Effects of Dietary Inclusion of Spirulina platensis on the Reproductive Performance of Female Mink
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet
2.3. Feed Analysis
2.3.1. Chemical Analysis of Feed
2.3.2. Diet Fatty Acid Profile
2.4. Body Weights
2.5. Reproductive Performance
2.6. Statistical Analysis
3. Results
3.1. Feed Analysis
3.2. Reproduction Performance of Mink Dams and Mortality of Kits
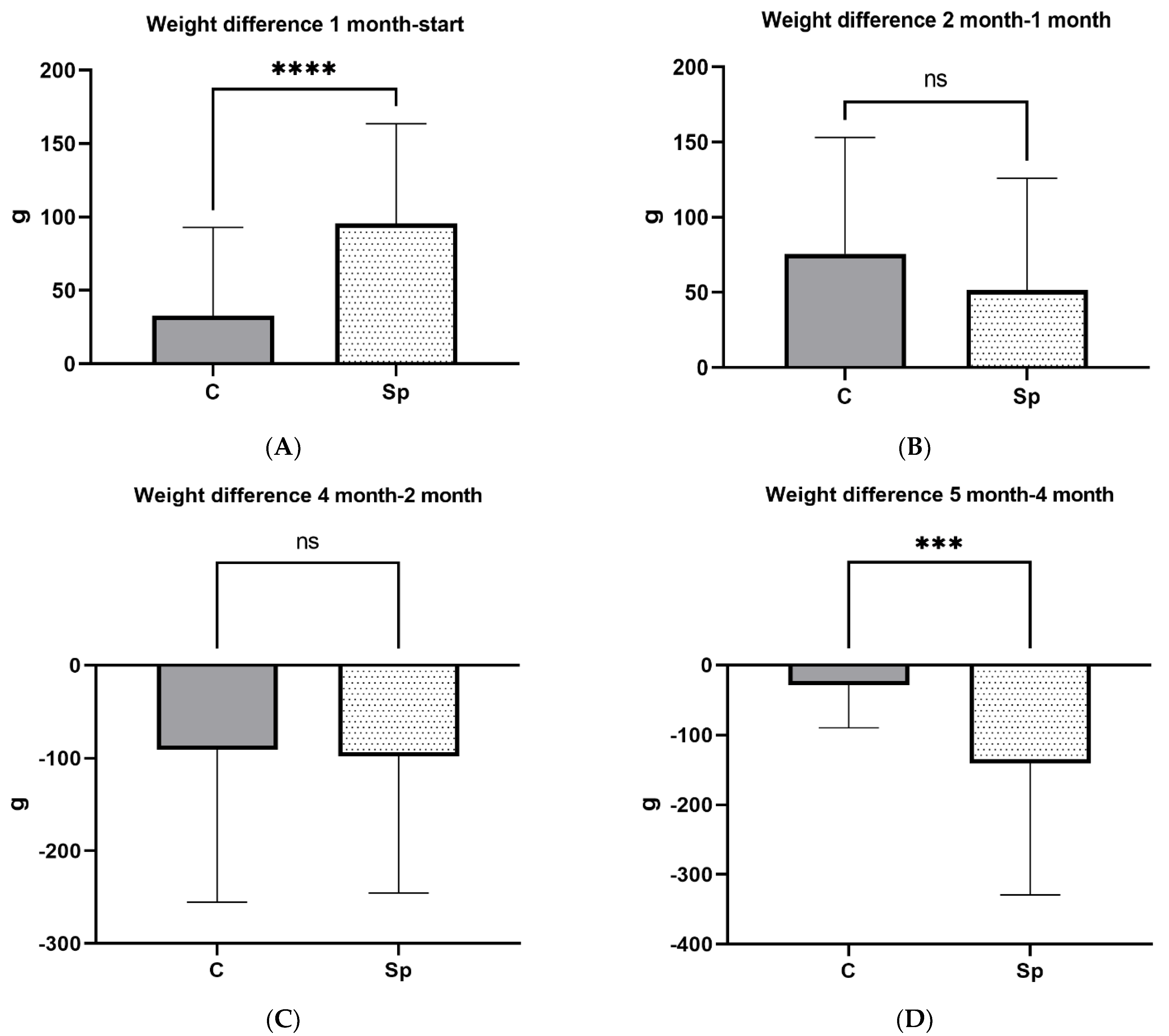
3.3. Weight of Female Mink and Kits at Weaning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korhonen, H.T.; Huuki, H. Effect of Carotenoid Supplement on Production Performance in Mink. Open J. Vet. Med. 2015, 5, 73–79. [Google Scholar] [CrossRef][Green Version]
- Madsen, M.D.; Villumsen, T.M.; Hansen, B.K.; Møller, S.H.; Jensen, J.; Shirali, M. Combined analysis of group recorded feed intake and individually recorded body weight and litter size in mink. Animal 2020, 14, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.; Benkel, B.; Astatkie, T.; Rouvinen-Watt, K. Ideal body condition improves reproductive performance and influences genetic health in female mink. Anim. Reprod. Sci. 2014, 145, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Lagerkvist, G.; Johansson, K.; Lundeheim, N. Selection for litter size, body weight, and pelt quality in mink (Mustela vison): Correlated responses. J. Anim. Sci. 1994, 72, 1126–1137. [Google Scholar] [CrossRef]
- Hunter, D.B.; Lemieux, N. Mink…Biology, Health and Disease; Hunter, D.B., Lemieux, N., Eds.; Graphic and Print Services: Guelph, ON, Canada, 1996. [Google Scholar]
- Rouvinen-Watt, K. Nursing sickness in the mink—A metabolic mystery or a familiar foe? Can. J. Vet. Res. 2003, 67, 161–168. [Google Scholar] [PubMed]
- Jiang, Q.; Li, G.; Zhang, T.; Zhang, H.; Gao, X.; Xing, X.; Zhao, J.; Yang, F. Effects of dietary protein level on nutrients digestibility and reproductive performance of female mink (Neovison vison) during gestation. Anim. Nutr. 2015, 1, 65–69. [Google Scholar] [CrossRef]
- Matthiesen, C.F.; Blache, D.; Thomsen, P.D.; Hansen, N.E.; Tauson, A.H. Effect of late gestation low protein supply to mink (Mustela vison) dams on reproductive performance and metabolism of dam and offspring. Arch. Anim. Nutr. 2010, 64, 56–76. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, K.; Zhong, W.; Li, G.; Liu, H. Effects of dietary vitamin E supplementation on the reproductive performance of yearling female mink (Neovison vison) fed wet fish-based feed. Anim. Reprod. Sci. 2020, 213, 106270. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, B.M.; Børsting, C.F.; Engberg, R.M.; Jensen, S.K. Effects of high dietary levels of fresh or oxidised fish oil on performance and blood parameters in female mink (Mustela vison) during the winter, reproduction, lactation and early growth periods. Acta Agric. Scand.-Sect. A Anim. Sci. 2003, 53, 136–146. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Muszyński, S.; Donaldson, J.; Jakubczak, A.; Żmuda, A.; Taszkun, I.; Rycerz, K.; Mielnik-Błaszczak, M.; Kuc, D.; Tomaszewska, E. The effects of prenatal supplementation with β-hydroxy-β-methylbutyrate and/or alpha-ketoglutaric acid on the development and maturation of mink intestines are dependent on the number of pregnancies and the sex of the offspring. Animals 2021, 11, 1468. [Google Scholar] [CrossRef]
- EL-Sabagh, M.R.; Abd Eldaim, M.A.; Mahboub, D.H.; Abdel-Daim, M. Effects of Spirulina Platensis Algae on Growth Performance, Antioxidative Status and Blood Metabolites in Fattening Lambs. J. Agric. Sci. 2014, 6, 92. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and Medical Applications of Spirulina Microalgae. Mini-Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two classes of pigments, carotenoids and c-phycocyanin, in spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; El-Hack, M.E.A.; Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016, 12, 36–51. [Google Scholar] [CrossRef]
- Gargouri, M.; Soussi, A.; Akrouti, A.; Magné, C.; El Feki, A. Potential protective effects of the edible alga arthrospira platensis against lead-induced oxidative stress, anemia, kidney injury, and histopathological changes in adult rats. Appl. Physiol. Nutr. Metab. 2019, 44, 271–281. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.S. In vitro antioxidant and antiproliferative activities of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. J. Agric. Food Chem. 2008, 56, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (oncorhynchus mykiss). Aquaculture 2013, 396–399, 14–19. [Google Scholar] [CrossRef]
- Mantog, S. Spirulina as a natural carotenoid source on growth, pigmentation and reproductive performance of yellow tail cichlid Pseudotropheus acei. Aquac. Int. 2012, 20, 869–878. [Google Scholar] [CrossRef]
- Berestov, V. Our Experience in Spirulina Feeding to Minks in the Reproduction Period. Scientifur 2001, 25, 11–16. [Google Scholar]
- Giannenas, I.; Tzora, A.; Bonos, E.; Sarakatsianos, I.; Karamoutsios, A.; Anastasiou, I.; Skoufos, I. Einfluss von zusätzen von essentiellen Ölen des Oreganos und des Lorbers sowie von attapulgit zum futter auf die chemische zusammensetzung, die oxidationsstabilität, das fettsäuremuster und den mineralstoffgehalt von broiler-brust-und schenkelfleisch. Eur. Poult. Sci. 2016, 80, 1–18. [Google Scholar] [CrossRef]
- Felska-Błaszczyk, L.; Seremak, B.; Ławrów, N. Mating system vs. litter size in farm mink (Neovison vison)—effect of multiple paternity. Acta Sci. Pol. Zootech. 2019, 18, 13–18. [Google Scholar] [CrossRef]
- Seremak, B.; Felska-Błaszczyk, L.; Opieka, P.; Wojciechowska, A. Supplementation of the diet with chelated selenium yeast and vitamin E and their effect on reproductive performance of farmed female American mink (Neovison vison). Wiadomości Zootechniczne 2017, 3, 9–12. [Google Scholar]
- Niu, Y.J.; Zhou, W.; Guo, J.; Nie, Z.W.; Shin, K.T.; Kim, N.H.; Lv, W.F.; Cui, X.S. C-Phycocyanin protects against mitochondrial dysfunction and oxidative stress in parthenogenetic porcine embryos. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 375–380. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose Effects of Orally Administered Spirulina Suspension on Colonic Microbiota in Healthy Mice. Front. Cell. Infect. Microbiol. 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, E.I.; Hassanien, H.A.M.; Mohamed, A.H.; Hussein, A.M.; Azza, A.M. Influence of Addition Spirulina Platensis Algae Powder on Reproductive and Productive Performance of Dairy Zaraibi. Egypt J. Nutr. Feed. 2016, 19, 211–225. [Google Scholar] [CrossRef]
- Yener, N.A.; Sinanoglu, O.; Ilter, E.; Celik, A.; Sezgin, G.; Midi, A.; Deveci, U.; Aksungar, F. Effects of spirulina on cyclophosphamide-induced ovarian toxicity in rats: Biochemical and histomorphometric evaluation of the ovary. Biochem. Res. Int. 2013, 2013, 764262. [Google Scholar] [CrossRef] [PubMed]
- Abadjieva, D.; Shumkov, K.; Kistanova, E.; Kacheva, D.; Georgiev, B. Opportunities for the improvement of the reproductive performances in female animals. Biotechnol. Anim. Husb. Biotehnol. Stoc. 2011, 27, 365–372. [Google Scholar] [CrossRef]
- El-ratel, I.T. Reproductive Performance, Oxidative Status and Blood Metabolites of Doe Rabbits Administrated with Spirulina Alga. Egypt. Poult. Sci. J. 2017, 37, 1153–1172. [Google Scholar] [CrossRef]
- Tauson, A. Meeting at the Danish Institute of Agricultural Sciences, Research Centre Foulum on September 21th 1999 on the subject: How to prepare mink dams for mating, gestation and lactation? Mating Eff. 2000, 24, 240–242. [Google Scholar]
- Selim, S.; Hussein, E.; Abou-Elkhair, R. Einfluss von Spirulina platensis als futterzusatzstoff auf die legeleistung, die eiqualität und den leberschutz von legehennen. Eur. Poult. Sci. 2018, 82, 1–13. [Google Scholar] [CrossRef]
- Kaoud, H. A Effect of spirulina platensis as a dietary supplement on broiler performance in comparison with prebiotics. Sci. J. Appl. Res. 2012, 2, 46–51. [Google Scholar]
- Fink, R.; Børsting, C.F. Quantitative glucose metabolism in lactating mink (Mustela vison)—Effects of dietary levels of protein, fat and carbohydrates. Acta Agric. Scand.-Sect. A Anim. Sci. 2002, 52, 34–42. [Google Scholar] [CrossRef]
- Bonos, E.; Kasapidou, E.; Kargopoulos, A.; Karampampas, A.; Christaki, E.; Florou-Paneri, P.; Nikolakakis, I. Spirulina as a functional ingredient in broiler chicken diets. S. Afr. J. Anim. Sci. 2016, 46, 94. [Google Scholar] [CrossRef]
- Toyomizu, M.; Sato, K.; Taroda, H.; Kato, T.; Akiba, Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001, 42, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, H.A.M.; Arafa, M.M.; Warda, M.A.A. Effect of Using Spirulina Platensis and Chlorella Vulgaris As Feed Additives on Growing Rabbit Performance. Egypt. J. Rabbit Sci. 2014, 24, 413–431. [Google Scholar] [CrossRef]

| Control | Spirulina | |
|---|---|---|
| Moisture (g/kg) | 3.1 | 3.6 |
| Protein (g/kg) | 43.7 | 43.7 |
| Fat (g/kg) | 13.8 | 14.3 |
| Ash (g/kg) | 9.7 | 8.9 |
| Total phenolics (ng GAE/g DM 1) | 20.5 | 21.4 |
| Arginine (g/kg) | 2.6 | 2.6 |
| Cysteine (g/kg) | 0.4 | 0.4 |
| Isoleucine (g/kg) | 1.5 | 1.4 |
| Leucine (g/kg) | 2.9 | 2.9 |
| Lysine (g/kg) | 2.8 | 2.9 |
| Methionine (g/kg) | 0.8 | 0.8 |
| Threonine (g/kg) | 1.6 | 1.6 |
| Tryptophan (g/kg) | 0.6 | 0.6 |
| Valine (g/kg) | 1.7 | 1.7 |
| Saturated FA 2 (g/kg) | 2.3 | 2.3 |
| Polyunsaturated FA 2 (g/kg) | 1.6 | 1.9 |
| Monounsaturated FA 2 (g/kg) | 6.0 | 5.8 |
| Total energy (TE) (KJ/g) | 20.737 | 20.881 |
| Metabolizable energy (ME) (KJ/g) | 15.194 | 15.311 |
| Components | g/kg |
|---|---|
| Protein | 65.75 |
| Fat | 2.57 |
| Fiber | 1.82 |
| Ash | 3.88 |
| Aspartic acid | 6.47 |
| Threonine | 2.13 |
| Serine | 2.93 |
| Glutamic acid | 9.47 |
| Proline | 3.40 |
| Glycine | 2.30 |
| Alanine | 2.13 |
| Cysteine | 0.88 |
| Valine | 2.47 |
| Methionnine | 0.23 |
| Isoleucine | 2.62 |
| Leucine | 1.47 |
| Tyrisine | 2.07 |
| Phenylalanine | 3.34 |
| Lysine | 2.14 |
| Histidine | 1.27 |
| Arginine | 3.03 |
| Tryptophan | 0.62 |
| Control | Spirulina | ||
|---|---|---|---|
| Mean (±SD 1) | Mean (±SD 1) | p-Value | |
| LS 2 birth | 7.4 (2.17) | 7.0 (1.54) | 0.270 |
| LS 2 d10 | 5.4 (2.47) | 5.3 (2.61) | 0.902 |
| LS 2 wean | 5.0 (2.42) | 4.9 (2.63) | 0.720 |
| Mortality of kits | 2.3(2.70) | 2.1 (2.38) | 0.668 |
| Control | Spirulina | |||||
|---|---|---|---|---|---|---|
| Traits (g) | Measurement | N | Mean (±SD 1) | N | Mean (±SD 1) | p-Value |
| Weight of females | Start | 50 | 1592.2 (70.37) | 50 | 1603.2 (69.94) | 0.435 |
| 1st month | 50 | 1624.4 (89.29) | 50 | 1696.9 (95.05) | <0.001 | |
| 2nd month | 40 | 1699.7 (116.31) | 40 | 1751.7 (120.89) | 0.067 | |
| 4th month | 39 | 1592.6 (189.15) | 44 | 1652.6 (156.32) | 0.160 | |
| 5th month | 39 | 1560.2 (220.62) | 44 | 1499.5 (270.25) | 0.219 | |
| Weight of kits | 100 | 1038.7 (181.62) | 100 | 1050.0 (178.15) | 0.657 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iatrou, A.M.; Papadopoulos, G.A.; Giannenas, I.; Lymberopoulos, A.; Fortomaris, P. Effects of Dietary Inclusion of Spirulina platensis on the Reproductive Performance of Female Mink. Vet. Sci. 2022, 9, 428. https://doi.org/10.3390/vetsci9080428
Iatrou AM, Papadopoulos GA, Giannenas I, Lymberopoulos A, Fortomaris P. Effects of Dietary Inclusion of Spirulina platensis on the Reproductive Performance of Female Mink. Veterinary Sciences. 2022; 9(8):428. https://doi.org/10.3390/vetsci9080428
Chicago/Turabian StyleIatrou, Anna Maria, Georgios A. Papadopoulos, Ilias Giannenas, Aristotelis Lymberopoulos, and Paschalis Fortomaris. 2022. "Effects of Dietary Inclusion of Spirulina platensis on the Reproductive Performance of Female Mink" Veterinary Sciences 9, no. 8: 428. https://doi.org/10.3390/vetsci9080428
APA StyleIatrou, A. M., Papadopoulos, G. A., Giannenas, I., Lymberopoulos, A., & Fortomaris, P. (2022). Effects of Dietary Inclusion of Spirulina platensis on the Reproductive Performance of Female Mink. Veterinary Sciences, 9(8), 428. https://doi.org/10.3390/vetsci9080428









