazi, M.H. Zinc deficiency induces epithelial barrier dysfunction and altered intestinal ion transport in novel murine model of Shigella flexneri diarrhea. Int. J. Infect. Dis. 2020, 101, 136. [Google Scholar] [CrossRef]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014, 5, e1307. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Song, Y.; Zou, Y.; Yang, Y.; Zhu, G. F4+ enterotoxigenic Escherichia coli (ETEC) adhesion mediated by the major fimbrial subunit FaeG. J. Basic Microbiol. 2015, 55, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, J.J.; Verdonck, F.; Ehrström, A.; Peltola, M.; Siljander-Rasi, H.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Teeri, T.H.; Cox, E.; Goddeeris, B.M.; et al. F4 (K88) fimbrial adhesin FaeG expressed in alfalfa reduces F4+ enterotoxigenic Escherichia coli excretion in weaned piglets. Vaccine 2006, 24, 2387–2394. [Google Scholar] [CrossRef]
- Chen, T.; Lin, R.; Avula, L.; Sarker, R.; Yang, J.; Cha, B.; Tse, C.M.; McNamara, G.; Seidler, U.; Waldman, S.; et al. NHERF3 is necessary for Escherichia coli heat-stable enterotoxin-induced inhibition of NHE3: Differences in signaling in mouse small intestine and Caco-2 cells. Am. J. Physiol.-Cell Physiol. 2019, 317, C737–C748. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Chen, Q.; Gan, L.; Du, X.; Li, Q.; Qiao, H.; Zhao, Y.; Huang, J.; Wang, J. Marginal Zinc Deficiency Aggravated Intestinal Barrier Dysfunction and Inflammation through ETEC Virulence Factors in a Mouse Model of Diarrhea. Vet. Sci. 2022, 9, 507. https://doi.org/10.3390/vetsci9090507
Wang P, Chen Q, Gan L, Du X, Li Q, Qiao H, Zhao Y, Huang J, Wang J. Marginal Zinc Deficiency Aggravated Intestinal Barrier Dysfunction and Inflammation through ETEC Virulence Factors in a Mouse Model of Diarrhea. Veterinary Sciences. 2022; 9(9):507. https://doi.org/10.3390/vetsci9090507
Chicago/Turabian StyleWang, Peng, Qianqian Chen, Liping Gan, Xinyu Du, Qiyue Li, Hanzhen Qiao, Yinli Zhao, Jin Huang, and Jinrong Wang. 2022. "Marginal Zinc Deficiency Aggravated Intestinal Barrier Dysfunction and Inflammation through ETEC Virulence Factors in a Mouse Model of Diarrhea" Veterinary Sciences 9, no. 9: 507. https://doi.org/10.3390/vetsci9090507



