Does Immunocastration Affect Behaviour and Body Lesions in Heavy Pigs?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments
2.2. Data Recordings
2.2.1. Behavioural Observations
2.2.2. Body Lesions
2.2.3. Testosterone Levels
2.3. Growth Performance and Carcass Traits
2.4. Statistical Analysis
3. Results
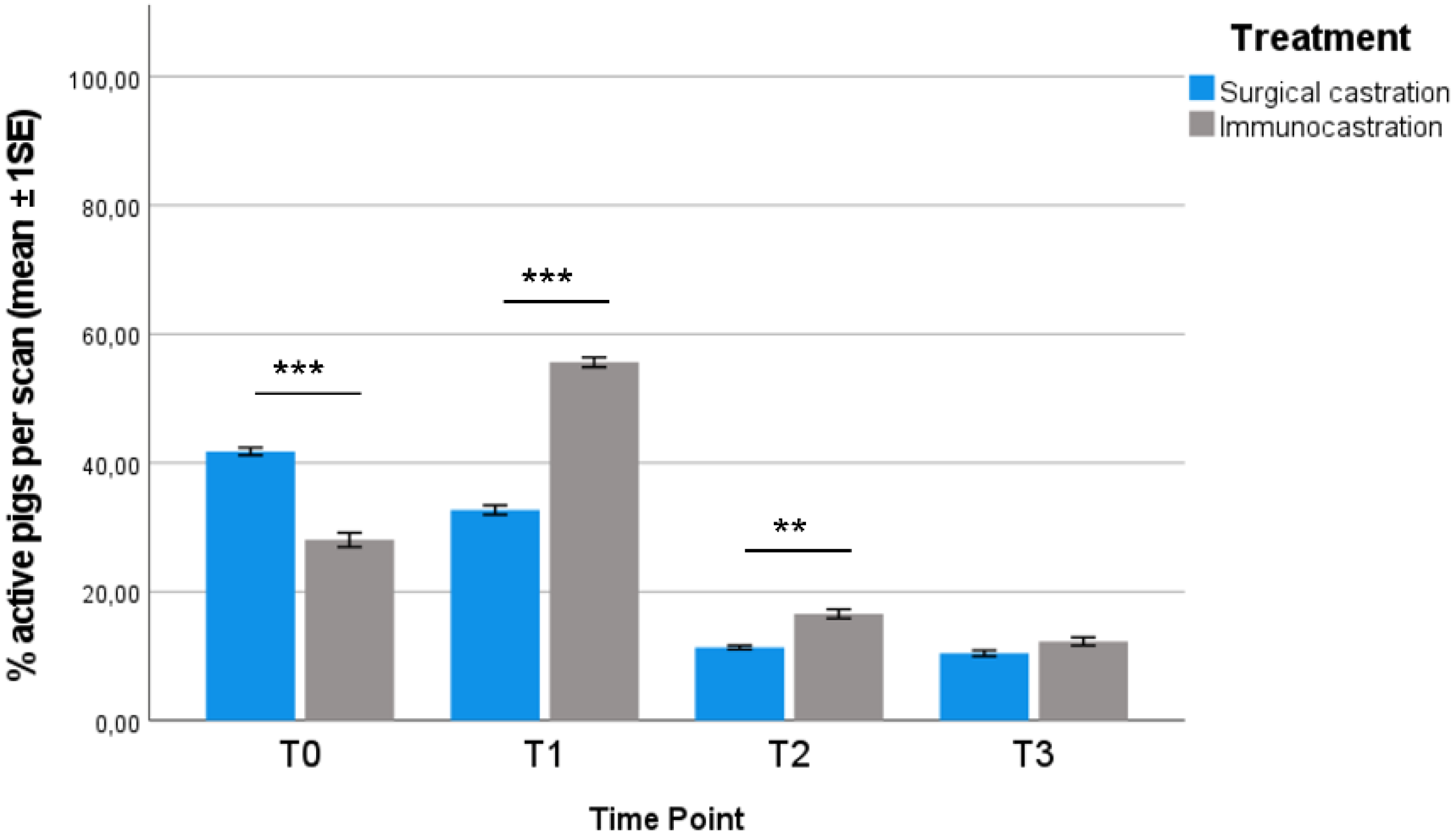
3.1. Behavioural Observations
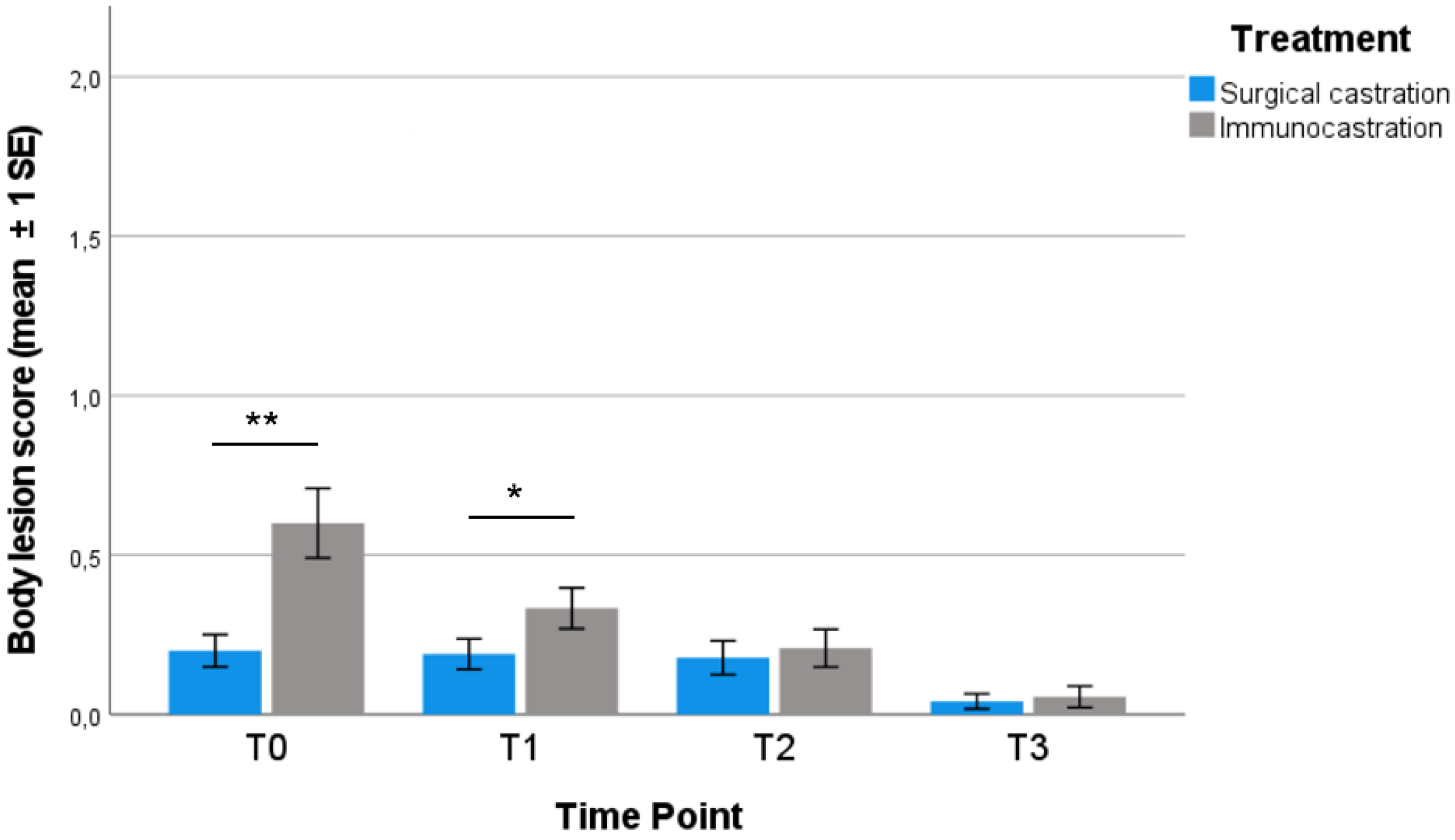
3.2. Body Lesions
3.3. Testosterone Levels
3.4. Growth Performance and Carcass Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backus, G.; Higuera, M.; Juul, N.; Og Fødevarer, L. Second Progress Report 2015–2017 on the European Declaration on Alternatives to Surgical Castration of Pigs; Brussels, Belgium, 2018; Available online: https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress-report-2015-2017-final-1.pdf (accessed on 20 July 2022).
- De Briyne, N.; Berg, C.; Blaha, T.; Temple, D. Pig Castration: Will the EU Manage to Ban Pig Castration by 2018? Porc. Health Manag. 2016, 2, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council Directive 2008/120/EC of 18 December 2008 down Minimum Standards for the Protection of Pigs (Codified). Off. J. Eur. Union 2009, L47, 5–13.
- Claus, R.; Weiler, U.; Herzog, A. Physiological Aspects of Androstenone and Skatole Formation in the Boar—A Review with Experimental Data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Patterson, R.L.S. 5α-Androst-16-Ene-3-One—Compound Responsible for Taint in Boar Fat. J. Sci. Food Agric. 1968, 19, 31–38. [Google Scholar] [CrossRef]
- Vold, E. Meat Production Properties in Entire Male Pigs and Castrates. IV. Organoleptic and Gas Chromatographic Investigations of Steam Volatile Compounds of Backfat of Entire Male Pigs. Meld. Fra Nor. Landbr. 1970, 49, 1–25. [Google Scholar]
- Walstra, P.; Maarse, H. Onderzoek Geslachtgeur van Mannelijke Mestvarkens (Investigation into Sex Odour of Entire Male Pigs); T.N.O., Rap. C-147 and 2: 1-30; Researchgroep Vlees en Vleesvare: Zeist, The Netherlands, 1970. [Google Scholar]
- Hay, M.; Vulin, A.; Génin, S.; Sales, P.; Prunier, A. Assessment of Pain Induced by Castration in Piglets: Behavioral and Physiological Responses over the Subsequent 5 Days. Appl. Anim. Behav. Sci. 2003, 82, 201–218. [Google Scholar] [CrossRef]
- Prunier, A.; Bonneau, M.; Von Borell, E.; Cinotti, S.; Gunn, M.; Fredriksen, B.; Giersing, M.; Morton, D.; Tuyttens, F.; Velarde, A. A Review of the Welfare Consequences of Surgical Castration in Piglets and the Evaluation of Non-Surgical Methods. Anim. Welf. 2006, 15, 277–289. [Google Scholar]
- Thompson, D.L., Jr. Immunization against GnRH in Male Species (Comparative Aspects). Anim. Reprod. Sci. 2000, 60–61, 459–469. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Andersson, H.K.; Chen, G.; Andersson, K.; Madej, A.; Lundström, K.L. Effect of a Gonadotropin-Releasing Hormone Vaccine (Improvac TM) on Steroid Hormones, Boar Taint Compounds and Performance in Entire Male Pigs. Reprod. Domest. Anim. 2008, 43, 351–359. [Google Scholar] [CrossRef]
- Zoels, S.; Reiter, S.; Ritzmann, M.; Weiß, C.; Numberger, J.; Schütz, A.; Lindner, P.; Stefanski, V.; Weiler, U. Influences of Immunocastration on Endocrine Parameters, Growth Performance and Carcass Quality, as Well as on Boar Taint and Penile Injuries. Animals 2020, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Čandek-Potokar, M.; Škrlep, M.; Zamaratskaia, G. Immunocastration as Alternative to Surgical Castration in Pigs. In Theriogenology; Payan Carreira, R., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Andersson, K.; Brunius, C.; Zamaratskaia, G.; Lundström, K. Early Vaccination with Improvac: Effects on Performance and Behaviour of Male Pigs. Animal 2012, 6, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of Boars with a GnRH Vaccine (Improvac) Eliminates Boar Taint and Increases Growth Performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef]
- Pinna, A.; Schivazappa, C.; Virgili, R.; Parolari, G. Effect of Vaccination against Gonadotropin-Releasing Hormone (GnRH) in Heavy Male Pigs for Italian Typical Dry-Cured Ham Production. Meat Sci. 2015, 110, 153–159. [Google Scholar] [CrossRef]
- Von Borell, E.; Bonneau, M.; Holinger, M.; Prunier, A.; Stefanski, V.; Zöls, S.; Weiler, U. Welfare Aspects of Raising Entire Male Pigs and Immunocastrates. Animals 2020, 10, 2140. [Google Scholar] [CrossRef]
- Kress, K.; Millet, S.; Labussière, É.; Weiler, U.; Stefanski, V. Sustainability of Pork Production with Immunocastration in Europe. Sustainability 2019, 11, 3335. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.M.; Ding, Y.; Wassie, T.; Jing, H.J.; Ahmed, S.; Liu, G.Q.; Jiang, X.P. Recent Advances in Immunocastration in Sheep and Goat and Its Animal Welfare Benefits: A Review. J. Integr. Agric. 2022, 21, 299–309. [Google Scholar] [CrossRef]
- Needham, T.; Lambrechts, H.; Hoffman, L.C. Castration of Male Livestock and the Potential of Immunocastration to Improve Animal Welfare and Production Traits: Invited Review. S. Afr. J. Anim. Sci. 2017, 47, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.; Dereu, A.; Manso, A.; De Frutos, L.; Piñeiro, C.; Manzanilla, E.G.; Wuyts, N. Surgical Castration with Pain Relief Affects the Health and Productive Performance of Pigs in the Suckling Period. Porc. Health Manag. 2017, 3, 18. [Google Scholar] [CrossRef]
- Velarde, A.; Gispert, M.; Oliver, M.A.; Soler, J.; Tibau, J.; Fàbrega, E. The Effect of Immunocastration on the Behaviour of Pigs. In Proceedings of the 41st International Congress of the the International Society for Applied Ethology, Merida, Mexico, 30 July–3 August 2007; Volume 117. [Google Scholar]
- Cronin, G.M.; Dunshea, F.R.; Butler, K.L.; McCauley, I.; Barnett, J.L.; Hemsworth, P.H. The Effects of Immuno- and Surgical-Castration on the Behaviour and Consequently Growth of Group-Housed, Male Finisher Pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- Rydhmer, L.; Lundström, K.; Andersson, K. Immunocastration Reduces Aggressive and Sexual Behaviour in Male Pigs. Animal 2010, 4, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Calabrese, J.M.; Grodzycki, M.; Paulick, M.; Pearce, M.C.; Rau, F.; Von Borell, E. Impact of Single-Sex and Mixed-Sex Group Housing of Boars Vaccinated against GnRF or Physically Castrated on Body Lesions, Feeding Behaviour and Weight Gain. Appl. Anim. Behav. Sci. 2011, 130, 42–52. [Google Scholar] [CrossRef]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; van Milgen, J. Meta-Analysis of the Effect of Immunocastration on Production Performance, Reproductive Organs and Boar Taint Compounds in Pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Poulsen Nautrup, B.; van Vlaenderen, I.; Aldaz, A.; Mah, C.K. The Effect of Immunization against Gonadotropin-Releasing Factor on Growth Performance, Carcass Characteristics and Boar Taint Relevant to Pig Producers and the Pork Packing Industry: A Meta-Analysis. Res. Vet. Sci. 2018, 119, 182–195. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Swine: 11th Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welfare Quality Welfare Quality Assessment Protocol for Pigs (Sow and Piglets, Growing and Finishing Pigs); Welfare Quality: Lelystad, The Netherlands, 2009.
- Pérez-Ciria, L.; Miana-Mena, F.J.; López-Mendoza, M.C.; Álvarez-Rodríguez, J.; Latorre, M.A. Influence of Immunocastration and Diet on Meat and Fat Quality of Heavy Female and Male Pigs. Animals 2021, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; García-Gudiño, J.; Izquierdo, M.; Brun, A.; Gispert, M.; Blanco-Penedo, I.; Hernández-García, F.I. Non-Destructive Evaluation of Carcass and Ham Traits and Meat Quality Assessment Applied to Early and Late Immunocastrated Iberian Pigs. Animal 2021, 15, 100189. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, A.; Velarde, A.; Rodríguez, P.; Pedernera, C.; Llonch, P.; Fàbrega, E.; Casal, N.; Mainau, E.; Gispert, M.; King, V.; et al. Use of an Anti-GnRF Vaccine to Suppress Estrus in Crossbred Iberian Female Pigs. Theriogenology 2015, 84, 342–347. [Google Scholar] [CrossRef]
- Di Martino, G.; Scollo, A.; Garbo, A.; Lega, F.; Stefani, A.L.; Vascellari, M.; Natale, A.; Zuliani, F.; Zanardello, C.; Tonon, F.; et al. Impact of Sexual Maturity on the Welfare of Immunocastrated v. Entire Heavy Female Pigs. Animal 2018, 12, 1631–1637. [Google Scholar] [CrossRef]
- Rydhmer, L.; Zamaratskaia, G.; Andersson, H.K.; Algers, B.; Guillemet, R.; Lundström, K. Aggressive and Sexual Behaviour of Growing and Finishing Pigs Reared in Groups, without Castration. Acta Agric. Scand. Sect. A Anim. Sci. 2006, 56, 109–119. [Google Scholar] [CrossRef]
- Fàbrega, E.; Velarde, A.; Cros, J.; Gispert, M.; Suárez, P.; Tibau, J.; Soler, J. Effect of Vaccination against Gonadotrophin-Releasing Hormone, Using Improvac®, on Growth Performance, Body Composition, Behaviour and Acute Phase Proteins. Livest. Sci. 2010, 132, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Brewster, V.; Nevel, A. Immunocastration with ImprovacTM Reduces Aggressive and Sexual Behaviours in Male Pigs. Appl. Anim. Behav. Sci. 2013, 145, 32–36. [Google Scholar] [CrossRef]
- Scollo, A.; Di Martino, G.; Bonfanti, L.; Stefani, A.L.; Schiavon, E.; Marangon, S.; Gottardo, F. Tail Docking and the Rearing of Heavy Pigs: The Role Played by Gender and the Presence of Straw in the Control of Tail Biting. Blood Parameters, Behaviour and Skin Lesions. Res. Vet. Sci. 2013, 95, 825–830. [Google Scholar] [CrossRef]
- Di Martino, G.; Scollo, A.; Gottardo, F.; Stefani, A.L.; Schiavon, E.; Capello, K.; Marangon, S.; Bonfanti, L. The Effect of Tail Docking on the Welfare of Pigs Housed under Challenging Conditions. Livest. Sci. 2015, 173, 78–86. [Google Scholar] [CrossRef]
- Claus, R.; Rottner, S.; Rueckert, C. Individual Return to Leydig Cell Function after GnRH-Immunization of Boars. Vaccine 2008, 26, 4571–4578. [Google Scholar] [CrossRef]
- Weiler, U.; Götz, M.; Schmidt, A.; Otto, M.; Müller, S. Influence of Sex and Immunocastration on Feed Intake Behavior, Skatole and Indole Concentrations in Adipose Tissue of Pigs. Animal 2013, 7, 300–308. [Google Scholar] [CrossRef]
- De Roest, K.; Montanari, C.; Fowler, T.; Baltussen, W. Resource Efficiency and Economic Implications of Alternatives to Surgical Castration without Anaesthesia. Animal 2009, 3, 1522–1531. [Google Scholar] [CrossRef]
- Vanhonacker, F.; Verbeke, W. Consumer Response to the Possible Use of a Vaccine Method to Control Boar Taint v. Physical Piglet Castration with Anaesthesia: A Quantitative Study in Four European Countries. Animal 2011, 5, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquale, J.; Vecchio, Y.; Martelli, G.; Sardi, L.; Adinolfi, F.; Nannoni, E. Health Risk Perception, Consumption Intention, and Willingness to Pay for Pig Products Obtained by Immunocastration. Animals 2020, 10, 1548. [Google Scholar] [CrossRef]

| Age | Treatment |
|---|---|
| 15 weeks | V1 |
| 22 weeks | V2 |
| 24 weeks | V2.2 |
| 32 weeks | V3 |
| 36 weeks | V4 |
| Behavioural Category | Description |
|---|---|
| Inactivity in lying | Animal is lying inactive (sleeping or resting) |
| Activity | Animal is busy in one of the following: walking, exploratory behaviour, social and agonistic interactions, sniffing, biting, chewing, or exploring environmental enrichment |
| SC No. of Pigs | IC No. of Pigs | Age Weeks | |
|---|---|---|---|
| Beginning of growing phase | 94 | 94 | 13 |
| Beginning of fattening phase | 83 | 83 | 22 |
| At slaughter | 68 | 71 | 41 (SC) 40 (IC) |
| Carcass Traits | SC | IC | Mann–Whitney U Test |
|---|---|---|---|
| Hot carcass weight (kg) | 145.10 ± 1.31 | 150.54 | p = 0.002 |
| Fat thickness (mm) | 32.31 ± 4.72 | 30.38 ± 0.59 | p = 0.034 |
| Muscle thickness (mm) | 58.47 ± 6.48 | 55.34 ± 8.94 | p = 0.028 |
| Lean meat content (%) | 50.86 | 51.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesenti Rossi, G.; Dalla Costa, E.; Filipe, J.F.S.; Mazzola, S.M.; Motta, A.; Borciani, M.; Gastaldo, A.; Canali, E.; Pilia, F.; Argenton, M.; et al. Does Immunocastration Affect Behaviour and Body Lesions in Heavy Pigs? Vet. Sci. 2022, 9, 410. https://doi.org/10.3390/vetsci9080410
Pesenti Rossi G, Dalla Costa E, Filipe JFS, Mazzola SM, Motta A, Borciani M, Gastaldo A, Canali E, Pilia F, Argenton M, et al. Does Immunocastration Affect Behaviour and Body Lesions in Heavy Pigs? Veterinary Sciences. 2022; 9(8):410. https://doi.org/10.3390/vetsci9080410
Chicago/Turabian StylePesenti Rossi, Gaia, Emanuela Dalla Costa, Joel Fernando Soares Filipe, Silvia Michela Mazzola, Ambra Motta, Marzia Borciani, Alessandro Gastaldo, Elisabetta Canali, Federica Pilia, Marco Argenton, and et al. 2022. "Does Immunocastration Affect Behaviour and Body Lesions in Heavy Pigs?" Veterinary Sciences 9, no. 8: 410. https://doi.org/10.3390/vetsci9080410
APA StylePesenti Rossi, G., Dalla Costa, E., Filipe, J. F. S., Mazzola, S. M., Motta, A., Borciani, M., Gastaldo, A., Canali, E., Pilia, F., Argenton, M., Caniatti, M., Pecile, A., Minero, M., & Barbieri, S. (2022). Does Immunocastration Affect Behaviour and Body Lesions in Heavy Pigs? Veterinary Sciences, 9(8), 410. https://doi.org/10.3390/vetsci9080410







