Metagenomic Characterization and Volatile Compounds Determination in Rumen from Saanen Goat Kids Fed Olive Leaves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Sampling
2.2. DNA Extraction and Illumina MiniSeq Sequencing
2.3. Evaluation of Volatile Compounds in Rumen
2.4. Bioinformatic and Statistical Analysis
3. Results
3.1. Acquisition of Metagenomic Data and Analysis
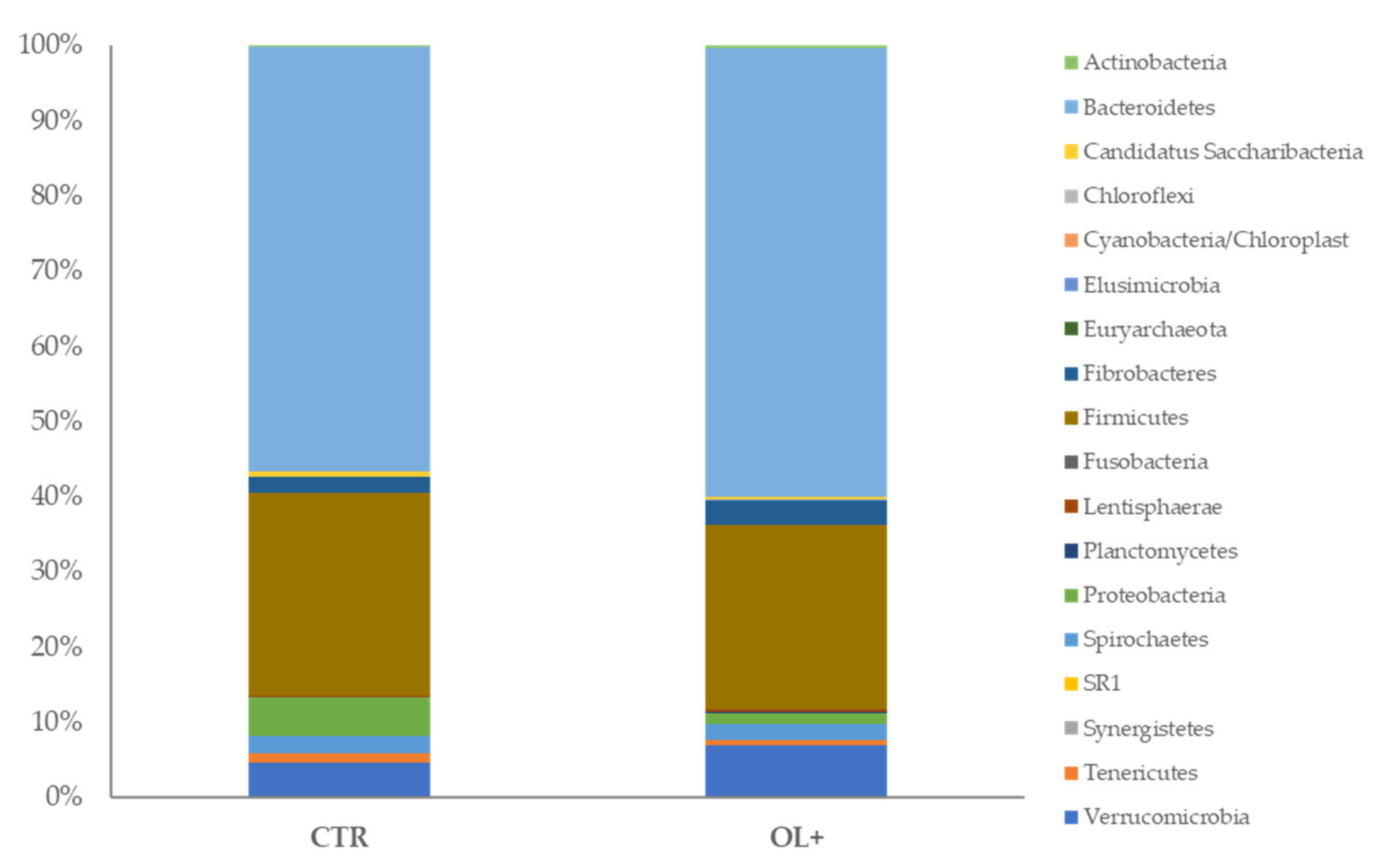
3.2. Effects of Diet on Microbial Community Composition
3.3. Identification of Volatile Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sirohi, S.; Singh, N.; Dagar, S.; Puniya, A. Molecular tools for deciphering the microbial community structure and diversity in rumen ecosystem. Appl. Microbiol. Biotechnol. 2012, 95, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, G.; Denman, S.E.; McSweeney, C.S. Sample Processing Methods Impacts on Rumen Microbiome. Front. Microbiol. 2019, 10, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xu, Q.; Kong, F.; Yang, Y.; Wu, D.; Mishra, S.; Li, Y. Exploring the goat rumen microbiome from seven days to two years. PLoS ONE 2016, 11, e0154354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmuthuge, N.; Guan, L.L. Understanding host-microbial interactions in rumen: Searching the best opportunity for microbiota manipulation. J. Anim. Sci. Biotechnol. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O.; Monteils, V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol. 2014, 116, 245–257. [Google Scholar] [CrossRef]
- Pitta, D.W.; Kumar, S.; Vecchiarelli, B.; Baker, L.D.; Ferguson, J.D.; Thomsen, N.; Shirley, D.J.; Bittinger, K. Temporal dynamics in the ruminal microbiome of dairy cows during the transition period. J. Anim. Sci. 2014, 92, 4014–4022. [Google Scholar] [CrossRef]
- Zhu, Z.; Kristensen, L.; Difford, G.F.; Poulsen, M.; Noel, S.J.; Abu Al-Soud, W.; Sorensen, S.J.; Lassen, J.; Lovendahl, P.; Hojberg, O. Changes in rumen bacterial and archaeal communities over the transition period in primiparous Holstein dairy cows. J. Dairy Sci. 2018, 101, 9847–9862. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Yanez-Ruiz, D.R.; Abecia, L.; Newbold, C.J. Manipulating rumen microbiome and fermentation through interventions during early life: A review. Front. Microbiol. 2015, 6, 1133. [Google Scholar] [CrossRef] [Green Version]
- Tapio, I.; Fischer, D.; Blasco, L.; Tapio, M.; Wallace, R.J.; Bayat, A.R.; Ventto, L.; Kahala, M.; Negussie, E.; Shingfield, K.J.; et al. Taxon abundance, diversity, co-occurrence and network analysis of the ruminal microbiota in response to dietary changes in dairy cows. PLoS ONE 2017, 12, e0180260. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Koziel, J.A.; Davis, J.; Lo, Y.C.; Xin, H. Characterization of volatile organic compounds and odors by in-vivo sampling of beef cattle rumen gas, by solid-phase microextraction, and gas chromatography–mass spectrometry–olfactometry. Anal. Bioanal. Chem. 2006, 386, 1791–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, M.; Wojnarowski, K.; Cholewińska, P.; Szeligowska, N.; Bawej, M.; Pacoń, J. Selected Alternative Feed Additives Used to Manipulate the Rumen Microbiome. Animals 2021, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Martín-García, A.I.; Moumen, A.; Yáñez-Ruiz, D.R.; Molina-Alcaide, E. Chemical composition and nutrients availability for goats and sheep of two-stage olive cake and olive leaves. Anim. Feed Sci. Technol. 2003, 107, 61–74. [Google Scholar] [CrossRef]
- Innosa, D.; Bennato, F.; Ianni, A.; Martino, C.; Grotta, L.; Pomilio, F.; Martino, G. Influence of olive leaves feeding on chemical-nutritional quality of goat ricotta cheese. Eur. Food Res. Technol. 2020, 246, 923–930. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Zervas, G. The effect of dietary inclusion of olive tree leaves and grape marc on the content of conjugated linoleic acid and vaccenic acid in the milk of dairy sheep and goats. J. Dairy Res. 2008, 75, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Innosa, D.; Oliva, E.; Bennato, F.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Sergi, M.; Martino, G. Effect of olive leaves feeding on phenolic composition and lipolytic volatile profile in goat milk. J. Dairy Sci. 2021, 104, 8835–8845. [Google Scholar] [CrossRef]
- Shakeri, P.; Durmic, Z.; Vadhanabhuti, J.; Vercoe, P.E. Products derived from olive leaves and fruits can alter in vitro ruminal fermentation and methane production. J. Sci. Food Agric. 2016, 97, 1367–1372. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Martín-García, A.I.; Moumen, A.; Carro, M.D. Ruminal fermentation, microbial growth and amino acid flow in single-flow continuous culture fermenters fed a diet containing olive leaves. J. Anim. Physiol. Anim. Nutr. 2009, 94, 227–236. [Google Scholar] [CrossRef]
- Ozturk, H.; Demirtas, A.; Salgirli, Y.; Pekcan, M.; Emre, B.; Fidanci, U.R. Effects of olive leaf extract on rumen microbial fermentation in in vitro semi-continuous culture system (RUSITEC). Ankara Üniv. Vet. Fak. Derg. 2012, 59, 17–21. [Google Scholar]
- Biscarini, F.; Palazzo, F.; Castellani, F.; Masetti, G.; Grotta, L.; Cichelli, A.; Martino, G. Rumen microbiome in dairy calves fed copper and grape pomace dietary supplementations: Composition and predicted functional profile. PLoS ONE 2018, 13, e0205670. [Google Scholar] [CrossRef] [PubMed]
- European Union Council. Directive 2008/119/EC of 18 December 2008 laying down minimum standards for the protection of calves. Off. J. Eur. Union 2009, L 10, 7–13. Available online: http://data.europa.eu/eli/dir/2008/119/oj (accessed on 6 October 2020).
- European Union Council. Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union 2009, L 303, 1–30. Available online: http://data.europa.eu/eli/reg/2009/1099/oj (accessed on 6 October 2020).
- Innosa, D.; Ianni, A.; Faccia, M.; Martino, C.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Martino, G. Physical, Nutritional, and Sensory Properties of Cheese Obtained from Goats Fed a Dietary Supplementation with Olive Leaves. Animals 2020, 10, 2238. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagent, Procedures and Some Applications); Agriculture Handbook No. 379; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Spinhirne, J.P.; Koziel, J.A.; Chirase, N.K. Characterizing volatile fatty acids and other gases in a rumen closed in vitro fermentation system using solid phase microextraction. Trans. ASABE 2003, 46, 585. [Google Scholar] [CrossRef]
- Ianni, A.; Iannaccone, M.; Martino, C.; Innosa, D.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of dairy cows: Effects on chemical composition, nutritional quality and volatile profile of Giuncata cheese. Int. Dairy J. 2019, 94, 65–71. [Google Scholar] [CrossRef]
- Github.com. Available online: https://github.com/swiftbiosciences/16S-SNAPP (accessed on 6 October 2020).
- Github.com. Available online: https://github.com/vegandevs/vegan (accessed on 6 October 2020).
- Github.com. Available online: https://github.com/gavinsimpson/ggvegan/ (accessed on 6 October 2020).
- Github.com. Available online: https://github.com/tidyverse/ggplot2/ (accessed on 6 October 2020).
- St-Pierre, B.; Cersosimo, L.M.; Ishaq, S.L.; Wright, A.D.G. Toward the identification of methanogenic archaeal groups as targets of methane mitigation in livestock animals. Front. Microbiol. 2015, 6, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonesi, P.; Conte, G.; Severgnini, M.; Turri, F.; Monni, A.; Capra, E.L.; Rapetti, S.; Colombini, S.; Chessa, S.; Battelli, G.; et al. Evaluation of the effects of different diets on microbiome diversity and fatty acid composition of rumen liquor in dairy goat. Animal 2018, 12, 1856–1866. [Google Scholar] [CrossRef]
- Cunha, I.S.; Barreto, C.C.; Costa, O.Y.A.; Bomfim, M.A.; Castro, A.P.; Kruger, R.H.; Quirino, B.F. Bacteria and Archaea community structure in the rumen microbiome of goats (Capra hircus) from the semiarid region of Brazil. Anaerobe 2011, 17, 118–124. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.D.G.; Kraft, J. Rumen bacterial communities shift across a lactation in Holstein, Jersey and Holstein × Jersey dairy cows and correlate to rumen function, bacterial fatty acid composition and production parameters. FEMS Microbiol. Ecol. 2016, 92, fiw059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.H.; Dao, T.K.; Nguyen, K.H.V.; Le, N.G.; Nguyen, T.M.P.; Le, T.L.; Phung, T.N.; van Straalen, N.M.; Roelofs, D.; Truong, N.H. Metagenomic analysis of bacterial community structure and diversity of lignocellulolytic bacteria in Vietnamese native goat rumen. Asian Australas. J. Anim. Sci. 2018, 31, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Y.; Yan, H.; Wang, X.; Qu, L.; Chen, Y. Rumen bacterial diversity of 80 to 110-day-old goats using 16S rRNA sequencing. PLoS ONE 2015, 10, e011781. [Google Scholar] [CrossRef]
- Mao, S.Y.; Huo, W.J.; Zhu, W.Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 2016, 18, 525–541. [Google Scholar] [CrossRef]
- Potu, R.B.; AbuGhazaleh, A.a.; Hastings, D.; Jones, K.; Ibrahim, S.a. The effect of lipid supplements on ruminal bacteria in continuous culture fermenters varies with the fatty acid composition. J. Microbiol. 2011, 49, 216–223. [Google Scholar] [CrossRef]
- Vargas-Bello-Perez, E.; Cancino-Padilla, N.; Romero, J.; Garnsworthy, P.C. Quantitative analysis of ruminal bacterial populations involved in lipid metabolism in dairy cows fed different vegetable oils. Animal 2016, 10, 1821–1828. [Google Scholar] [CrossRef]
- Lourenco, M.; Ramos-Morales, E.; Wallace, R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, 832–833. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, C.V.; Picoloto, R.S.; Cichoski, A.J.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Da Croce, D.M.; Barin, J.S. Olive leaves offer more than phenolic compounds—Fatty acids and mineral composition of varieties from Southern Brazil. Ind. Crops Prod. 2015, 71, 122–127. [Google Scholar] [CrossRef]
- Paillard, D.; McKain, N.; Rincon, M.T.; Shingfield, K.J.; Givens, D.I.; Wallace, R.J. Quantification of ruminal Clostridium proteoclasticum by real-time PCR using a molecular beacon approach. J. Appl. Microbiol. 2007, 103, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Boeckaert, C.; Vlaeminck, B.; Fievez, V.; Maignien, L.; Dijkstra, J.; Boon, N. Accumulation of trans C18:1 fatty acids in the rumen after dietary algal supplementation is associated with changes in the Butyrivibrio community. Appl. Environ. Microbiol. 2008, 74, 6923–6930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, C.D.; Pacheco, D.M.; Kelly, W.J.; Leahy, S.C.; Li, D.; Kopečný, J.; Attwood, G.T. Reclassification of Clostridium proteoclasticum as Butyrivibrio proteoclasticus comb. nov., a butyrateproducing ruminal bacterium. Int. J. Syst. Evol. Microbiol. 2008, 58, 2041–2045. [Google Scholar] [CrossRef] [Green Version]
- Pallara, G.; Buccioni, A.; Pastorelli, R.; Minieri, S.; Mele, M.; Rapaccini, S.; Messini, A.; Pauselli, M.; Servili, M.; Giovannetti, L.; et al. Effect of stoned olive pomace on rumen microbial communities and polyunsaturated fatty acids biohydrogenation: An in vitro study. BMC Vet. Res. 2014, 10, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbeddou, S.; Rischkowsky, B.; Richter, E.K.; Hess, H.D.; Kreuzer, M. Modification of milk fatty acid composition by feeding forages and agro-industrial by-products from dry areas to Awassi sheep. J. Dairy Sci. 2011, 94, 4657–4668. [Google Scholar] [CrossRef] [PubMed]
- Bekele, A.Z.; Koike, S.; Kobayashi, Y. Genetic diversity and diet specificity of ruminal prevotella revealed by 16s rrna gene-based analysis. FEMS Microbiol. Lett. 2010, 305, 49–57. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Q.; Wang, L.; Wang, J.; Guo, W.; Zhou, M. The impact of diet on the composition and relative abundance of rumen microbes in goat. Asian-Australas J. Anim. Sci. 2016, 30, 531–537. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, W.; Mao, S. Impact of subacute ruminal acidosis on the diversity of liquid and solid-associated bacteria in the rumen of goats. J. Microbiol. Biotechnol. 2014, 30, 669–680. [Google Scholar] [CrossRef]
- Fernando, S.C.; Purvis, H.T.; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen Microbial Population Dynamics during Adaptation to a High-Grain Diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, C.S.; Palmer, B.; Krause, D.O.; Brooker, J.D. Rumen microbial ecology and physiology in sheep and goats fed a tannin containing diet. In Tannins in Livestock and Human Nutrition, Proceedings of the International Workshop, Adelaide, Australia, 31 May–2 June 1999; Australian Centre for International Agricultural Research: Canberra, Australia, 1999; pp. 140–145. [Google Scholar]
- Salama, Z.A.; Aboul-Enein, A.M.; Gaafar, A.A.; Asker, M.S.; Aly, H.F.; Ahmed, H.A. In-vitro antioxidant, antimicrobial and anticancer activities of banana leaves (Musa acuminata) and olive leaves (Olea europaea L.) as by-products. Res. J. Pharm. Technol. 2020, 13, 687–696. [Google Scholar] [CrossRef]
- Spinhirne, J.P.; Koziel, J.A.; Chirase, N.K. Sampling and analysis of volatile organic compounds in bovine breath by solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1025, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Chao, W.; Askew, E.; Roberts, D.; Wood, S.; Perkins, J. Oxidative stress in humans during work at moderate altitude. J. Nutr. 2019, 129, 2009–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| CTR | OL+ | |
|---|---|---|
| Ingredients (%) | ||
| Soybean, meal | 17.50 | 15.50 |
| Wheat, bran | 20.00 | 20.00 |
| Barley, meal | 32.00 | 27.00 |
| Corn, meal | 28.50 | 25.50 |
| Olive leaves | - | 10.00 |
| Vitamin and mineral | 2.00 | 2.00 |
| Chemical composition (%) | ||
| Dry matter (DM) | 89.10 | 88.50 |
| Ash 1, % | 5.10 | 5.26 |
| Ether extract (EE) 1, % | 3.25 | 3.36 |
| Crude protein (CP) 1, % | 18.20 | 17.95 |
| Neutral detergent fiber (NDF) 1, % | 12.55 | 13.32 |
| Acid detergent fiber (ADF) 1, % | 5.44 | 5.95 |
| Acid detergent lignin (ADL) 1, % | 1.16 | 1.39 |
| Starch 1, % | 48.59 | 43.88 |
| ME 1, MJ/kg | 7.47 | 7.18 |
| CTR | OL+ | |
|---|---|---|
| Starting reads | 236,831 ± 114,396 | 277,576 ± 48,923 |
| Trimmed | 220,392 ± 110,678 | 257,589 ± 47,028 |
| Filtered | 219,302 ± 110,140 | 256,299 ± 46,772 |
| Denoised R1 | 218,575 ± 109,722 | 255,226 ± 46,491 |
| Denoised R2 | 218,290 ± 109,463 | 254,648 ± 46,359 |
| Merged | 217,631 ± 109,077 | 253,679 ± 46,116 |
| Non-chimera | 1,955,481 ± 91,487 | 221,149 ± 42,834 |
| Reads for analysis (%) | 83 | 80 |
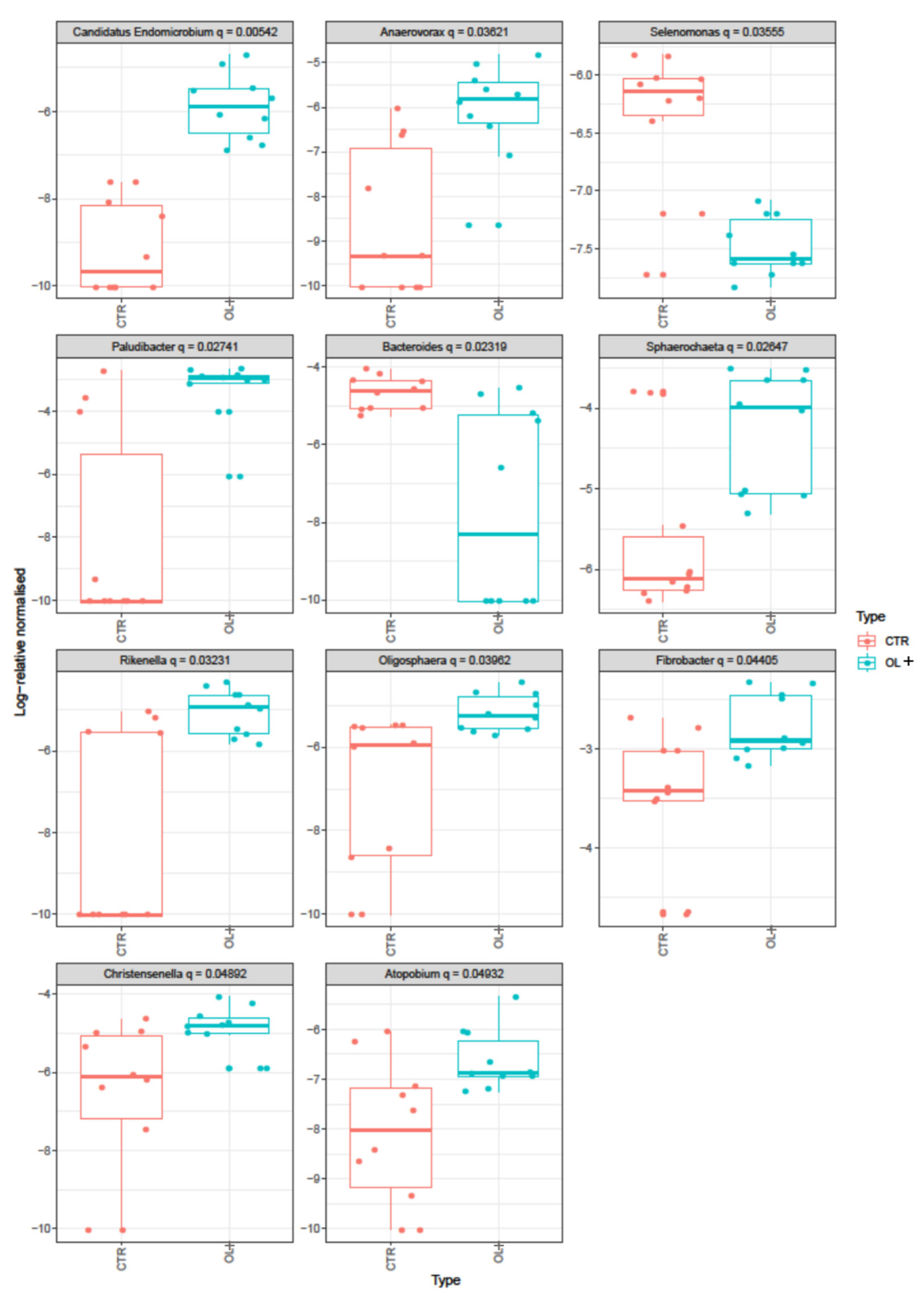
| Genus | Average | S.D. | Ratio | CTR | OL+ | Cumulative Sum | p-Value | q-Value (≤0.05) |
|---|---|---|---|---|---|---|---|---|
| Prevotella | 0.0691659 | 0.0399527 | 1.7312 | 9207.3 | 8482.8 | 0.1828 | 0.290 | |
| Subdivision5_genera_incertae_sedis | 0.0490198 | 0.0492473 | 0.9954 | 1759 | 3719.2 | 0.3124 | 0.023 | |
| Paraprevotella | 0.040015 | 0.0494914 | 0.8085 | 2459.2 | 790.5 | 0.4182 | 0.019 | |
| Oscillibacter | 0.0256097 | 0.0256221 | 0.9995 | 937.6 | 1385.1 | 0.4859 | 0.070 | |
| Ruminobacter | 0.0248105 | 0.0331442 | 0.7486 | 1207.4 | 326 | 0.5515 | 0.050 | |
| Anaerocella | 0.022674 | 0.0263719 | 0.8598 | 1033.3 | 33 | 0.6115 | 0.041 | |
| Paludibacter | 0.0202838 | 0.0106447 | 1.9055 | 259 | 1061.5 | 0.6651 | 0.003 | 0.027 |
| Fibrobacter | 0.0166172 | 0.0120337 | 1.3809 | 849.2 | 1502.7 | 0.709 | 0.010 | 0.044 |
| Anaerobacterium | 0.0131868 | 0.0117324 | 1.124 | 728 | 204.9 | 0.7439 | 0.082 | |
| Ruminococcus | 0.0122287 | 0.0090614 | 1.3495 | 584.9 | 991.6 | 0.7762 | 0.034 | |
| Anaeroplasma | 0.0096275 | 0.0118004 | 0.8159 | 453.2 | 270.3 | 0.8016 | 0.384 | |
| Sphaerochaeta | 0.0068604 | 0.0046886 | 1.4632 | 142.3 | 386.2 | 0.8198 | 0.004 | 0.026 |
| Treponema | 0.0067165 | 0.0040503 | 1.6583 | 574.2 | 623.6 | 0.8375 | 0.705 | |
| Butyrivibrio | 0.0059045 | 0.0060017 | 0.9838 | 373.7 | 266.2 | 0.8531 | 0.677 | |
| Acetobacteroides | 0.0053678 | 0.0087699 | 0.6121 | 38.4 | 224.9 | 0.8673 | 0.249 | |
| Saccharibacteria_genera_incertae_sedis | 0.0048374 | 0.0052325 | 0.9245 | 260.9 | 132.2 | 0.8801 | 0.406 | |
| Clostridium IV | 0.0044499 | 0.0049431 | 0.9002 | 129 | 268.9 | 0.8919 | 0.112 | |
| Lachnospiracea_incertae_sedis | 0.003994 | 0.0029685 | 1.3454 | 313.7 | 298 | 0.9024 | 0.520 | |
| Bacteroides | 0.0039234 | 0.0023095 | 1.6988 | 233.2 | 67.3 | 0.9128 | 0.003 | 0.023 |
| Succiniclasticum | 0.0036021 | 0.0045189 | 0.7971 | 174.2 | 56.6 | 0.9223 | 0.173 | |
| Christensenella | 0.0029613 | 0.0020312 | 1.4579 | 76.1 | 192.9 | 0.9302 | 0.013 | 0.049 |
| Mogibacterium | 0.0029331 | 0.0024578 | 1.1934 | 106.9 | 170.3 | 0.9379 | 0.151 | |
| Rikenella | 0.0029132 | 0.0018389 | 1.5842 | 44.4 | 165.7 | 0.9456 | 0.005 | 0.032 |
| Clostridium XlVa | 0.0022393 | 0.0020527 | 1.0909 | 29.3 | 118.9 | 0.9515 | 0.07 | |
| Saccharofermentans | 0.0021554 | 0.0012938 | 1.6659 | 121.8 | 168.3 | 0.9572 | 0.034 | |
| Pseudobutyrivibrio | 0.0020747 | 0.0014231 | 1.4579 | 128.9 | 60.7 | 0.9627 | 0.082 | |
| Oligosphaera | 0.0019913 | 0.0015864 | 1.2552 | 49.7 | 137 | 0.968 | 0.008 | 0.040 |
| Succinivibrio | 0.0018624 | 0.001527 | 1.2196 | 83.4 | 69.8 | 0.9729 | 0.733 | |
| Candidatus Endomicrobium | 0.0016373 | 0.0013349 | 1.2266 | 3.4 | 77.6 | 0.9772 | 0.0001 | 0.005 |
| Anaerovorax | 0.0013937 | 0.0010073 | 1.3836 | 12.3 | 71.3 | 0.9809 | 0.002 | 0.036 |
| Olsenella | 0.0011188 | 0.0006048 | 1.8497 | 44.4 | 75.4 | 0.9839 | 0.29 | |
| Flavonifractor | 0.0010989 | 0.0014461 | 0.7599 | 49.7 | 0.8 | 0.9868 | 0.49 | |
| Victivallis | 0.0010159 | 0.001484 | 0.6846 | 19.2 | 58.8 | 0.9895 | 0.069 | |
| Vampirovibrio | 0.0008977 | 0.0006995 | 1.2833 | 46.5 | 29.8 | 0.9918 | 0.650 | |
| Selenomonas | 0.0007026 | 0.0004087 | 1.7191 | 43 | 11.6 | 0.9937 | 0.003 | 0.036 |
| Atopobium | 0.0006991 | 0.0005682 | 1.2303 | 12.7 | 40.6 | 0.9956 | 0.014 | 0.049 |
| Elusimicrobium | 0.000696 | 0.0004842 | 1.4373 | 33.8 | 51.5 | 0.9974 | 0.211 | |
| Blautia | 0.0005036 | 0.0003512 | 1.434 | 21.6 | 33.7 | 0.9987 | 0.211 | |
| Intestinimonas | 0.0004819 | 0.0004932 | 0.9772 | 14.2 | 32.8 | 1 | 0.017 |
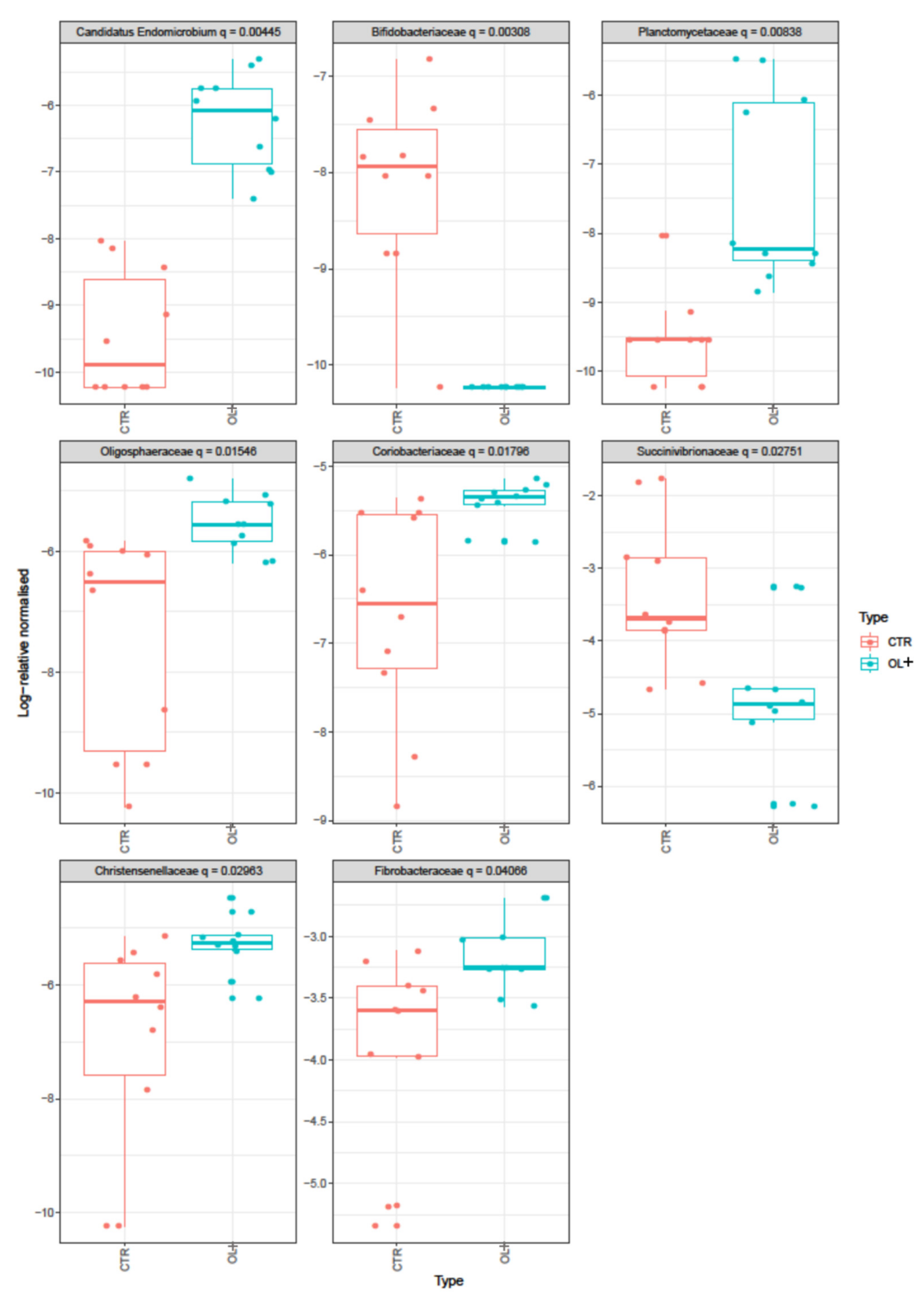
| Families | Average | S.D. | Ratio | CTR | OL+ | Cumulative Sum | p-Value | q-Value (≤ 0.05) |
|---|---|---|---|---|---|---|---|---|
| Prevotellaceae | 0.058200 | 0.035000 | 1.6624 | 10449 | 8134.1 | 0.2359 | 0.096 | |
| Subdivision5_genera_incertae_sedis | 0.031800 | 0.031900 | 0.9972 | 1563.9 | 3041.7 | 0.3648 | 0.023 | |
| Succinivibrionaceae | 0.026700 | 0.028900 | 0.9233 | 1699.2 | 347.1 | 0.4729 | 0.005 | 0.028 |
| Ruminococcaceae | 0.024600 | 0.017300 | 1.4234 | 4425.6 | 5237.3 | 0.5724 | 0.290 | |
| Porphyromonadaceae | 0.024500 | 0.021900 | 1.118 | 3670.3 | 4806.1 | 0.6716 | 0.112 | |
| Rikenellaceae | 0.017900 | 0.017000 | 1.057 | 1068.7 | 268.6 | 0.7443 | 0.140 | |
| Lachnospiraceae | 0.015400 | 0.010000 | 1.5428 | 1983 | 2245.7 | 0.8069 | 0.650 | |
| Fibrobacteraceae | 0.011200 | 0.008560 | 1.3092 | 684.2 | 1249.5 | 0.8523 | 0.010 | 0.041 |
| Spirochaetaceae | 0.007300 | 0.005480 | 1.3321 | 754.7 | 934.7 | 0.8819 | 0.290 | |
| Anaeroplasmataceae | 0.006220 | 0.007280 | 0.8532 | 383.9 | 248.8 | 0.9071 | 0.910 | |
| Acidaminococcaceae | 0.003170 | 0.005220 | 0.6065 | 191.6 | 50.5 | 0.9199 | 0.174 | |
| Clostridiales_Incertae Sedis XIII | 0.003160 | 0.002490 | 1.2672 | 115.1 | 223.9 | 0.9328 | 0.034 | |
| Saccharibacteria_genera_incertae_sedis | 0.003000 | 0.002850 | 1.0543 | 195.8 | 109.4 | 0.9449 | 0.571 | |
| Bacteroidaceae | 0.002530 | 0.001650 | 1.5293 | 185.7 | 72.6 | 0.9552 | 0.015 | |
| Christensenellaceae | 0.002150 | 0.001450 | 1.4783 | 71.7 | 165.2 | 0.9639 | 0.006 | 0.030 |
| Coriobacteriaceae | 0.001590 | 0.000809 | 1.9603 | 67.3 | 131.5 | 0.9703 | 0.003 | 0.018 |
| Oligosphaeraceae | 0.001550 | 0.001070 | 1.4428 | 39 | 121.3 | 0.9766 | 0.002 | 0.015 |
| Candidatus Endomicrobium | 0.001050 | 0.000740 | 1.425 | 2.6 | 61.1 | 0.9808 | 0.0001 | 0.004 |
| Erysipelotrichaceae | 0.000655 | 0.000820 | 0.7987 | 25.5 | 54.2 | 0.9835 | 0.363 | |
| Planctomycetaceae | 0.000635 | 0.000791 | 0.8022 | 0.9 | 36.1 | 0.9861 | 0.001 | 0.008 |
| Victivallaceae | 0.000624 | 0.000857 | 0.7283 | 18.9 | 46.4 | 0.9886 | 0.272 | |
| Bdellovibrionaceae | 0.000541 | 0.000460 | 1.1771 | 28.8 | 25.7 | 0.9908 | 0.597 | |
| Elusimicrobiaceae | 0.000535 | 0.000386 | 1.3869 | 31.9 | 44.5 | 0.993 | 0.427 | |
| Veillonellaceae | 0.000431 | 0.000313 | 1.379 | 50.5 | 32.2 | 0.9947 | 0.064 | |
| Verrucomicrobiaceae | 0.000252 | 0.000760 | 0.3317 | 0 | 14 | 0.9957 | 0.317 | |
| Eubacteriaceae | 0.000240 | 0.000217 | 1.1074 | 10.6 | 20.8 | 0.9967 | 0.120 | |
| Rhodospirillaceae | 0.000170 | 0.000255 | 0.6662 | 9.3 | 5.5 | 0.9974 | 0.85 | |
| Acholeplasmataceae | 0.000152 | 0.000160 | 0.952 | 7.6 | 3.8 | 0.998 | 0.831 | |
| Sutterellaceae | 0.000151 | 0.000169 | 0.8924 | 2.1 | 9.6 | 0.9986 | 0.029 | |
| Bifidobacteriaceae | 0.000132 | 0.000108 | 1.2206 | 7.3 | 0 | 0.9992 | 0.0002 | 0.003 |
| Streptococcaceae | 0.000126 | 0.000089 | 1.4141 | 7.3 | 6.4 | 0.9997 | 0.210 | |
| Anaerolineaceae | 0.000083 | 0.000073 | 1.1437 | 3 | 6.7 | 1 | 0.207 |
| VOCs 1 | CTR | OL+ | p-Value |
|---|---|---|---|
| Acids | |||
| Acetic acid | 3.58 ± 0.49 | 3.12 ± 0.45 | ns |
| Butanoic acid | 40.83 ± 3.94 | 36.03 ± 3.79 | ns |
| Butanoic acid, 3-methyl | 4.02 ± 0.32 | 4.24 ± 0.35 | ns |
| Pentanoic acid | 0.81 ± 0.09 | 0.91 ± 0.11 | ns |
| Hexanoic acid | 7.07 ± 0.76 | 9.35 ± 0.89 | * |
| Eicosanoic acid | 0.41 ± 0.05 | 0.40 ± 0.06 | ns |
| Aldehydes | |||
| Pentanal, 2-methyl | 6.17 ± 0.57 | 6.21 ± 0.76 | ns |
| Decanal | 1.89 ± 0.17 | 0.85 ± 0.10 | ** |
| Undecanal | 0.39 ± 0.05 | 0.33 ± 0.04 | ns |
| Tridecanal | 0.24 ± 0.04 | 0.22 ± 0.03 | ns |
| Hexadecanal | 1.44 ± 0.13 | 1.33 ± 0.16 | ns |
| Pentadecanal | 1.08 ± 0.14 | 1.00 ± 0.12 | ns |
| Alcohol | |||
| 2-hexanol | 1.00 ± 0.09 | 0.88 ± 0.09 | ns |
| α-terpineol | nd | 3.06 ± 0.26 | ** |
| Ketones | |||
| 2-nonanone | 5.47 ± 0.47 | 5.57 ± 0.53 | ns |
| 2-hexanone, 5-methyl | 0.26 ± 0.03 | 0.27 ± 0.03 | ns |
| 2-heptadecanone | 0.23 ± 0.02 | 0.31 ± 0.04 | ns |
| Esters | |||
| Pentanoic acid, ethyl ester | 8.82 ± 0.95 | 10.31 ± 0.93 | ns |
| Heptanoic acid, ethyl ester | 1.27 ± 0.11 | 1.11 ± 0.10 | ns |
| Octadecanoic acid, phenylmethyl ester | 0.80 ± 0.08 | 0.71 ± 0.08 | ns |
| Eicosanoic acid, phenylmethyl ester | 0.46 ± 0.05 | 0.48 ± 0.04 | ns |
| Oxalic acid, allyl octadecyl ester | 0.21 ± 0.03 | 0.19 ± 0.02 | ns |
| Hydrocarbons | |||
| Octadecane, 3-ethyl-5-(2-ethylbutyl) | 2.06 ± 0.23 | 1.82 ± 0.17 | ns |
| Octanoic acid | 1.04 ± 0.13 | 1.09 ± 0.08 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennato, F.; Martino, C.; Di Domenico, M.; Ianni, A.; Chai, B.; Di Marcantonio, L.; Cammà, C.; Martino, G. Metagenomic Characterization and Volatile Compounds Determination in Rumen from Saanen Goat Kids Fed Olive Leaves. Vet. Sci. 2022, 9, 452. https://doi.org/10.3390/vetsci9090452
Bennato F, Martino C, Di Domenico M, Ianni A, Chai B, Di Marcantonio L, Cammà C, Martino G. Metagenomic Characterization and Volatile Compounds Determination in Rumen from Saanen Goat Kids Fed Olive Leaves. Veterinary Sciences. 2022; 9(9):452. https://doi.org/10.3390/vetsci9090452
Chicago/Turabian StyleBennato, Francesca, Camillo Martino, Marco Di Domenico, Andrea Ianni, Benli Chai, Lisa Di Marcantonio, Cesare Cammà, and Giuseppe Martino. 2022. "Metagenomic Characterization and Volatile Compounds Determination in Rumen from Saanen Goat Kids Fed Olive Leaves" Veterinary Sciences 9, no. 9: 452. https://doi.org/10.3390/vetsci9090452
APA StyleBennato, F., Martino, C., Di Domenico, M., Ianni, A., Chai, B., Di Marcantonio, L., Cammà, C., & Martino, G. (2022). Metagenomic Characterization and Volatile Compounds Determination in Rumen from Saanen Goat Kids Fed Olive Leaves. Veterinary Sciences, 9(9), 452. https://doi.org/10.3390/vetsci9090452






