Identification and Characterization of Eimeria tenella Rhoptry Protein 35 (EtROP35)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites and Animals
2.2. Molecular Cloning and Bioinformatic Analysis of EtROP35
2.3. Localization of EtROP35
2.4. Sporozoite Secretion Experiment
2.5. The Transcription and Expression Levels of EtROP35 in Different Developmental Stages of E. tenella
2.6. E. tenella Sporozoite Invasion-Blocking Assay
2.7. The Immune Protection of EtROP35 Proteins
2.8. Detection of Serum Antibody Level
2.9. Statistical Analysis
3. Results
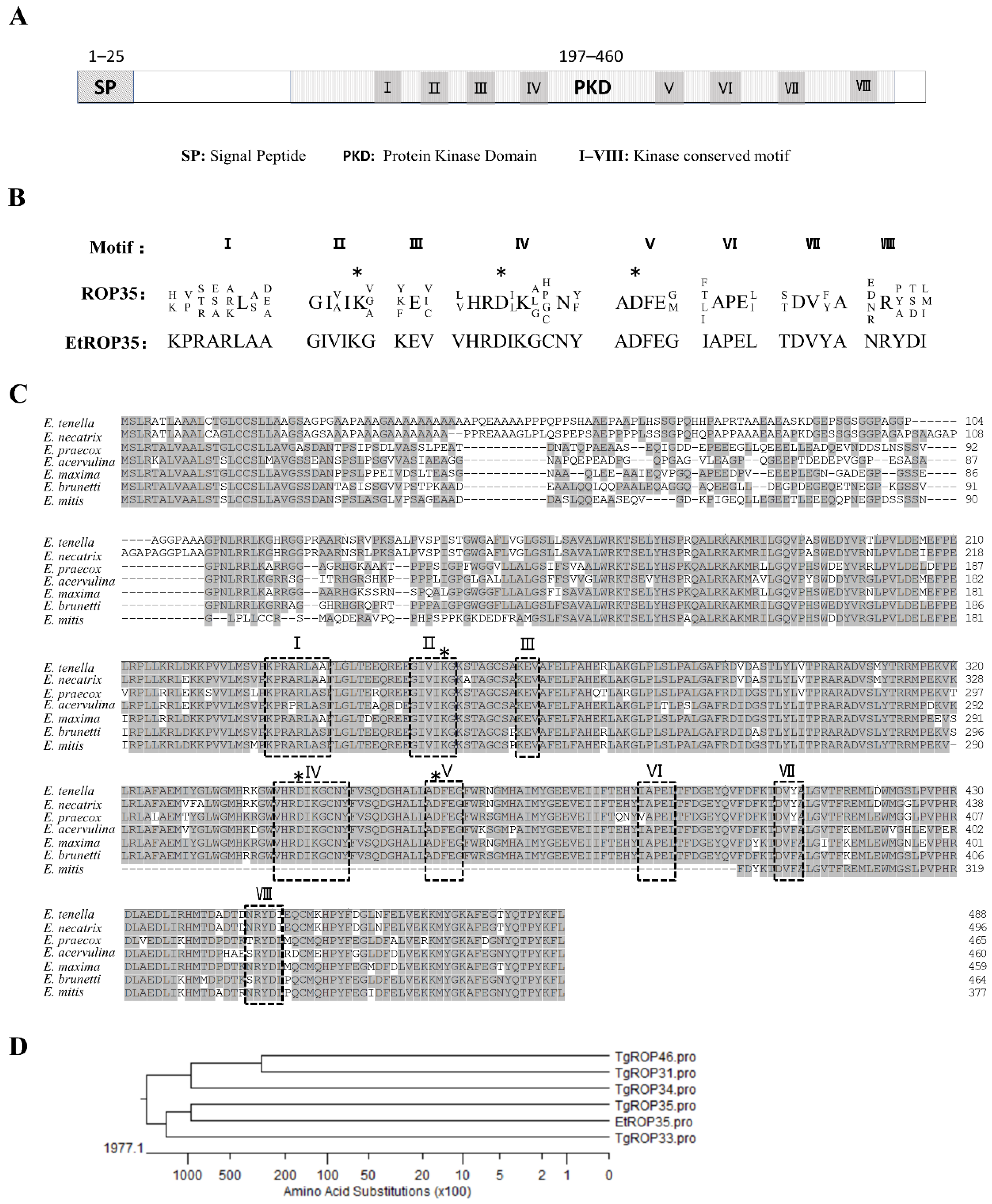
3.1. Identification of EtROP35
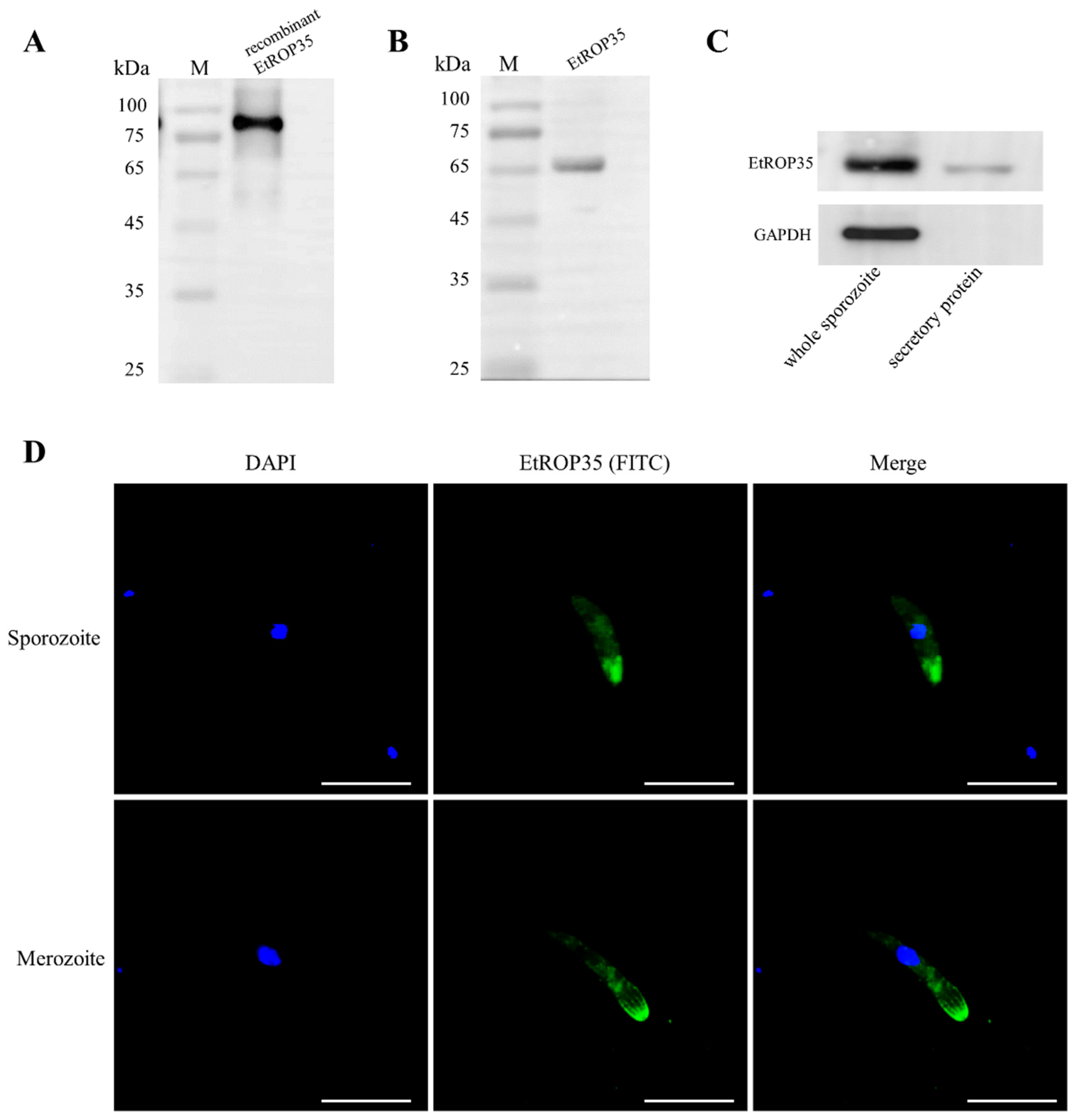
3.2. The Localization of EtROP35
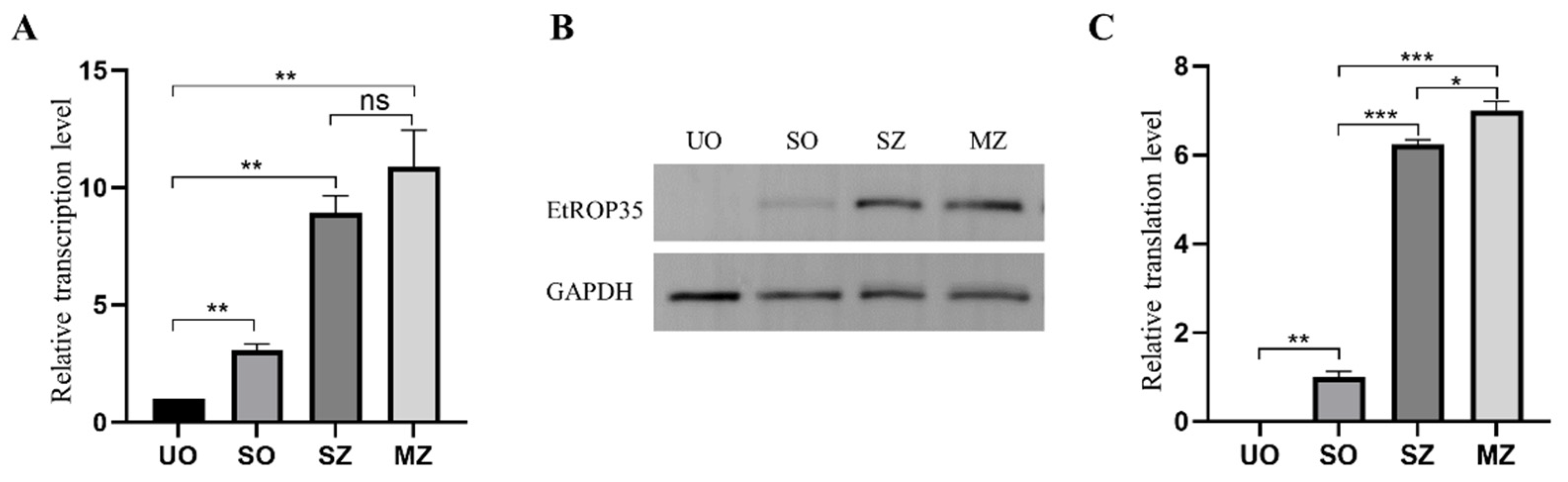
3.3. Expression of EtROP35 during Different Developmental Stages of E. tenella
3.4. Effect of EtROP35 on E. tenella Sporozoite Invasion of Host Cells
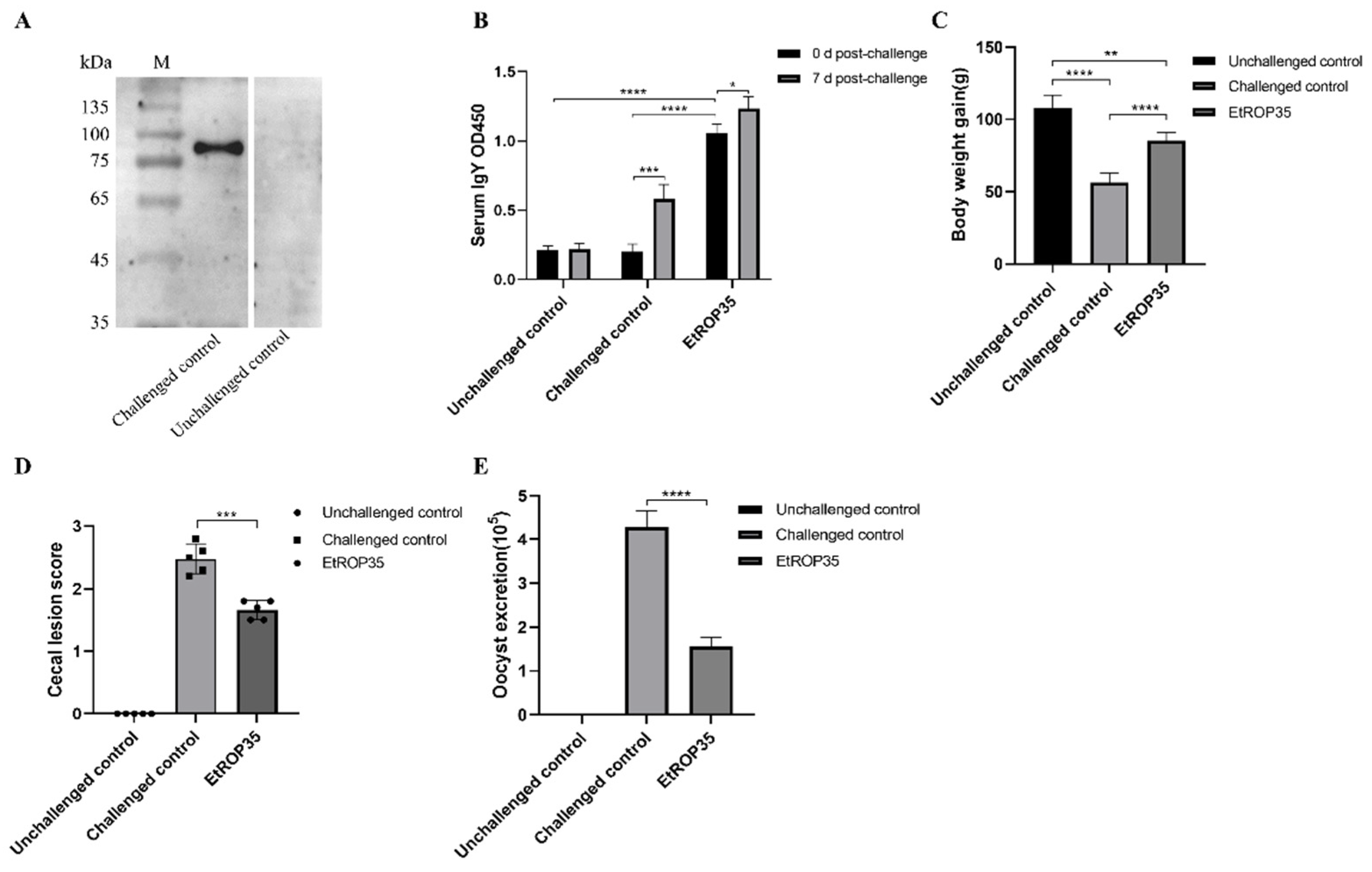
3.5. Immune Protection Induced against E. tenella by Recombinant EtROP35
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, H.D.; Barta, J.R.; Blake, D.; Gruber, A.; Jenkins, M.; Smith, N.C.; Suo, X.; Tomley, F.M. A Selective Review of Advances in Coccidiosis Research. Adv. Parasitol. 2013, 83, 93–171. [Google Scholar] [CrossRef] [PubMed]
- Kats, L.; Black, C.; Proellocks, N.I.; Coppel, R. Plasmodium rhoptries: How things went pear-shaped. Trends Parasitol. 2006, 22, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Preiser, P.; Kaviratne, M.; Khan, S.; Bannister, L.; Jarra, W. The apical organelles of malaria merozoites: Host cell selection, invasion, host immunity and immune evasion. Microbes Infect. 2000, 2, 1461–1477. [Google Scholar] [CrossRef]
- Dogga, S.K.; Mukherjee, B.; Jacot, D.; Kockmann, T.; Molino, L.; Hammoudi, P.-M.; Hartkoorn, R.C.; Hehl, A.B.; Soldati-Favre, D. A druggable secretory protein maturase of Toxoplasma essential for invasion and egress. eLife 2017, 6, e27480. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, S.; Charron, A.J.; Sibley, L. Toxoplasma evacuoles: A two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 2001, 20, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Kemp, L.E.; Yamamoto, M.; Soldati-Favre, D. Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol. Rev. 2013, 37, 607–631. [Google Scholar] [CrossRef]
- Peixoto, L.; Chen, F.; Harb, O.; Davis, P.H.; Beiting, D.P.; Brownback, C.S.; Ouloguem, D.; Roos, D.S. Integrative Genomic Approaches Highlight a Family of Parasite-Specific Kinases that Regulate Host Responses. Cell Host Microbe 2010, 8, 208–218. [Google Scholar] [CrossRef]
- Reid, A.J.; Blake, D.P.; Ansari, H.R.; Billington, K.; Browne, H.P.; Bryant, J.; Dunn, M.; Hung, S.S.; Kawahara, F.; Miranda-Saavedra, D.; et al. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef]
- Diallo, M.A.; Sausset, A.; Gnahoui-David, A.; Silva, A.R.E.; Brionne, A.; Le Vern, Y.; Bussière, F.I.; Tottey, J.; Lacroix-Lamandé, S.; Laurent, F.; et al. Eimeria tenella ROP kinase EtROP1 induces G0/G1 cell cycle arrest and inhibits host cell apoptosis. Cell. Microbiol. 2019, 21, e13027. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Zhang, T.; Liu, J.; Liu, Q. A Novel Rhoptry Protein as Candidate Vaccine against Eimeria tenella Infection. Vaccines 2020, 8, 452. [Google Scholar] [CrossRef]
- Liu, X.; Mu, B.; Zheng, W.; Meng, Y.; Yu, L.; Gao, W.; Zhu, X.; Liu, Q. Identification and Protective Efficacy of Eimeria tenella Rhoptry Kinase Family Protein 17. Animals 2022, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, N.; Wang, Y.; Sun, L.; Li, H.; Zhang, X.; Zhao, X. Characterization of the Eimeria tenella rhoptry protein with a nuclear localization sequence (EtROP30). Parasitol. Res. 2022, 121, 1507–1516. [Google Scholar] [CrossRef]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B.; et al. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Liang, Y.; Wang, S.; Xie, Q.; Nan, X.; Li, P.; Hong, G.; Liu, Q.; Li, X. Molecular characterization and protective immunity of rhoptry protein 35 (ROP35) of Toxoplasma gondii as a DNA vaccine. Veter-Parasitol. 2018, 260, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Shi, W.; Sun, H.; Zhang, J.; Li, H.; Xiao, Y.; Wang, F.; Zhao, X. Preparation and initial application of monoclonal antibodies that recognize Eimeria tenella microneme proteins 1 and 2. Parasitol. Res. 2014, 113, 4151–4161. [Google Scholar] [CrossRef]
- Zhao, N.; Ming, S.; Lu, Y.; Wang, F.; Li, H.; Zhang, X.; Zhao, X. Identification and Application of Epitopes in EtMIC1 of Eimeria tenella Recognized by the Monoclonal Antibodies 1-A1 and 1-H2. Infect. Immun. 2019, 87, e00596-19. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Wang, H.; Sun, L.; Wang, B.; Jiang, Y.; Li, H.; Zhang, X.; Li, H.; Zhao, X. The role of Eimeria tenella EtCab protein in the attachment and invasion of host cells. Veter-Parasitol. 2021, 292, 109415. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Wang, J.; Fu, Y.; Nan, H.; Liu, Q. Identification and characterization of a microneme protein (NcMIC6) in Neospora caninum. Parasitol. Res. 2015, 114, 2893–2902. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Zhao, N.; Lv, J.; Lu, Y.; Jiang, Y.; Li, H.; Liu, Y.; Zhang, X.; Zhao, X. Prolonging and enhancing the protective efficacy of the EtMIC3-C-MAR against eimeria tenella through delivered by attenuated salmonella typhimurium. Veter-Parasitol. 2020, 279, 109061. [Google Scholar] [CrossRef]
- Talevich, E.; Kannan, N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol. Biol. 2013, 13, 117. [Google Scholar] [CrossRef]
- Maskus, D.J.; Bethke, S.; Seidel, M.; Kapelski, S.; Addai-Mensah, O.; Boes, A.; Edgü, G.; Spiegel, H.; Reimann, A.; Fischer, R.; et al. Isolation, production and characterization of fully human monoclonal antibodies directed to Plasmodium falciparum MSP10. Malar. J. 2015, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Reddy, K.S.; Pandey, A.K.; Mian, S.Y.; Singh, H.; Mittal, S.A.; Amlabu, E.; Bassat, Q.; Mayor, A.; Chauhan, V.S.; et al. A novel Plasmodium falciparum rhoptry associated adhesin mediates erythrocyte invasion through the sialic-acid dependent pathway. Sci. Rep. 2016, 6, 29185. [Google Scholar] [CrossRef] [PubMed]
- Ekka, R.; Gupta, A.; Bhatnagar, S.; Malhotra, P.; Sharma, P. Phosphorylation of Rhoptry Protein RhopH3 Is Critical for Host Cell Invasion by the Malaria Parasite. mBio 2020, 11, e00166-20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Ming, S.; Sun, L.; Wang, B.; Li, H.; Zhang, X.; Zhao, X. Identification and Characterization of Eimeria tenella Microneme Protein (EtMIC8). Microbiol. Spectr. 2021, 9, e0022821. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Q.; Zhu, S.; Wang, Q.; Wang, H.; Yu, S.; Yu, Y.; Liang, S.; Zhao, H.; Huang, B.; et al. Eimeria tenella Eimeria-specific protein that interacts with apical membrane antigen 1 (EtAMA1) is involved in host cell invasion. Parasites Vectors 2020, 13, 373. [Google Scholar] [CrossRef]
- Rezaei, F.; Sarvi, S.; Sharif, M.; Hejazi, S.H.; Pagheh, A.S.; Aghayan, S.; Daryani, A. A systematic review of Toxoplasma gondii antigens to find the best vaccine candidates for immunization. Microb. Pathog. 2018, 126, 172–184. [Google Scholar] [CrossRef]
- Richards, J.S.; Arumugam, T.U.; Reiling, L.; Healer, J.; Hodder, A.N.; Fowkes, F.J.I.; Cross, N.; Langer, C.; Takeo, S.; Uboldi, A.D.; et al. Identification and Prioritization of Merozoite Antigens as Targets of Protective Human Immunity to Plasmodium falciparum Malaria for Vaccine and Biomarker Development. J. Immunol. 2013, 191, 795–809. [Google Scholar] [CrossRef]
- Roque-Reséndiz, J.L.; Rosales, R.; Herion, P. MVA ROP2 vaccinia virus recombinant as a vaccine candidate for toxoplasmosis. Parasitology 1999, 128, 397–405. [Google Scholar] [CrossRef]
- Zheng, B.; Lu, S.; Tong, Q.; Kong, Q.; Lou, D. The virulence-related rhoptry protein 5 (ROP5) of Toxoplasma Gondii is a novel vaccine candidate against toxoplasmosis in mice. Vaccine 2013, 31, 4578–4584. [Google Scholar] [CrossRef]
- Yuan, Z.-G.; Zhang, X.-X.; He, X.-H.; Petersen, E.; Zhou, D.-H.; He, Y.; Lin, R.-Q.; Li, X.-Z.; Chen, X.-L.; Shi, X.-R.; et al. Protective Immunity Induced by Toxoplasma gondii Rhoptry Protein 16 against Toxoplasmosis in Mice. Clin. Vaccine Immunol. 2011, 18, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhao, N.; Sun, J.; Sun, L.; Li, H.; Wu, Z.; Li, H.; Zhang, X.; Zhao, X. Identification and Characterization of Eimeria tenella Rhoptry Protein 35 (EtROP35). Vet. Sci. 2022, 9, 465. https://doi.org/10.3390/vetsci9090465
Wang B, Zhao N, Sun J, Sun L, Li H, Wu Z, Li H, Zhang X, Zhao X. Identification and Characterization of Eimeria tenella Rhoptry Protein 35 (EtROP35). Veterinary Sciences. 2022; 9(9):465. https://doi.org/10.3390/vetsci9090465
Chicago/Turabian StyleWang, Bingxiang, Ningning Zhao, Jinkun Sun, Lingyu Sun, Huihui Li, Zhiyuan Wu, Hongmei Li, Xiao Zhang, and Xiaomin Zhao. 2022. "Identification and Characterization of Eimeria tenella Rhoptry Protein 35 (EtROP35)" Veterinary Sciences 9, no. 9: 465. https://doi.org/10.3390/vetsci9090465
APA StyleWang, B., Zhao, N., Sun, J., Sun, L., Li, H., Wu, Z., Li, H., Zhang, X., & Zhao, X. (2022). Identification and Characterization of Eimeria tenella Rhoptry Protein 35 (EtROP35). Veterinary Sciences, 9(9), 465. https://doi.org/10.3390/vetsci9090465





