Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
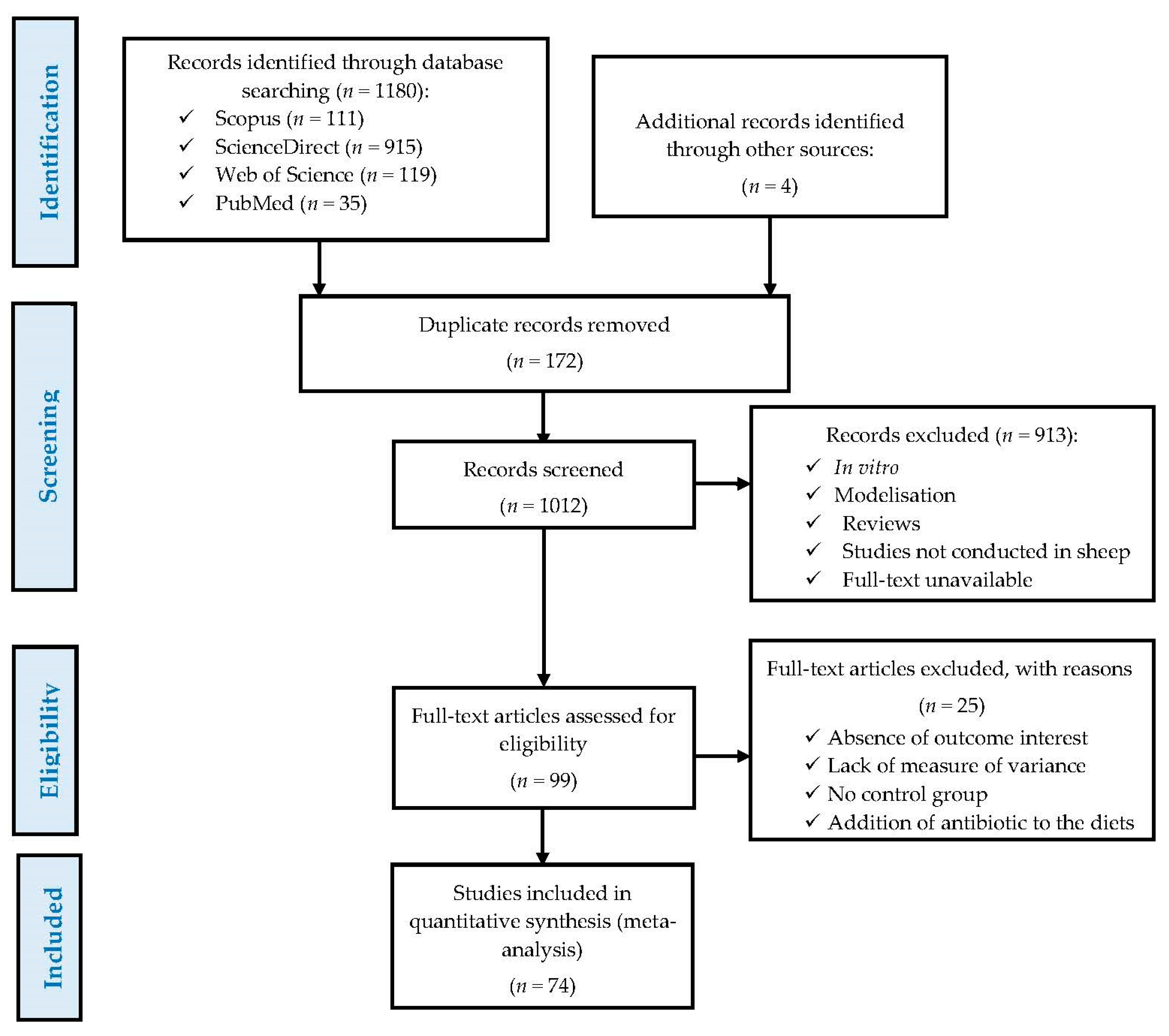
2.1. Literature Search and Study Selection
2.2. Data Extraction
2.3. Calculations and Statistical Analysis
2.4. Heterogeneity and Publication Bias
2.5. Meta-Regression and Subgroup Analysis
3. Results
3.1. Study Attributes and Excluded Studies
3.2. Dry Matter Intake and Digestibility
3.3. Growth Performance and Carcass Characteristics
3.4. Ruminal Parameters and Ruminal Microorganisms
3.5. Blood Metabolites
3.6. Meat Quality
3.7. Milk Yield and Quality
3.8. Publication Bias and Meta-Regression
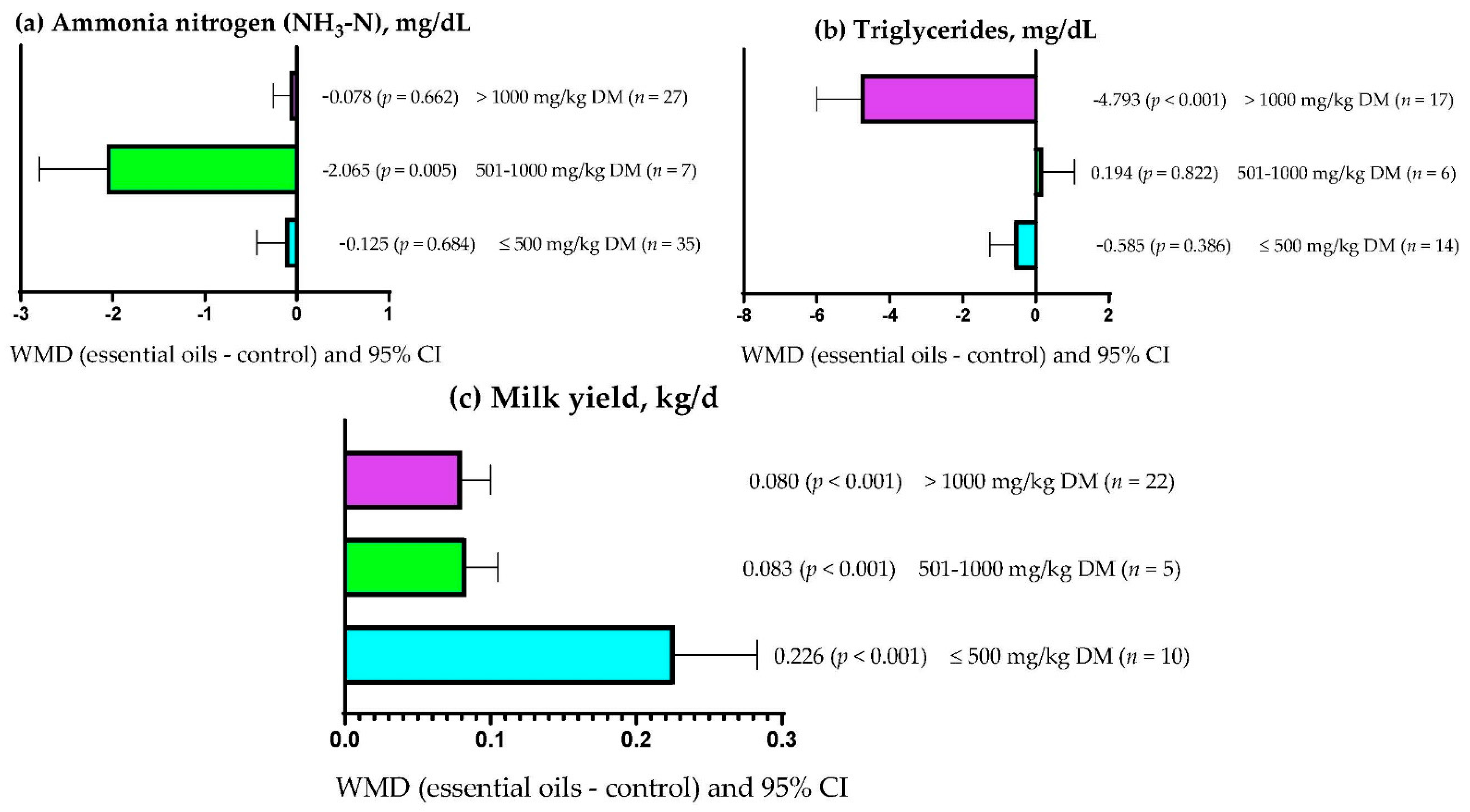
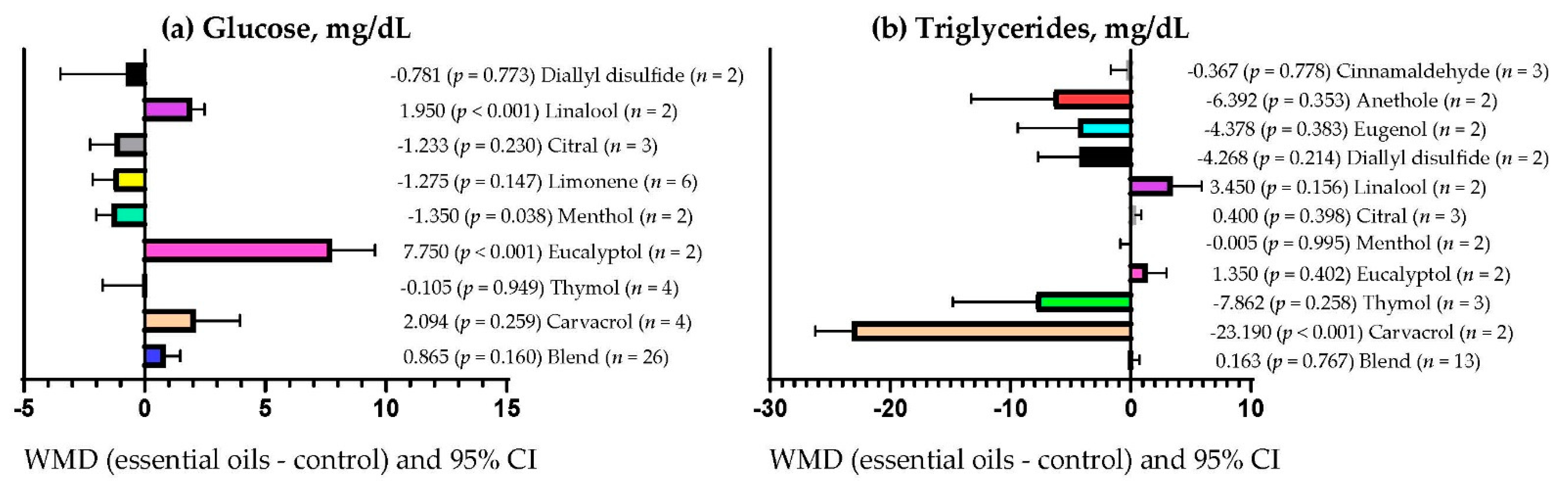
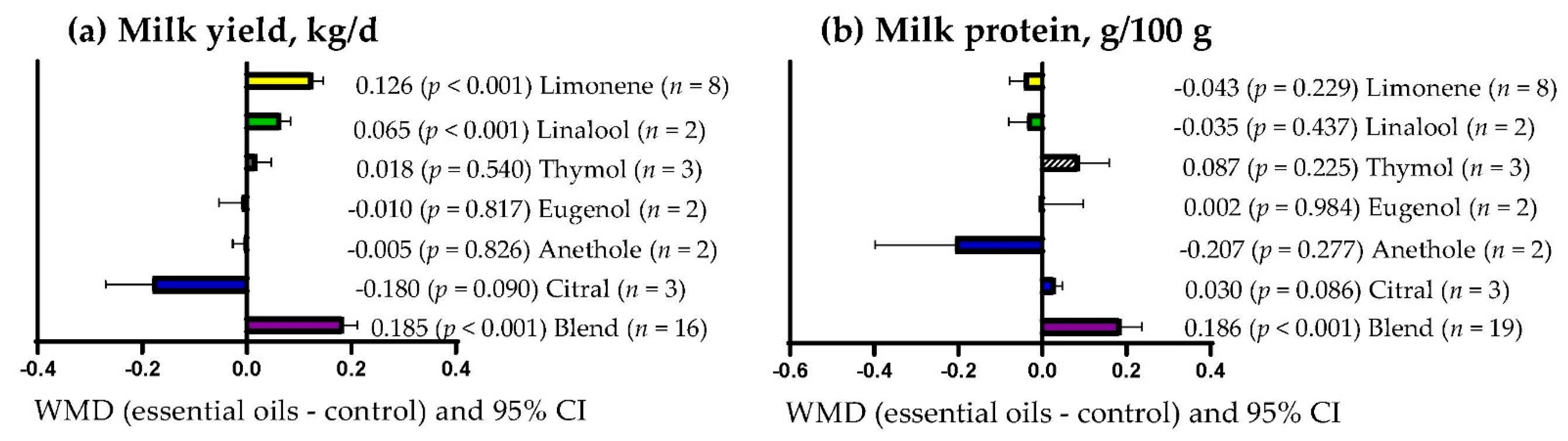
3.9. Subgroup Analysis
4. Discussion
4.1. Dry Matter Intake and Digestibility
4.2. Growth Performance and Carcass Characteristics
4.3. Ruminal Fermentation and Ruminal Microorganisms
4.4. Blood Metabolites
4.5. Meat Quality
4.6. Milk Production and Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author | Country | Specie | Duration, d | Primary Bioactive Compound | Dose, mg/kg DM |
|---|---|---|---|---|---|
| Abd El Tawab et al. [109] | Egypt | Sheep | 90 | Limonene, thymol | 1195, 1272 |
| Abdalla et al. [110] | Brazil | Sheep | 28 | Blend (n = 2) | 8333, 15,384 |
| Ahmed et al. [111] | Japan | Sheep | 84 | Allicin (n = 3) | 10, 50, 100 |
| An et al. [55] | China | Sheep | 60 | Blend (n = 2) | 50, 80 |
| Anasoori et al. [112] | Iran | Sheep | 28 | Diallyl disulfide (n = 2) | 500, 750 |
| Anasoori et al. [113] | Iran | Sheep | 28 | Diallyl disulfide (n = 2) | 500, 750 |
| Aouadi et al. [114] | Tunisia | Sheep | 90 | Eucalyptol, Camphor | 400, 400 |
| Arteaga-Wences et al. [15] | Mexico | Sheep | 56 | Blend | 129 |
| Bañón et al. [115] | Spain | Sheep | 21 | Blend | 667 |
| Baytok et al. [116] | Turkey | Sheep | 56 | Carvacrol (n = 2) | 280, 419 |
| Birick et al. [117] | Turkey | Sheep | 70 | Carvacrol (n = 2), thymol (n = 2), blend (n = 2) | 100 (n = 3), 300 (n = 3) |
| Canaes et al. [17] | Brazil | Goats | 84 | Citral (n = 3) | 2470, 5007, 7795 |
| Chaves et al. [118] | Canada | Sheep | 126 | Cinnamaldehyde (n = 3) | 100, 200, 400 |
| Cobellis et al. [119] | Italy | Sheep | 84 | Carnosic acid (n = 3) | 250, 250, 175 |
| Cobellis et al. [52] | Italy | Sheep | 84 | Carnosic acid (n = 3) | 250, 250, 175 |
| El-Azrak et al. [19] | Egypt | Goats | 45 | Blend | 750.00 |
| El-Essawy et al. [120] | Egypt | Sheep | 120 | Anethole, eugenol, thymol | 3069, 2920, 2780 |
| El-Essawy et al. [18] | Egypt | Goats | 88 | Anethole, eugenol, thymol | 1706, 1813, 1712 |
| Estrada-Angulo et al. [16] | Mexico | Sheep | 87, 100 | Blend (n = 2) | 115, 162 |
| Favaretto et al. [121] | Brazil | Sheep | 40 | Blend(n = 2) | 500, 1000 |
| Giannenas et al. [23] | Greece | Sheep | 150 | Blend(n = 3) | 50, 100, 150 |
| Güney et al. [57] | Turkey | Sheep | 70 | Eucalyptol (n = 2) | 250, 500 |
| Hashem et al. [122] | Egypt | Goats | 63 | Limonene (n = 2) | 523, 1051 |
| Hundal et al. [123] | India | Goats | 90 | Blend | 123.00 |
| Jiao et al. [124] | China | Sheep | 63 | Blend (n = 8) | 45 (n = 4), 79 (n = 4) |
| Kalaitsidis et al. [24] | Greece | Sheep | 45 | Blend | 15 |
| Katheri et al. [125] | Iran | Sheep | 48 | Blend (n = 2) | 800, 1600 |
| Khattab et al. [126] | Egypt | Sheep | 90 | Blend | 1232 |
| Kholif et al. [127] | Egypt | Goats | 90 | Blend (n = 3) | 1428, 1449, 1428 |
| Kholif et al. [99] | Egypt | Sheep | 84 | Blend | 2475 |
| Kholif et al. [103] | Egypt | Goats | 90 | Linalool (n = 2) | 946, 1902 |
| Klevenhusen et al. [128] | Switzerland | Sheep | 69 | Diallyl disulfide (n = 2) | 1775, 2000 |
| Kotsampasi et al. [129] | Greece | Sheep | 60 | Limonene (n = 3) | 86, 171, 254 |
| Leal et al. [130] | Spain | Sheep | 14 | Carnosic acid (n = 6) | 200 (n = 2), 400 (n = 2), 800 (n = 2) |
| Lei et al. [131] | China | Goats | 90 | Blend (n = 2) | 58, 101 |
| Lin et al. [132] | China | Sheep | 21 | Blend (n = 3) | 1111, 555, 1111 |
| Ma et al. [133] | China | Sheep | 29, 42 | Allicin (n = 2) | 2000 (n = 2) |
| Malekkhani et al. [134] | Iran | Sheep | 50 | Blend | 486 |
| Morsy et al. [135] | Egypt | Goats | 90 | Blend (n = 3) | 1428, 1418, 1379 |
| Moura et al. [136] | Brazil | Goats | 56 | β-caryophyllene (n = 3) | 500, 1000, 1500 |
| Naseri et al. [42] | Iran | Sheep | 56 | alpha-pinene | 852 |
| Nieto et al. [137] | Spain | Sheep | NR | Thymol (n = 2) | 1538, 3076 |
| Ortuño et al. [138] | Spain | Sheep | 80 | Carnosic acid (n = 2) | 200, 400 |
| Ortuño et al. [139] | Spain | Sheep | 80 | Blend | 400 |
| Ortuño et al. [140] | Spain | Sheep | 50 | Blend | 500 |
| Ortuño et al. [141] | Spain | Sheep | 80 | Blend (n = 2) | 200, 400 |
| Ortuño et al. [142] | Spain | Sheep | 50 | Blend | 500 |
| Özdoğan et al. [143] | Turkey | Sheep | 56 | Blend (n = 2) | 1000 (n = 2) |
| Panthee et al. [144] | Japan | Sheep | 44 | Alliin | 123 |
| Paraskevakis [145] | Greece | Goats | 28 | Carvacrol | 495 |
| Parvar et al. [146] | Iran | Sheep | 90 | Blend (n = 3) | 250, 500, 750 |
| Passetti et al. [147] | Canada | Sheep | 100 | Blend (n = 4) | 1100 (n = 2), 125 (n = 2) |
| Patindra et al. [148] | Thailand | Goats | 42 | Eugenol | 290 |
| Patra et al. [149] | Germany | Sheep | 28 | Menthol (n = 2) | 64, 126 |
| Ranucci et al. [150] | Italy | Sheep | 30 | Blend | 2000 |
| Sahraei et al. [151] | Iran | Sheep | 84 | Carnosic acid (n = 3) | 40, 80, 160 |
| Selmi et al. [152] | Tunisia | Sheep | 84 | Blend (n = 2) | 150, 300 |
| Serrano et al. [153] | Spain | Sheep | 80 | Carnosic acid (n = 2) | 600 (n = 2) |
| Shaaban et al. [20] | Egypt | Sheep | 288 | Limonene, thymol, blend | 1466, 1486, 1476 |
| Simitzis et al. [154] | Greece | Sheep | 35 | Cinnamaldehyde | 413 |
| Smeti et al. [155] | Tunisia | Sheep | 60 | Eucalyptol | 600 |
| Smeti et al. [156] | Tunisia | Goats | 56 | Blend | 599 |
| Smeti et al. [21] | Tunisia | Sheep | 100 | Blend (n = 3) | 900, 477, 957 |
| Smeti et al. [22] | Tunisia | Goats | 67 | alpha-pinene (n = 2) | 3000, 6000 |
| Soltan et al. [157] | Egypt | Goats | 63 | Limonene (n = 2) | 523, 1051 |
| Soltan et al. [77] | Brazil | Sheep | 111 | Blend (n = 2) | 200, 400 |
| Ünlü et al. [158] | Turkey | Sheep | 56 | Blend, capsaicin | 300, 300 |
| Wu et al. [56] | China | Sheep | 72 | Carvacrol (n = 2) | 2750, 5500 |
| Yanza et al. [159] | Poland | Sheep | 48, 30 | Rosmarinic acid (n = 2) | 3920 (n = 2) |
| Yesilbag et al. [160] | Turkey | Goats | 60 | alpha-pinene (n = 3) | 400, 800, 2000 |
| Zhang et al. [48] | China | Sheep | 24 | Carvacrol (n = 3) | 10,000, 20,000, 40,000 |
| Zhou et al. [41] | China | Sheep | 36 | Blend (n = 2) | 52, 91 |
| Zhu et al. [161] | China | Goats | 60 | Blend | 1481 |
| Zhu et al. [162] | China | Goats | 30 | Blend (n = 3) | 570, 1140, 1710 |
| Parameter | Covariates | QM | df | p-Value | R2 (%) |
|---|---|---|---|---|---|
| Meat pH | Essential oils dose | 0.80 | 1 | 0.370 | 0.0 |
| Supplementation period | 11.11 | 1 | 0.065 | 0.0 | |
| Primary bioactive compound | 97.07 | 7 | <0.001 | 100 | |
| Glucose | Essential oils dose | 0.44 | 1 | 0.508 | 1.32 |
| Supplementation period | 0.92 | 1 | 0.336 | 4.64 | |
| Primary bioactive compound | 20.54 | 9 | 0.015 | 30.28 | |
| Cholesterol | Essential oils dose | 1.71 | 1 | 0.191 | 0.0 |
| Supplementation period | 2.31 | 1 | 0.128 | 5.68 | |
| Primary bioactive compound | 14.79 | 10 | 0.140 | 0.0 | |
| Triglycerides | Essential oils dose | 14.64 | 1 | <0.001 | 2.65 |
| Supplementation period | 1.078 | 1 | 0.299 | 0.0 | |
| Primary bioactive compound | 327.36 | 11 | <0.001 | 80.30 | |
| Milk yield | Essential oils dose | 22.22 | 1 | <0.001 | 28.20 |
| Supplementation period | 2.61 | 1 | 0.106 | 0.00 | |
| Primary bioactive compound | 38.58 | 9 | <0.001 | 47.17 | |
| Milk fat | Essential oils dose | 0.03 | 1 | 0.863 | 0.00 |
| Supplementation period | 5.55 | 1 | 0.068 | 0.00 | |
| Primary bioactive compound | 13.05 | 1 | 0.071 | 25.18 | |
| Milk protein | Essential oils dose | 0.078 | 1 | 0.780 | 0.00 |
| Supplementation period | 6.40 | 1 | 0.011 | 10.41 | |
| Primary bioactive compound | 26.17 | 7 | <0.001 | 32.38 | |
| Milk lactose | Essential oils dose | 0.826 | 1 | 0.363 | 0.00 |
| Supplementation period | 7.43 | 1 | 0.106 | 2.05 | |
| Primary bioactive compound | 13.29 | 7 | 0.065 | 0.00 |
References
- Benchaar, C.; Hristov, A.N.; Greathead, H. Essential Oils as Feed Additives in Ruminant Nutrition. In Phytogenics in Animal Nutrition; Steiner, T., Ed.; Nottingham University Press: Nottingham, UK, 2009; pp. 111–146. [Google Scholar]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin—A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Iturbide, G.; Orzuna-Orzuna, J.F.; Lara-Bueno, A.; Miranda-Romero, L.A.; Mendoza-Martínez, G.D.; Hernández-García, P.A. Effects of a Polyherbal Dietary Additive on Performance, Dietary Energetics, Carcass Traits, and Blood Metabolites of Finishing Lambs. Metabolites 2022, 12, 413. [Google Scholar] [CrossRef]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef]
- Brenes, A.; Roura, E. Essential Oils in Poultry Nutrition: Main Effects and Modes of Action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential Oil and Aromatic Plants as Feed Additives in Non-Ruminant Nutrition: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Benchaar, C.; Greathead, H. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 2011, 166, 338–355. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.; Colombatto, D.; McAllister, T.A.; Beauchemin, K. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Mucha, W.; Witkowska, D. The Applicability of Essential Oils in Different Stages of Production of Animal-Based Foods. Molecules 2021, 26, 3798. [Google Scholar] [CrossRef]
- Arteaga-Wences, Y.J.; Estrada-Angulo, A.; Ríos-Rincón, F.G.G.; Castro-Pérez, B.; Mendoza-Cortéz, D.A.; Manriquez-Núñez, O.M.; Barreras, A.; Corona-Gochi, L.; Zinn, R.A.; Perea-Domínguez, X.P.; et al. The effects of feeding a standardized mixture of essential oils vs monensin on growth performance, dietary energy and carcass characteristics of lambs fed a high-energy finish- ing diet. Small Rumin. Res. 2021, 205, 106557. [Google Scholar] [CrossRef]
- Estrada-Angulo, A.; Arteaga-Wences, Y.J.; Castro-Pérez, B.I.; Urías-Estrada, J.D.; Gaxiola-Camacho, S.; Angulo-Montoya, C.; Ponce-Barraza, E.; Barreras, A.; Corona, L.; Zinn, R.A.; et al. Blend of Essential Oils Supplemented Alone or Combined with Exogenous Amylase Compared with Virginiamycin Supplementation on Finishing Lambs: Performance, Dietary Energetics, Carcass Traits, and Nutrient Digestion. Animals 2021, 11, 2390. [Google Scholar] [CrossRef] [PubMed]
- Canaes, T.S.; Zanferari, F.; Maganhe, B.L.; Takiya, C.S.; Silva, T.H.; Del Valle, T.A.; Rennó, F.P. Increasing dietary levels of citral oil on nutrient total tract digestibility, ruminal fermentation, and milk composition in Saanen goats. Anim. Feed Sci. Technol. 2017, 229, 47–56. [Google Scholar] [CrossRef]
- El-Essawy, A.M.; Anele, U.Y.; Abdel-Wahed, A.M.; Abdou, A.R.; Khattab, I.M. Effects of anise, clove and thyme essential oils supplementation on rumen fermentation, blood metabolites, milk yield and milk composition in lactating goats. Anim. Feed Sci. Technol. 2021, 271, 114760. [Google Scholar] [CrossRef]
- El-Azrak, K.M.; Morsy, A.S.; Soltan, Y.; Hashem, N.M.; Sallam, S.M. Impact of specific essential oils blend on milk production, serum biochemical parameters and kid performance of goats. Anim. Biotechnol. 2021, 1–9. [Google Scholar] [CrossRef]
- Shaaban, M.M.; Kholif, A.E.; Abd El Tawab, A.M.; Radwan, M.A.; Hadhoud, F.I.; Khattab, M.S.A.; Saleh, H.M.; Anele, U.Y. Thyme and celery as potential alternatives to ionophores use in livestock production: Their effects on feed utilization, growth performance and meat quality of Barki lambs. Small Ruminant Res. 2021, 200, 106400. [Google Scholar] [CrossRef]
- Smeti, S.; Hajji, H.; Mekki, I.; Mahouachi, M.; Atti, N. Effects of dose and administration form of rosemary essential oils on meat quality and fatty acid profile of lamb. Small Rumin. Res. 2018, 158, 62–68. [Google Scholar] [CrossRef]
- Smeti, S.; Tibaoui, S.; Bertolin, J.R.; Yagoubi, Y.; Mekki, I.; Joy, M.; Atti, N. Effects of myrtle (Myrtus communis L.) essential oils as dietary antioxidant supplementation on carcass and meat quality of goat meat. J. Anim. Physiol. Anim. Nutr. 2020, 105, 452–461. [Google Scholar] [CrossRef]
- Giannenas, I.; Skoufos, J.; Giannakopoulos, C.; Wiemann, M.; Gortzi, O.; Lalas, S.; Kyriazakis, I. Effects of essential oils on milk production, milk composition, and rumen microbiota in Chios dairy ewes. J. Dairy Sci. 2011, 94, 5569–5577. [Google Scholar] [CrossRef] [PubMed]
- Kalaitsidis, K.; Sidiropoulou, E.; Tsiftsoglou, O.; Mourtzinos, I.; Moschakis, T.; Basdagianni, Z.; Vasilopoulos, S.; Chatzigavriel, S.; Lazari, D.; Giannenas, I. Effects of cornus and Its mixture with oregano and thyme essential oils on dairy sheep performance and milk, yoghurt, and cheese quality under heat stress. Animals 2021, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Miranda-Romero, L.A.; Mendoza-Martínez, G.D.; Santiago-Figueroa, I. A Meta-Analysis of Essential Oils Use for Beef Cattle Feed: Rumen Fermentation, Blood Metabolites, Meat Quality, Performance and, Environmental and Economic Impact. Fermentation 2022, 8, 254. [Google Scholar] [CrossRef]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A Meta-analysis Describing the Effects of the Essential oils Blend Agolin Ruminant on Performance, Rumen Fermentation and Methane Emissions in Dairy Cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Khiaosa-Ard, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Hernández-García, P.A. Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis. Sustainability 2021, 13, 7410. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Lee-Rangel, H.A. Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis. Animals 2021, 11, 3184. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons: Chichester, UK, 2009; p. 413. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Viechtbauer, W. Conducting meta-analysis in R with the metaphor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). SAS/STAT User’s Guide (Release 6.4); SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care, 2nd ed.; MBJ Publishing Group: London, UK, 2001; pp. 109–121. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Amer. Statist. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Littell, J.H.; Corcoran, J.; Pillai, V. Systematic Reviews and Meta-Analysis, 1st ed.; Oxford University Press: Oxford, UK, 2008; pp. 111–132. [Google Scholar]
- Zhou, R.; Wu, J.; Zhang, L.; Liu, L.; Casper, D.P.; Jiao, T.; Liu, T.; Wang, J.; Lang, X.; Song, S.; et al. Effects of oregano essential oil on the ruminal pH and microbial population of sheep. PLoS ONE. 2019, 14, e0217054. [Google Scholar] [CrossRef]
- Naseri, V.; Kafilzadeh, F.; Jahani-Azizabadi, H. Effects of Pistacia atlantica gum essential oil on ruminal methanogen, protozoa, selected bacteria species and fermentation characteristics in sheep. Small Rumin. Res. 2022, 209, 106650. [Google Scholar] [CrossRef]
- Clouard, C.; Val-Laillet, D. Impact of sensory feed additives on feed intake, feed preferences, and growth of female piglets during the early postweaning period. J. Anim. Sci. 2014, 92, 2133–2140. [Google Scholar] [CrossRef]
- Li, S.; Du, M.; Zhang, C.; Wang, Y.; Lee, Y.; Zhang, G. Diet Type Impacts Production Performance of Fattening Lambs by Manipulating the Ruminal Microbiota and Metabolome. Front. Microbiol. 2022, 13, 824001. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef]
- Kim, H.; Jung, E.; Lee, H.G.; Kim, B.; Cho, S.; Lee, S.; Kwon, I.; Seo, J. Essential oil mixture on rumen fermentation and microbial community—An in vitro study. Asian-Australas. J. Anim. Sci. 2019, 32, 808–814. [Google Scholar] [CrossRef]
- Koike, S.; Kobayashi, Y. Fibrolytic rumen bacteria: Their ecology and functions. Asian-Australas. J. Anim. 2009, 22, 131–138. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of origanum oil, hydrolysable tannins and tea saponin in mitigating ruminant methane: In vitro and in vivo methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Lang, X.; Liu, L.; Casper, D.P.; Wang, C.; Zhang, L.; Wei, S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J. Dairy Sci. 2020, 103, 2303–2314. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhang, L.; Wu, J.; Zhao, S.; Jiao, T. Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community. Animals 2022, 12, 118. [Google Scholar] [CrossRef]
- Kong, F.; Liu, Y.; Wang, S.; Zhang, Y.; Wang, W.; Yang, H.; Lu, N.; Li, S. Nutrient Digestibility, Microbial Fermentation, and Response in Bacterial Composition to Methionine Dipeptide: An In Vitro Study. Biology 2022, 11, 93. [Google Scholar] [CrossRef]
- Cobellis, G.; Yu, Z.; Forte, C.; Acuti, G.; Trabalza-Marinucci, M. Dietary supplementation of Rosmarinus officinalis L. leaves in sheep affects the abundance of rumen methanogens and other microbial populations. J. Anim. Sci. Biotechnol. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Le, J.; Wu, P.; Liu, J.; Guan, L.L.; Wang, J. Alfalfa intervention alters rumen microbial community development in Hu lambs during early life. Front. Microbiol. 2018, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, Y.; Yun, Y.; Ji, W.; Jin, Z.; Wang, C.; Yu, Z. Weaning age affects the development of the ruminal bacterial and archaeal community in hu lambs during early life. Front. Microbiol. 2021, 12, 636865. [Google Scholar] [CrossRef]
- An, X.; Wang, Y.; Wang, R.; Hao, X.; Hu, Y.; Guo, T.; Zhang, J.; Wang, W.; Shi, X.; Han, S.; et al. Effects of a blend of cinnamaldehyde, eugenol and capsicum oleoresin (CEC) on growth performance, nutrient digestibility, immune response and antioxidant status of growing ewes. Livest. Sci. 2020, 234, 103982. [Google Scholar] [CrossRef]
- Wu, J.P.; Zhou, R.; Liu, L.S.; Casper, D.P.; Lang, X.; Wang, C.L.; Zhang, L.P.; Wei, S.; Liu, H.B. Growth performance, nutrient digestibility, blood parameters, and carcass characteristics by lambs fed an oregano and cobalt blend. Animal 2021, 15, 100365. [Google Scholar] [CrossRef] [PubMed]
- Güney, M.; Karaca, S.; Erdogan, S.; Kor, A.; Kale, C.; Onalan, S.; Demirel, M.; Bingol, N.T. Effects of dietary supplementation with rosemary oil on methanogenic bacteria density, blood and rumen parameters and meat quality of fattening lambs. Ital. J. Anim. Sci. 2021, 20, 794–805. [Google Scholar] [CrossRef]
- Gatford, K.L.; Fletcher, T.P.; Clarke, I.J.; Owens, P.C.; Quinn, K.J.; Walton, P.E.; Grant, P.A.; Hosking, B.J.; Egan, A.R.; Ponnampalam, E.N. Sexual dimorphism of circulating somatotropin, insulin-like growth factor I and II, insulin-like growth factor binding proteins, and insulin: Relationships to growth rate and carcass characteristics in growing lambs. J. Anim. Sci. 1996, 74, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cheng, Z.; Zhao, Y.; Wang, Y.; Wang, H.; Ren, Z. Influence of increasing levels of oregano essential oil on intestinal morphology, intestinal flora and performance of Sewa sheep. Ital. J. Anim. Sci. 2022, 21, 463–472. [Google Scholar] [CrossRef]
- Corazzin, M.; Del Bianco, S.; Bovolenta, S.; Piasentier, E. Carcass characteristics and meat quality of sheep and goat. In More than Beef, Pork and Chicken—The Production, Processing, and Quality Traits of Other Sources of Meat for Human Diet; Lorenzo, J.M., Munekata, P.E.S., Barba, F., Toldrá, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 119–165. ISBN 978-3-030-05483-0. [Google Scholar]
- Laliotis, G.P.; Bizelis, I.; Rogdakis, E. Comparative Approach of the de Novo Fatty Acid Synthesis (Lipogenesis) between Ruminant and Non Ruminant Mammalian Species: From Biochemical Level to the Main Regulatory Lipogenic Genes. Curr. Genom. 2010, 11, 168–183. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, S.S.; Singh, S.; Dabur, R. Natural products: Potential therapeutic agents to prevent skeletal muscle atrophy. Eur. J. Pharm. 2022, 925, 174995. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef]
- Ebert, S.M.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Murry, D.J.; Fox, D.K.; Bongers, K.S.; Lira, V.A.; Meyerholz, D.K.; Talley, J.J.; et al. Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-dependent Pathway to Age-related Skeletal Muscle Weakness and Atrophy. J. Biol. Chem. 2015, 290, 25497–25511. [Google Scholar] [CrossRef]
- Monteschio, J.O.; Vargas-Junior, F.M.; Almeida, F.L.; Pinto, L.A.D.M.; Kaneko, I.N.; Almeida, A.A.; Freitas, L.W.; Alves, S.P.; Bessa, R.J.; Prado, I.N. The effect of encapsulated active principles (eugenol, thymol and vanillin) and clove and rosemary essential oils on the structure, collagen content, chemical composition and fatty acid profile of Nellore heifers muscle. Meat Sci. 2019, 155, 27–35. [Google Scholar] [CrossRef]
- McIntosh, F.M.; Williams, P.; Losa, R.; Wallace, R.J.; Beever, D.A.; Newbold, C.J. Effects of essential oils on ruminal microorganisms and their protein metabolism. Appl. Environ. Microbiol. 2003, 69, 5011–5014. [Google Scholar] [CrossRef]
- Chen, J.; Harstad, O.M.; McAllister, T.; Dörsch, P.; Holo, H. Propionic acid bacteria enhance ruminal feed degradation and reduce methane production in vitro. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 169–175. [Google Scholar] [CrossRef]
- Qiu, X.; Qin, X.; Chen, L.; Chen, Z.; Hao, R.; Zhang, S.; Yang, S.; Wang, L.; Cui, Y.; Li, Y.; et al. Serum Biochemical Parameters, Rumen Fermentation, and Rumen Bacterial Communities Are Partly Driven by the Breed and Sex of Cattle When Fed High-Grain Diet. Microorganisms 2022, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; De La Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The Role of Ciliate Protozoa in the Rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef] [PubMed]
- Guyader, J.; Eugène, M.; Nozière, P.; Morgavi, D.P.; Doreau, M.; Martin, C. Influence of rumen protozoa on methane emission in ruminants: A meta-analysis approach. Animal 2014, 8, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Franzolin, R.; Dehority, B.A. The role of pH on the survival of rumen protozoa in steers. Rev. Bras. Zootec. 2010, 39, 2262–2267. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 3, 545–546. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Skliros, D.; Simoni, M.; Righi, F.; Flemetakis, E.; Tsiplakou, E. Alterations in the Rumen Particle-Associated Microbiota of Goats in Response to Dietary Supplementation Levels of Schizochytrium spp. Sustainability 2021, 13, 607. [Google Scholar] [CrossRef]
- McCabe, M.S.; Cormican, P.; Keogh, K.; O’Connor, A.; O’Hara, E.; Palladino, R.A.; Kenny, D.A.; Waters, S.M. Illumina miseq phylogenetic amplicon sequencing shows a large reduction of an uncharacterised Succinivibrionaceae and an increase of the Methanobrevibacter gottschalkii clade in feed restricted cattle. PLoS ONE 2015, 10, e0133234. [Google Scholar] [CrossRef]
- Wallace, R.J.; Rooke, J.A.; Duthie, C.-A.; Hyslop, J.J.; Ross, D.W.; McKain, N.; de Souza, S.M.; Snelling, T.J.; Waterhouse, A.; Roehe, R. Archaeal abundance in post-mortem ruminal digesta may help predict methane emissions from beef cattle. Sci. Rep. 2014, 4, 5892. [Google Scholar] [CrossRef]
- Paengkoum, P.; Chen, S.; Paengkoum, S. Effects of crude protein and undegradable intake protein on growth performance, nutrient utilization, and rumen fermentation in growing Thai-indigenous beef cattle. Trop. Anim. Health Prod. 2019, 51, 1151–1159. [Google Scholar] [CrossRef]
- Ran, T.; Shen, Y.Z.; Saleem, A.M.; AlZahal, O.; Beauchemin, K.A.; Yang, W.Z. Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: Growth performance, carcass traits, blood metabolites, and fecal Escherichia coli. J. Anim. Sci. 2018, 96, 4385–4397. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Morsy, A.S.; Hashem, N.M.; Sallam, S.M. Boswellia sacra resin as a phytogenic feed supplement to enhance ruminal fermentation, milk yield, and metabolic energy status of early lactating goats. Anim. Feed Sci. Technol. 2021, 277, 114963. [Google Scholar] [CrossRef]
- Jun, H.-J.; Lee, J.H.; Jia, Y.; Hoang, M.-H.; Byun, H.; Kim, K.H.; Lee, S.-J. Melissa officinalis essential oil reduces plasma triglycerides in human apolipoprotein E2 transgenic mice by inhibiting sterol regulatory element-binding protein-1c–dependent fatty acid synthesis. J. Nutr. 2012, 142, 432–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Celi, P. Oxidative stress in ruminants. In Studies on Veterinary Medicine. Oxidative Stress in Applied Basic Research and Clinical Practice; Mandelker, L., Vajdovich, P., Eds.; Humana Press: Totowa, NJ, USA; New York, NY, USA, 2011; pp. 191–231. ISBN 978-1-61779-070-6. [Google Scholar]
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Ablikim, B.; Liu, Y.; Kerim, A.; Shen, P.; Abdurerim, P.; Zhou, G.H. Effects of breed, muscle type, and frozen storage on physico-chemical characteristics of lamb meat and its relationship with tenderness. CyTA J. Food 2016, 14, 109–116. [Google Scholar] [CrossRef]
- Toldrá, F. Lawrie’s Meat Science, 8th ed.; Woodhead Publishing Limited: Cambridge, UK, 2017; 713p. [Google Scholar]
- Okeudo, N.J.; Moss, B.W. Interrelationships amongst carcass and meat quality characteristics of sheep. Meat Sci. 2005, 69, 1–8. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Hernández-García, P.A. Growth Performance, Carcass Characteristics, and Blood Metabolites of Lambs Supplemented with a Polyherbal Mixture. Animals 2021, 11, 955. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; López-Ordaz, R.; Hernández-García, P.A. Productive Performance, Carcass Traits, and Meat Quality in Finishing Lambs Supplemented with a Polyherbal Mixture. Agriculture 2021, 11, 942. [Google Scholar] [CrossRef]
- Calnan, H.; Jacob, R.; Pethick, D.; Gardner, G. Factors affecting the colour of lamb meat from the longissimus muscle during display: The influence of muscle weight and muscle oxidative capacity. Meat Sci. 2014, 96, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Węglarz, A. Meat quality defined based on pH and colour depending on cattle category and slaughter season. colour and pH as determinants of meat quality dependent on cattle category and slaughter season. Czech J. Anim. Sci. 2010, 55, 548–556. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Tian, Q.; Piao, X. Essential Oil Blend Could Decrease Diarrhea Prevalence by Improving Antioxidative Capability for Weaned Pigs. Animals 2019, 9, 847. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Golian, A.; Buyse, J.; Wang, Y.; De Smet, S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult. Sci. 2014, 93, 1930–1941. [Google Scholar] [CrossRef]
- Jordán, M.J.; Castillo, J.; Bañón, S.; Martínez-Conesa, C.; Sotomayor, J.A. Relevance of the carnosic acid/carnosol ratio for the level of rosemary diterpene transfer and for improving lamb meat antioxidant status. Food Chem. 2014, 151, 212–218. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Kholif, A.E.; Kassab, A.Y.; Azzaz, H.H.; Matloup, O.H.; Hamdon, H.A.; Olafadehan, O.A.; Morsy, T.A. Essential oils blend with a newly developed enzyme cocktail works synergistically to enhance feed utilization and milk production of Farafra ewes in the subtropics. Small Rumin. Res. 2018, 161, 43–50. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Skliros, D.; Flemetakis, E.; Tsiplakou, E. Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp. Microorganisms 2021, 9, 1528. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, K.; Li, C.; Jiao, T.; Wu, J.; Wei, Y.; Tian, K.; Li, C.; Tang, D.; Davis, D.I.; et al. Ruminal metagenomic analyses of goat data reveals potential functional microbiota by supplementation with essential oil-cobalt complexes. BMC Microbiol. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Mizrahi, I. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef]
- Kholif, A.E.; Elazab, M.A.; Matloup, O.H.; Olafadehan, O.A.; Sallam, S.M.A. Crude coriander oil in the diet of lactating goats enhanced lactational performance, ruminal fermentation, apparent nutrient digestibility, and blood chemistry. Small Rumin. Res. 2021, 204, 106522. [Google Scholar] [CrossRef]
- Seymour, W.M.; Campbell, D.R.; Johnson, Z.B. Relationships between rumen volatile fatty acid concentrations and milk production in dairy cows: A literature study. Anim. Feed Sci. Technol. 2005, 119, 155–169. [Google Scholar] [CrossRef]
- Al-Suwaiegh, S.B.; Morshedy, S.A.; Mansour, A.T.; Ahmed, M.H.; Zahran, S.M.; Alnemr, T.M.; Sallam, S.M.A. Effect of an Essential Oil Blend on Dairy Cow Performance during Treatment and Post-Treatment Periods. Sustainability 2020, 12, 9123. [Google Scholar] [CrossRef]
- Malik, T.A.; Mohini, M.; Mir, S.H.; Ganaie, B.A.; Singh, D.; Varun, T.K.; Howal, S.; Thakur, S. Somatic Cells in Relation to Udder Health and Milk Quality-A Review. J. Anim. Health Prod. 2018, 6, 18–26. [Google Scholar] [CrossRef]
- Juozaitene, V.; Juozaitis, A.; Mikicikeviciene, R. Relationship between somatic cell count and milk production or morphological traits of udder in black-and-white cows. Turk. J. Vet. Anim. Sci. 2006, 30, 47–51. [Google Scholar]
- Cinar, M.; Serbester, U.; Ceyhan, A.; Gorgulu, M. Effect of somatic cell count on milk yield and composition of first and second lactation dairy cows. Ital. J. Anim. Sci. 2015, 14, 3646. [Google Scholar] [CrossRef]
- Abd El Tawab, A.M.; Kholif, A.E.; Khattab, M.S.A.; Shaaban, M.M.; Hadhoud, F.I.; Mostafa, M.M.M.; Olafadehan, O.A. Feed utilization and lactational performance of Barki sheep fed diets containing thyme or celery. Small Rumin. Res. 2020, 192, 106249. [Google Scholar] [CrossRef]
- Abdalla, A.L.; Louvandini, H.; Sallam, S.M.A.H.; Bueno, I.C.D.S.; Tsai, S.M.; Figueira, A.V.D.O. In vitro evaluation, in vivo quantification, and microbial diversity studies of nutritional strategies for reducing enteric methane production. Trop. Anim. Health Prod. 2012, 44, 953–964. [Google Scholar] [CrossRef]
- Ahmed, E.; Batbekh, B.; Fukuma, N.; Kand, D.; Hanada, M.; Nishida, T. A garlic and citrus extract: Impacts on behavior, feed intake, rumen fermentation, and digestibility in sheep. Anim. Feed Sci. Technol. 2021, 278, 115007. [Google Scholar] [CrossRef]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Taghizadeh, A.; Asri-Rezaei, S.; Maham, M.; Farahmand-Azar, S.; Farhoomand, P. Garlic: A potential alternative for monensin as a rumen modifier. Livest. Sci. 2011, 142, 276–287. [Google Scholar] [CrossRef]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Hadian, M. Changes in blood profile in sheep receiving raw garlic, garlic oil or monensin. J. Anim. Physiol. Anim. Nutr. 2015, 99, 114–122. [Google Scholar] [CrossRef]
- Aoudi, D.; Luciano, G.; Vasta, V.; Nasri, S.; Brogna, D.M.R.; Abidi, S.; Priolo, A.; Ben Salem, H. The antioxidant status and oxidative stability of muscle from lambs receiving oral administration of Artemisia herba alba and Rosmarinus officinalis essential oils. Meat Sci. 2014, 97, 237–243. [Google Scholar] [CrossRef]
- Bañón, S.; Méndez, L.; Almela, E. Effects of dietary rosemary extract on lamb spoilage under retail display conditions. Meat Sci. 2012, 90, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Baytok, E.; Kara, K.; Aksu, T.; Guclu, B.; Özkaya, S.; Denek, N.; Kamalak, A.; Kaya, D.A.; Önel, S.E.; Akçay, A. The effect of Mediterranean thyme (Thymbra spicata L. var. spicata) essential oil on fattening performance and ruminal parameters in lamb. J. Anim. Feed Sci. 2017, 26, 319–325. [Google Scholar] [CrossRef]
- Biricik, H.; Oral, H.H.; Taluğ, A.M.; Cengiz, S.S.; Koyuncu, M.; Dikmen, S. The effects of carvacrol and/or thymol on the performance, blood and rumen parameters, and carcass traits of Merino sheep. Turk. J. Vet. Anim. Sci. 2016, 40, 19. [Google Scholar] [CrossRef]
- Chaves, A.V.; Dugan, M.E.R.; Stanford, K.; Gibson, L.L.; Bystrom, J.M.; McAllister, T.A.; Van Herk, F.; Benchaar, C. A dose-response of cinnamaldehyde supplementation on intake, ruminal fermentation, blood metabolites, growth performance, and carcass characteristics of growing lambs. Livest. Sci. 2011, 141, 213–220. [Google Scholar] [CrossRef]
- Cobellis, G.; Acuti, G.; Forte, C.; Menghini, L.; de Vincenzi, S.; Orrù, M.; Valiani, A.; Pacetti, D.; Trabalza-Marinucci, M. Use of Rosmarinus officinalis in sheep diet formulations: Effects on ruminal fermentation, microbial numbers and in situ degradability. Small Rumin. Res. 2015, 126, 10–18. [Google Scholar] [CrossRef]
- El-Essawy, A.M.; Abdou, A.R.; El-Gendy, M.H. Impact of anise, clove and thyme essential oils as feed supplements on the productive performance and digestion of Barki ewes. Aust. J. Basic Appl. Sci. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Favaretto, J.A.; Alba, D.F.; Marchiori, M.S.; Marcon, H.J.; Souza, C.F.; Baldissera, M.D.; Bianchi, A.E.; Zanluchi, M.; Klein, B.; Wagner, R.; et al. Supplementation with a blend based on micro-encapsulated carvacrol, thymol, and cinnamaldehyde in lambs feed inhibits immune cells and improves growth performance. Livest. Sci. 2020, 240, 104144. [Google Scholar] [CrossRef]
- Hashem, N.M.; Morsy, A.S.; Soltan, Y.A.; Sallam, S.M. Potential Benefits of Boswellia sacra resin on immunity, metabolic status, udder and uterus health, and milk production in transitioning goats. Agriculture 2021, 11, 900. [Google Scholar] [CrossRef]
- Hundal, J.S.; Wadhwa, M.; Bakshi, M.P.S. Effect of supplementing herbal feed additive anethum sowa on nutrient utilization, productive performance and carcass characteristics of male beetal kids. Anim. Nutr. Feed Technol. 2020, 20, 25–38. [Google Scholar] [CrossRef]
- Jiao, T.; Wu, J.; Casper, D.P.; Davis, D.I.; Brown, M.A.; Zhao, S.; Liang, J.; Lei, Z.; Holloway, B. Feeding sheep cobalt and oregano essential oil alone or in combination on ruminal nutrient digestibility, fermentation, and fiber digestion combined with scanning electron microscopy. Front. Vet. Sci. 2021, 8, 639432. [Google Scholar] [CrossRef]
- Khateri, N.; Azizi, O.; Jahani-Azizabadi, H. Effects of a specific blend of essential oils on apparent nutrient digestion, rumen fermentation and rumen microbial populations in sheep fed a 50:50 alfalfa hay: Concentrate diet. Asian Australas. J. Anim. Sci. 2017, 30, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.S.A.; Kholif, A.E.; Abd El Tawab, A.M.; Shaaban, M.M.; Hadhoud, F.I.; El-Fouly, H.A.; Olafadehan, O.A. Effect of replacement of antibiotics with thyme and celery seed mixture on the feed intake and digestion, ruminal fermentation, blood chemistry, and milk lactation of lactating Barki ewes. Food Funct. 2020, 11, 6889–6898. [Google Scholar] [CrossRef] [PubMed]
- Kholif, S.M.; Morsy, T.A.; Abdo, M.M.; Matloup, O.H.; Abu El-Ella, A.A. Effect of supplementing lactating goats rations with garlic, cinnamon or ginger oils on milk yield, milk composition and milk fatty acids profile. J. Life Sci. 2012, 4, 27–34. [Google Scholar] [CrossRef]
- Klevenhusen, F.; Zeitz, J.O.; Duval, S.; Kreuzer, M.; Soliva, C.R. Garlic oil and its principal component diallyl disulfide fail to mitigate methane, but improve digestibility in sheep. Anim. Feed Sci. Technol. 2011, 166–167, 356–363. [Google Scholar] [CrossRef]
- Kotsampasi, B.; Tsiplakou, E.; Christodoulou, C.; Mavrommatis, A.; Mitsiopoulou, C.; Karaiskou, C.; Sossidou, E.; Fragioudakis, N.; Kpsomenos, I.; Bampidis, V.A.; et al. Effects of dietary orange peel essential oil supplementation on milk yield and composition, and blood and milk antioxidant status of dairy ewes. Anim. Feed Sci. Technol. 2018, 245, 20–31. [Google Scholar] [CrossRef]
- Leal, L.N.; Beltrán, J.A.; Bellés, M.; Bello, J.M.; den Hartog, L.A.; Hendriks, W.H.; Martín-Tereso, J. Supplementation of lamb diets with vitamin E and rosemary extracts on meat quality parameters. J. Sci. Food Agri. 2020, 100, 2922–2931. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, K.; Li, C.; Wu, J.; Davis, D.; Casper, D.; Jiang, H.; Jiao, T.; Wang, X.; Wang, J. Dietary supplementation with Essential-oils-cobalt for improving growth performance, meat quality and skin cell capacity of goats. Sci. Rep. 2018, 8, 11634. [Google Scholar] [CrossRef]
- Lin, B.; Lu, Y.; Salem, A.Z.M.; Wang, J.H.; Liang, Q.; Liu, J.X. Effects of essential oil combinations on sheep ruminal fermentation and digestibility of a diet with fumarate included. Anim. Feed Sci. Technol. 2013, 184, 24–32. [Google Scholar] [CrossRef]
- Ma, T.; Chen, D.; Tu, Y.; Zhang, N.; Si, B.; Deng, K.; Diao, Q. Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes. J. Anim. Sci. Biotechnol. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Malekkhahi, M.; Tahmasbi, A.M.; Naserian, A.A.; Danesh Mesgaran, M.; Kleen, J.; Parand, A. Effects of essential oils, yeast culture and malate on rumen fermentation, blood metabolites, growth performance and nutrient digestibility of Baluchi lambs fed high-concentrate diets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Morsy, T.A.; Kholif, S.M.; Matloup, O.H.; Abdo, M.M.; El-Shafie, M.H. Impact of anise, clove and juniper oils as Feed additives on the productive performance of lactating goats. Int. J. Dairy Sci. 2012, 7, 20–28. [Google Scholar] [CrossRef]
- Moura, L.V.; Oliveira, E.R.; Fernandes, A.R.M.; Gabriel, A.M.A.; Silva, L.H.X.; Takiya, C.S.; Cônsolo, N.R.B.; Rodrigues, G.C.G.; Lemos, T.; Gandra, J.R. Feed efficiency and carcass traits of feedlot lambs supplemented either monensin or increasing doses of copaiba (Copaifera spp.) essential oil. Anim. Feed Sci. Technol. 2017, 232, 110–118. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañon, S.; Garrido, M.D.; Nieto, G. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci. 2010, 85, 82–88. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañón, S. Antioxidant and antimicrobial effects of dietary supplementation with rosemary diterpenes (carnosic acid and carnosol) vs vitamin E on lamb meat packed under protective atmosphere. Meat Sci. 2015, 110, 62–69. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañon, S.; Casanova, J.O. Use of dietary rosemary diterpenes to extend the preservation of sulphited-lamb products. Small Rumin. Res. 2015, 123, 269–277. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañón, S. Incorporating rosemary diterpenes in lamb diet to improve microbial quality of meat packed in different environments. Anim. Sci. J. 2017, 88, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, J.; Serrano, R.; Jordán, M.; Bañón, S. Relationship between antioxidant status and oxidative stability in lamb meat reinforced with dietary rosemary diterpenes. Food Chem. 2016, 190, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Ortuno, J.; Serrano, R.; Jordan, M.J.; Banon, S. Shelf life of meat from lambs given essential oil-free rosemary extract containing carnosic acid plus carnosol at 200 or 400 mg kg−1. Meat Sci. 2014, 96, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Özdoğan, M.; Önenç, S.S.; Önenç, A. Fattening performance, blood parameters and slaughter traits of Karya lambs consuming blend of essential oil compounds. Afr. J. Biotechnol. 2011, 10, 6663–6669. [Google Scholar]
- Panthee, A.; Matsuno, A.; Al-Mamun, M.; Sano, H. Effect of feeding garlic leaves on rumen fermentation, methane emission, plasma glucose kinetics, and nitrogen utilization in sheep. J. Anim. Sci. Technol. 2017, 59, 14. [Google Scholar] [CrossRef][Green Version]
- Paraskevakis, N. Effects of dietary dried Greek Oregano (Origanum vulgare ssp. hirtum) supplementation on blood and milk enzymatic antioxidant indices, on milk total antioxidant capacity and on productivity in goats. Anim. Feed Sci. Technol. 2015, 209, 90–97. [Google Scholar] [CrossRef]
- Parvar, R.; Ghoorchi, T.; Kashfi, H.; Parvar, K. Effect of Ferulago angulata (Chavil) essential oil supplementation on lamb growth performance and meat quality characteristics. Small Rumin. Res. 2018, 167, 48–54. [Google Scholar] [CrossRef]
- Passetti, L.C.; Passetti, R.A.; McAllister, T.A. Effect of essential oil blends and a nonionic surfactant on rumen fermentation, anti-oxidative status, and growth performance of lambs. Trans. Anim. Sci. 2021, 5, 118. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkum, S.; Yuangklang, C.; Paengkum, P.; Salem, A.Z.M.; Boo, L.J. Mammary gene expressions and oxidative indicators in ruminal fluid, blood, milk, and mammary tissue of dairy goats fed a total mixed ration containing piper meal (Piper betle L.). Ital. J. Anim. Sci. 2022, 21, 129–141. [Google Scholar] [CrossRef]
- Patra, A.K.; Geiger, S.; Schrapers, K.T.; Braun, H.S.; Gehlen, H.; Starke, A.; Pieper, R.; Cieslak, A.; Szumacher-Strabe, M.; Aschenbach, J.R. Effects of dietary menthol-rich bioactive lipid compounds on zootechnical traits, blood variables and gastrointestinal function in growing sheep. J. Anim. Sci. Biotechnol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Ranucci, D.; Branciari, R.; Cobellis, G.; Acuti, G.; Miraglia, D.; Olivieri, O.; Roila, R.; Trabalza-Marinucci, M. Dietary essential oil mix improves oxidative stability and hygienic characteristic of lamb meat. Small Ruminant Res. 2019, 175, 104–109. [Google Scholar] [CrossRef]
- Sahraei, M.; Pirmohammadi, R.; Payvastegan, S. The effect of rosemary (Rosmarinus officinalis L.) essential oil on digestibility, ruminal fermentation and blood metabolites of Ghezel sheep fed barley-based diets. Span. J. Agric. Res. 2014, 12, 448–454. [Google Scholar] [CrossRef]
- Selmi, H.; Bahri, A.; Ferchichi, A.; Rouissi, H. Effect of supplementing Moringa oleifera essential oils on milk quality and fatty acid profile in dairy sheep. Indian J. Anim. Res. 2020, 54, 879–882. [Google Scholar] [CrossRef]
- Serrano, R.; Jordán, M.J.; Bañón, S. Use of dietary rosemary extract in ewe and lamb to extend the shelf life of raw and cooked meat. Small Rumin. Res. 2014, 116, 144–152. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Bronis, M.; Charismiadou, M.A.; Mountzouris, K.C.; Deligeorgis, S.G. Effect of cinnamon (Cinnamomum zeylanicum) essential oil supplementation on lamb growth performance and meat quality characteristics. Animal 2014, 8, 1554–1560. [Google Scholar] [CrossRef]
- Smeti, S.; Atti, N.; Mahouachi, M.; Munoz, F. Use of dietary rosemary (Rosmarinus officinalis L.) essential oils to increase the shelf life of Barbarine light lamb meat. Small Rumin. Res. 2013, 113, 340–345. [Google Scholar] [CrossRef]
- Smeti, S.; Hajji, H.; Bouzid, K.; Abdelmoula, J.; Munoz, F.; Mahouachi, M.; Atti, N. Effects of Rosmarinus officinalis L. as essential oils or in form of leaves supplementation on goat’s production and metabolic statute. Trop. Anim. Health Prod. 2015, 47, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Soltan, Y.A.; Natel, A.S.; Araujo, R.C.; Morsy, A.S.; Abdalla, A.L. Progressive adaptation of sheep to a microencapsulated blend of essential oils: Ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Anim. Feed Sci. Technol. 2018, 237, 8–18. [Google Scholar] [CrossRef]
- Ünlü, H.B.; İpçak, H.H.; Kandemir, Ç.; Özdoğan, M.; Canbolat, Ö.N.D.E.R. Effects of oregano essential oil and capsicum extract on fattening, serum constituents, and rumen fermentation of lambs. S. Afr. J. Anim. Sci. 2021, 51, 172–179. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Lechniak, D.; Ślusarczyk, S.; Kolodziejski, P.; Patra, A.K.; Váradyová, Z.; Lisiak, D.; Vazirigohar, M.; Cieslak, A. Dietary Coleus amboinicus Lour. decreases ruminal methanogenesis and biohydrogenation, and improves meat quality and fatty acid composition in longissimus thoracis muscle of lambs. J. Animal. Sci. Biotechnol. 2022, 13, 5. [Google Scholar] [CrossRef]
- Yesilbag, D.; Biricik, H.; Cetin, I.; Kara, C.; Meral, Y.; Cengiz, S.S.; Orman, A.; Udum, D. Effects of juniper essential oil on growth performance, some rumen protozoa, rumen fermentation and antioxidant blood enzyme parameters of growing Saanen kids. J. Anim. Physiol. Anim. Nutr. 2017, 101, e67–e76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hang, S.; Zhu, H.; Zhong, S.; Mao, S.; Zhu, W. Effects of garlic oil on milk fatty acid profile and lipogenesis-related gene expression in mammary gland of dairy goats. J. Sci. Food Agric. 2013, 93, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Mao, S.; Zhu, W. Effects of ruminal infusion of garlic oil on fermentation dynamics, fatty acid profile and abundance of bacteria involved in biohydrogenation in rumen of goats. Asian Aust. J. Anim. Sci. 2012, 25, 962. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Mean | Median | Minimum | Maximum | SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Features | NC | Control | Eos | Control | Eos | Control | Eos | Control | Eos | Control | Eos |
| Concentrate, g/kg DM | 140 | 479.9 | 479.9 | 500.0 | 500.0 | 210.0 | 210.0 | 790.0 | 790.0 | 172.3 | 172.3 |
| Forage, g/kg DM | 140 | 520.1 | 520.1 | 500.0 | 500.0 | 100.0 | 100.0 | 900.0 | 900.0 | 172.3 | 172.3 |
| DM, g/kg DM | 131 | 863.1 | 864.1 | 896.0 | 896.0 | 455.0 | 455.0 | 973.0 | 989.0 | 105.5 | 106.9 |
| OM, g/kg DM | 62 | 913.1 | 914.8 | 912.0 | 912.0 | 808.0 | 808.0 | 949.0 | 972.0 | 24.1 | 26.2 |
| CP, g/kg DM | 106 | 148.4 | 147.4 | 149.5 | 150.0 | 80.0 | 80.0 | 259.0 | 253.0 | 33.1 | 30.7 |
| EE, g/kg DM | 93 | 29.1 | 29.4 | 30.0 | 30.1 | 2.6 | 2.6 | 63.0 | 63.0 | 12.1 | 12.2 |
| NDF, g/kg DM | 102 | 400.8 | 400.8 | 397.3 | 397.3 | 118.2 | 118.2 | 594.0 | 594.0 | 99.6 | 99.7 |
| ADF, g/kg DM | 105 | 220.8 | 220.9 | 225.0 | 225.0 | 50.4 | 50.4 | 382.3 | 382.3 | 62.2 | 62.2 |
| Starch, g/kg DM | 21 | 225.1 | 225.1 | 193.0 | 193.0 | 33.0 | 33.0 | 405.0 | 405.0 | 110.9 | 110.9 |
| Ca, g/kg DM | 79 | 9.46 | 9.49 | 8.0 | 8.0 | 1.0 | 1.0 | 24.3 | 24.3 | 4.93 | 4.91 |
| P, g/kg DM | 79 | 5.24 | 5.23 | 4.2 | 4.2 | 1.0 | 1.0 | 14.5 | 14.5 | 2.83 | 2.82 |
| ME, Mcal/kg DM | 64 | 2.77 | 2.74 | 2.51 | 2.51 | 1.48 | 1.48 | 4.59 | 4.54 | 1.02 | 1.03 |
| Eos, mg/kg DM | 164 | - | 1452 | - | 500 | - | 10 | - | 40,000 | - | 3844 |
| Duration, days | 162 | 71 | 69 | 14 | 288 | 42 | |||||
| Item | N (NC) | Heterogeneity | Egger Test 1 | ||||
|---|---|---|---|---|---|---|---|
| Control Means (SD) | WMD (95 % CI) | p-Value | p-Value | I2 (%) | p-Value | ||
| DMI, kg/d | 35 (76) | 1.146 (0.302) | 0.021 (0.013; 0.030) | <0.001 | 0.115 | 17.27 | 0.245 |
| Digestibility, g/kg of DM | |||||||
| DMD | 23 (46) | 652.4 (78.8) | 14.11 (9.50; 18.72) | <0.001 | <0.001 | 99.24 | 0.073 |
| OMD | 20 (35) | 662.5 (81.4) | 8.81 (0.08; 17.54) | 0.048 | <0.001 | 99.31 | 0.080 |
| CPD | 26 (49) | 662.8 (93.1) | 12.93 (6.64; 19.21) | <0.001 | <0.001 | 99.64 | 0.092 |
| EED | 9 (18) | 631.6 (108.5) | 3.13 (−21.32; 27.58 | 0.802 | <0.001 | 99.86 | 0.775 |
| NDFD | 25 (48) | 504.2 (118.6) | 13.00 (3.72; 22.28) | 0.006 | <0.001 | 99.87 | 0.116 |
| ADFD | 17 (34) | 409.5 (123.2) | 31.04 (16.51; 45.57) | <0.001 | <0.001 | 99.74 | 0.066 |
| Item | N (NC) | Heterogeneity | Egger Test 1 | ||||
|---|---|---|---|---|---|---|---|
| Control Means (SD) | WMD (95 % CI) | p-Value | p-Value | I2 (%) | p-Value | ||
| ADG, kg/d | 21 (51) | 0.224 (0.08) | 0.008 (0.000; 0.016) | 0.037 | <0.001 | 62.34 | 0.537 |
| FCR, kg/kg | 13 (33) | 6.54 (3.61) | −0.111 (−0.220; −0.003) | 0.045 | 0.129 | 22.26 | 0.075 |
| Carcass characteristics | |||||||
| HCW, kg | 12 (24) | 19.68 (5.17) | −0.001 (−0.294; 0.292) | 0.996 | 0.113 | 28.87 | 0.906 |
| HCY, % | 11 (23) | 48.30 (4.51) | 0.552 (−0.022; 1.126) | 0.049 | 0.110 | 27.83 | 0.306 |
| CCW, kg | 8 (17) | 17.80 (5.81) | −0.160 (−0.433; 0.113) | 0.248 | 0.184 | 23.88 | 0.619 |
| BFT, mm | 6 (12) | 2.27 (1.11) | −0.033 (−0.152; 0.085) | 0.583 | 0.412 | 3.39 | 0.062 |
| LMA, cm2 | 6 (11) | 15.07 (4.70) | 2.074 (0.674; 3.474) | 0.004 | <0.001 | 85.01 | 0.839 |
| Item | N (NC) | Heterogeneity | Egger Test 1 | ||||
|---|---|---|---|---|---|---|---|
| Control Means (SD) | WMD (95 % CI) | p-Value | p-Value | I2 (%) | p-Value | ||
| pH | 31 (78) | 6.25 (0.33) | 0.00 (−0.037; −0.038) | 0.985 | <0.001 | 71.86 | 0.839 |
| NH3-N, mg/dL | 29 (69) | 19.40 (8.25) | −0.310 (−0.60; −0.02) | 0.038 | <0.001 | 62.58 | 0.241 |
| SCFA, mol/100 mol | |||||||
| Acetate | 30 (73) | 5.04 (11.80) | 0.165 (−0.71; 1.04) | 0.713 | <0.001 | 94.90 | 0.212 |
| Propionate | 30 (73) | 21.96 (6.28) | 0.726 (0.20; 1.25) | 0.006 | <0.001 | 85.88 | 0.223 |
| Butyrate | 30 (73) | 11.53 (3.68) | 0.050 (−0.24; 0.34) | 0.743 | <0.001 | 83.46 | 0.412 |
| Protozoa, ×105/mL | |||||||
| Total | 14 (34) | 7.59 (3.62) | −1.426 (−1.85; −1.00) | <0.001 | <0.001 | 97.91 | 0.268 |
| Entodinium | 6 (16) | 5.51 (3.14) | −0.008 (−0.05; 0.03) | 0.687 | <0.001 | 80.97 | 0.522 |
| Diplodidium | 4 (11) | 0.49 (0.33) | −0.107 (−0.23; 0.02) | 0.094 | 0.086 | 40.772 | 0.177 |
| Isotrichae | 4 (11) | 0.31 (0.08) | 0.021 (−0.05; 0.09) | 0.574 | 0.240 | 21.31 | 0.074 |
| Epidinium | 3 (7) | 0.85 (0.36) | −0.12 (−0.17; −0.08) | <0.001 | 0.474 | 0.00 | NA |
| Microbial population, per mL of ruminal fluid | |||||||
| Total bacteria, ×1010 | 8 (17) | 6.61 (3.24) | 0.046 (−0.12; 0.21) | 0.579 | <0.001 | 71.65 | 0.353 |
| R. flavefaciens, ×108 | 6 (11) | 9.99 (6.46) | 0.43 (0.013; 0.86) | 0.043 | <0.001 | 81.33 | 0.741 |
| R. albus, ×107 | 4 (8) | 7.70 (1.55) | 0.34 (−0.32; 0.99) | 0.311 | <0.001 | 93.94 | NA |
| F. succinogenes, ×105 | 6 (11) | 4.99 (2.51) | −0.42 (−0.96; 0.12) | 0.129 | <0.001 | 94.18 | 0.082 |
| Methanogens, ×107 | 6 (12) | 6.319 (2.77) | −0.60 (−0.88; −0.33) | <0.001 | <0.001 | 83.88 | 0.065 |
| CH4, L/d | 7 (13) | 32.66 (11.71) | −3.93 (−4.68; −3.19) | <0.001 | 0.352 | 9.34 | 0.789 |
| Item | N (NC) | Heterogeneity | Egger Test 1 | ||||
|---|---|---|---|---|---|---|---|
| Control Means (SD) | WMD (95 % CI) | p-Value | p-Value | I2 (%) | p-Value | ||
| Blood metabolites, mg/dL | |||||||
| Urea | 21 (44) | 39.07 (15.32) | −0.688 (−1.206; −0.170) | 0.009 | 0.103 | 21.91 | 0.978 |
| Glucose | 24 (52) | 62.52 (18.91) | 0.587 (−0.266; 1.440) | 0.178 | <0.001 | 79.74 | 0.306 |
| NEFA, mmol/L | 6 (12) | 0.361 (0.16) | −0.027 (−0.053; −0.002) | 0.034 | <0.001 | 73.76 | 0.616 |
| BHB, mmol/L | 3 (8) | 0.446 (0.15) | −0.020 (−0.033; −0.007) | 0.003 | 0.189 | 29.98 | NA |
| Albumin | 17 (32) | 4.94 (1.05) | 0.029 (−0.003; 0.061) | 0.078 | 0.280 | 11.70 | 0.063 |
| Globulin | 13 (24) | 5.99 (1.81) | 0.003 (−0.088; 0.093) | 0.953 | 0.119 | 29.28 | 0.253 |
| Protein total | 19 (28) | 13.31 (2.71) | −0.104 (−0.220; 0.012) | 0.080 | 0.138 | 48.41 | 0.305 |
| Cholesterol | 20 (45) | 114.30 (30.6) | −5.789 (−8.651; −2.926) | <0.001 | <0.001 | 86.83 | 0.936 |
| Triglycerides | 16 (37) | 29.90 (10.18) | −2.310 (−3.667; −0.954) | <0.001 | <0.001 | 98.70 | 0.073 |
| Thyroxine, ng/mL | 3 (6) | 79.05 (4.33) | 7.06 (5.51; 8.61) | <0.001 | 0.678 | 0.00 | NA |
| Antioxidant status | |||||||
| MDA, ng/mL | 5 (9) | 164.40 (92.50) | −3.88 (−8.48; 0.718) | 0.098 | 0.521 | 0.00 | NA |
| CAT, ng/mL | 4 (7) | 1.27 (0.42) | 0.204 (0.13; 0.28) | <0.001 | 0.699 | 0.00 | NA |
| SOD, ng/mL | 6 (12) | 1.12 (0.76) | 0.037 (0.004; 0.07) | 0.028 | 0.149 | 31.26 | 0.642 |
| GPx, nmol/mL | 7 (14) | 57.20 (39.30) | 2.65 (−17.85; 23.15) | 0.800 | <0.001 | 99.98 | 0.346 |
| TAC, U/mL | 4 (10) | 6.01 (2.45) | 0.749 (0.183; 1.31) | 0.009 | <0.001 | 85.01 | 0.811 |
| Item | N (NC) | Heterogeneity | Egger Test 1 | ||||
|---|---|---|---|---|---|---|---|
| Control Means (SD) | WMD (95 % CI) | p-Value | p-Value | I2 (%) | p-Value | ||
| pH 24 h | 15 (26) | 5.824 (0.37) | −0.012 (−0.056; 0.033) | 0.604 | <0.001 | 77.13 | 0.080 |
| CL, g/100 g | 8 (17) | 25.48 (9.02) | −0.617 (−1.174; −0.061) | 0.030 | 0.760 | 0.00 | 0.369 |
| ShF, kgf/cm2 | 4 (8) | 4.027 (0.20) | −0.171 (−0.337; −0.009) | 0.038 | 0.993 | 0.00 | NA |
| Meat color | |||||||
| Lightness (L*) | 17 (31) | 40.808 (4.69) | −0.207 (−0.505; 0.091) | 0.173 | 0.159 | 20.61 | 0.240 |
| Redness (a*) | 17 (31) | 16.701 (12.29) | 0.123 (−0.133; 0.378) | 0.347 | 0.132 | 22.57 | 0.359 |
| Yellowness (b*) | 15 (29) | 6.445 (4.33) | −0.316 (−0.481; −0.151) | <0.001 | 0.453 | 0.75 | 0.860 |
| Lipid oxidation (mg MDA/kg of meat) | |||||||
| Day 1 | 12 (24) | 0.435 (0.38) | −0.029 (−0.045; −0.014) | <0.001 | 0.493 | 0.26 | 0.069 |
| Day 3 | 5 (8) | 1.591 (1.12) | −0.368 (−0.650; −0.085) | 0.011 | 0.005 | 65.45 | NA |
| Day 6 | 9 (20) | 2.887 (1.37) | −0.551 (−0.816; −0.286) | <0.001 | <0.001 | 75.02 | 0..278 |
| Day 9 | 3 (9) | 2.180 (0.76) | −0.189 (−0.337; −0.041) | 0.012 | 0.727 | 0.00 | NA |
| Day 14 | 8 (16) | 5.888 (2.19) | −1.607 (−2.354; −0.859) | <0.001 | <0.001 | 89.24 | 0.094 |
| Chemical composition, g/100 g of DM | |||||||
| Moisture | 9 (18) | 74.141 (1.48) | 0.042 (−0.168; 0.251) | 0.696 | 0.406 | 4.15 | 0.288 |
| Protein | 9 (18) | 25.28 (13.78) | −0.780 (−1.050; −0.509) | 0.061 | 0.198 | 31.55 | 0.112 |
| Fat | 11 (20) | 5.72 (4.70) | 0.055 (−0.140; 0.251) | 0.578 | 0.110 | 30.07 | 0.223 |
| Ash | 8 (16) | 1.797 (1.59) | −0.001 (−0.006; 0.004) | 0.645 | 0.702 | 0.00 | 0.740 |
| Bacterial counts of raw lamb meat after 7 days of storage, expressed as log CFU/g | |||||||
| TVC | 8 (11) | 3.957 (1.98) | −0.605 (−0.857; −0.353) | <0.001 | <0.001 | 68.03 | 0.480 |
| ENT | 6 (9) | 1.079 (1.52) | −0.139 (−0.233; −0.045) | 0.004 | 0.805 | 0.00 | NA |
| PSY | 4 (7) | 3.084 (0.91) | −0.600 (−0.867; −0.332) | <0.001 | 0.941 | 0.00 | NA |
| MY | 4 (7) | 1.411 (0.45) | −0.275 (−0.537; −0.014) | 0.039 | 0.697 | 0.00 | NA |
| Item | N (NC) | Heterogeneity | Egger Test 1 | ||||
|---|---|---|---|---|---|---|---|
| Control Means (SD) | WMD (95 % CI) | p-Value | p-Value | I2 (%) | p-Value | ||
| Milk yield, kg/d | 18 (37) | 1.18 (0.76) | 0.113 (0.077; 0.148) | <0.001 | <0.001 | 87.35 | 0.067 |
| FE, kg/kg | 10 (21) | 0.776 (0.39) | 0.039 (0.022; 0.056) | <0.001 | 0.119 | 29.56 | 0.522 |
| Milk composition, g/100 g | |||||||
| Fat | 19 (40) | 4.426 (1.33) | −0.003 (−0.099; 0.09) | 0.959 | <0.001 | 93.47 | 0.079 |
| Protein | 19 (40) | 3.947 (1.15) | 0.059 (0.005; 0.113) | 0.031 | <0.001 | 91.08 | 0.424 |
| Lactose | 17 (36) | 4.811 (0.96) | 0.100 (0.048; 0.152) | <0.001 | <0.001 | 86.74 | 0.269 |
| SCC, ×103 cell/mL | 6 (14) | 3.081 (1.50) | −0.916 (−1.37; −0.46) | <0.001 | <0.001 | 97.05 | 0.480 |
| Urea, mg/dL | 3 (6) | 40.74 (5.46) | −7.73 (−11.77; −3.70) | <0.001 | 0.043 | 56.33 | NA |
| pH | 3 (6) | 6.62 (0.0465) | 0.003 (−0.028; 0.034) | 0.845 | 0.989 | 0.00 | NA |
| Parameter | Covariates | QM | Df | p-Value | R2 (%) |
|---|---|---|---|---|---|
| Average daily gain (ADG) | Essential oils dose | 0.002 | 1 | 0.968 | 0.0 |
| Supplementation period | 0.824 | 1 | 0.364 | 0.0 | |
| Primary Bioactive Compound | 6.56 | 11 | 0.834 | 0.0 | |
| Dry matter digestibility (DMD) | Essential oils dose | 1.44 | 1 | 0.230 | 0.0 |
| Supplementation period | 3.31 | 1 | 0.069 | 3.23 | |
| Primary bioactive compound | 36.01 | 10 | <0.001 | 17.16 | |
| Organic matter digestibility (OMD) | Essential oils dose | 1.99 | 1 | 0.158 | 5.86 |
| Supplementation period | 0.258 | 1 | 0.612 | 0.0 | |
| Primary bioactive compound | 6.63 | 8 | 0.577 | 0.0 | |
| Crude protein digestibility (CPD) | Essential oils dose | 0.039 | 1 | 0.842 | 6.61 |
| Supplementation period | 0.479 | 1 | 0.489 | 0.0 | |
| Primary bioactive compound | 19.281 | 11 | 0.066 | 0.0 | |
| Neutral detergent fiber digestibility (NDFD) | Essential oils dose | 3.23 | 1 | 0.072 | 7.15 |
| Supplementation period | 2.35 | 1 | 0.125 | 0.0 | |
| Primary bioactive compound | 26.55 | 11 | 0.005 | 7.97 | |
| Acid detergent fiber digestibility (ADFD) | Essential oils dose | 2.44 | 1 | 0.118 | 4.27 |
| Supplementation period | 0.38 | 1 | 0.541 | 9.29 | |
| Primary bioactive compound | 38.50 | 9 | <0.001 | 62.55 | |
| Ruminal pH | Essential oils dose | 0.15 | 1 | 0.696 | 0.0 |
| Supplementation period | 8.55 | 1 | 0.003 | 3.52 | |
| Primary bioactive compound | 56.31 | 16 | <0.001 | 56.20 | |
| Ammonia nitrogen (NH3-N) | Essential oils dose | 8.30 | 1 | 0.004 | 16.39 |
| Supplementation period | 2.19 | 1 | 0.139 | 0.0 | |
| Primary bioactive compound | 48.30 | 15 | <0.001 | 40.93 | |
| Acetate | Essential oils dose | 0.03 | 1 | 0.853 | 0.0 |
| Supplementation period | 3.26 | 1 | 0.071 | 5.78 | |
| Primary bioactive compound | 44.27 | 16 | <0.001 | 7.63 | |
| Propionate | Essential oils dose | 0.56 | 1 | 0.452 | 2.99 |
| Supplementation period | 1.72 | 1 | 0.189 | 0.0 | |
| Primary bioactive compound | 28.69 | 16 | 0.026 | 18.77 | |
| Butyrate | Essential oils dose | 1.15 | 1 | 0.284 | 3.98 |
| Supplementation period | 0.002 | 1 | 0.962 | 8.65 | |
| Primary bioactive compound | 32.71 | 16 | 0.008 | 40.95 | |
| Total ruminal protozoa | Essential oils dose | 2.43 | 1 | 0.119 | 0.0 |
| Supplementation period | 8.89 | 1 | 0.003 | 7.3 | |
| Primary bioactive compound | 31.43 | 8 | <0.001 | 42.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorantes-Iturbide, G.; Orzuna-Orzuna, J.F.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Lee-Rangel, H.A. Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality. Vet. Sci. 2022, 9, 475. https://doi.org/10.3390/vetsci9090475
Dorantes-Iturbide G, Orzuna-Orzuna JF, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, Lee-Rangel HA. Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality. Veterinary Sciences. 2022; 9(9):475. https://doi.org/10.3390/vetsci9090475
Chicago/Turabian StyleDorantes-Iturbide, Griselda, José Felipe Orzuna-Orzuna, Alejandro Lara-Bueno, Germán David Mendoza-Martínez, Luis Alberto Miranda-Romero, and Héctor Aarón Lee-Rangel. 2022. "Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality" Veterinary Sciences 9, no. 9: 475. https://doi.org/10.3390/vetsci9090475
APA StyleDorantes-Iturbide, G., Orzuna-Orzuna, J. F., Lara-Bueno, A., Mendoza-Martínez, G. D., Miranda-Romero, L. A., & Lee-Rangel, H. A. (2022). Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality. Veterinary Sciences, 9(9), 475. https://doi.org/10.3390/vetsci9090475





