Expression Analysis of Outer Membrane Protein HPS_06257 in Different Strains of Glaesserella parasuis and Its Potential Role in Protective Immune Response against HPS_06257-Expressing Strains via Antibody-Dependent Phagocytosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Glaesserella parasuis
2.2. DNA Isolation and PCR
2.3. Preparation of Polyclonal Antibody against HPS_06257
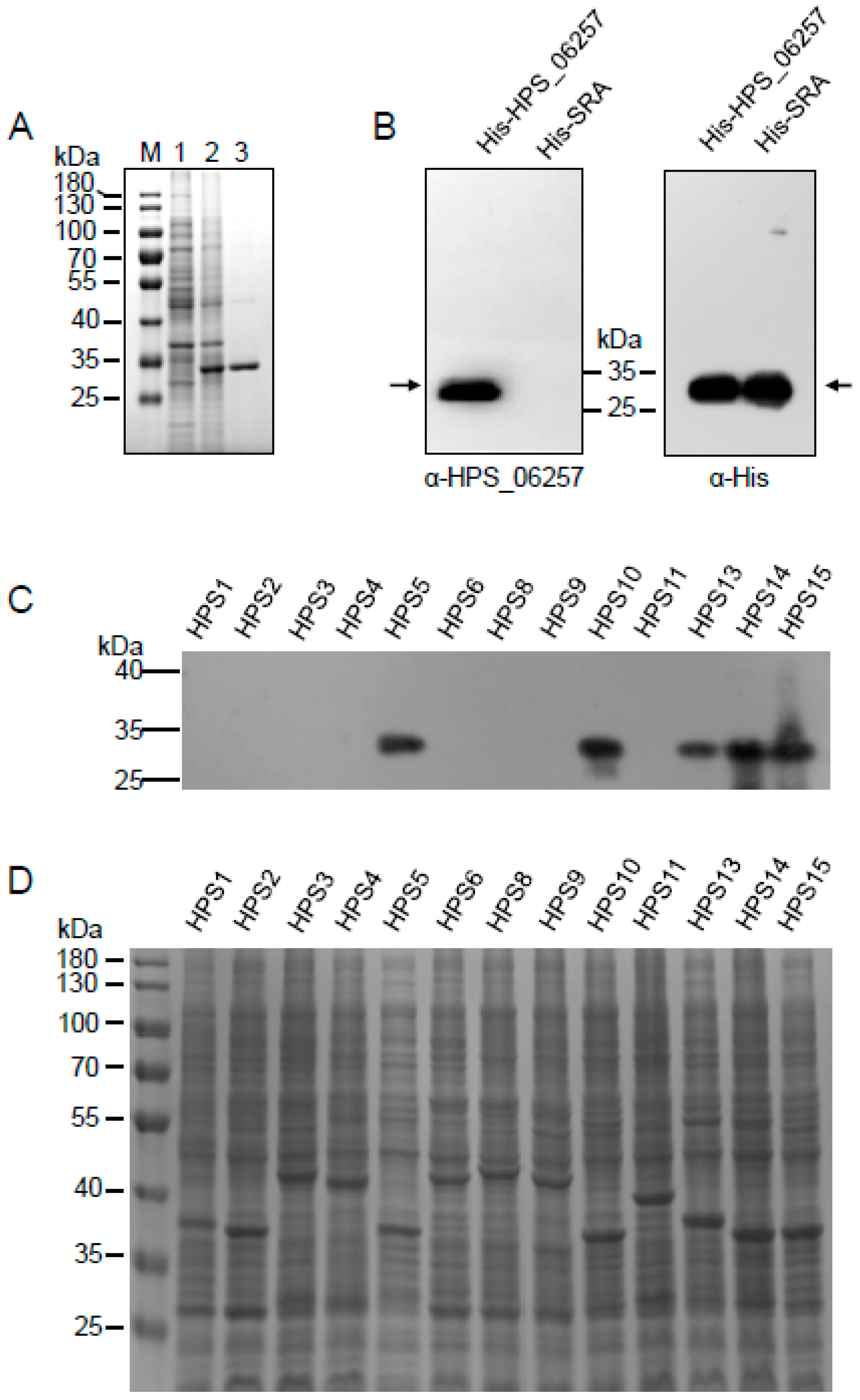
2.4. Western Blotting
2.5. Immunization of Mice and Challenge with G. parasuis
2.6. Antibody-Mediated Phagocytosis
2.7. Statistical Analysis
3. Results
3.1. Differential Presence of HPS_06257 Gene in Different G. parasuis Strains
3.2. Differential Expression of HPS_06257 Protein in Different Strains of G. parasuis
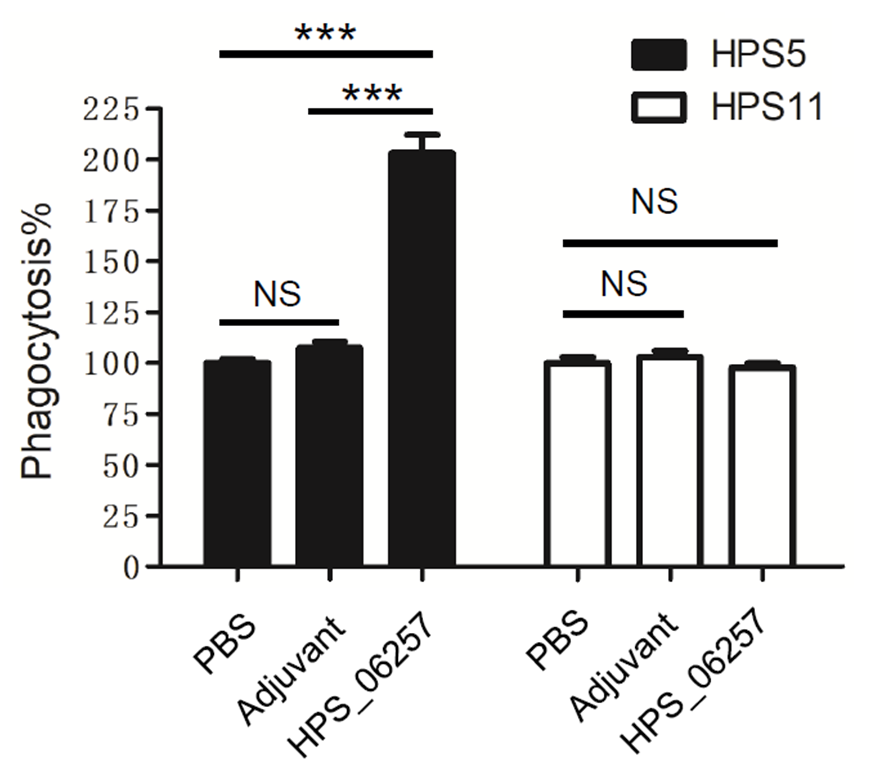
3.3. Differential Immunoprotective Effects of HPS_06257 against HPS_06257-Expressing Strains and HPS_06257-Null Strains
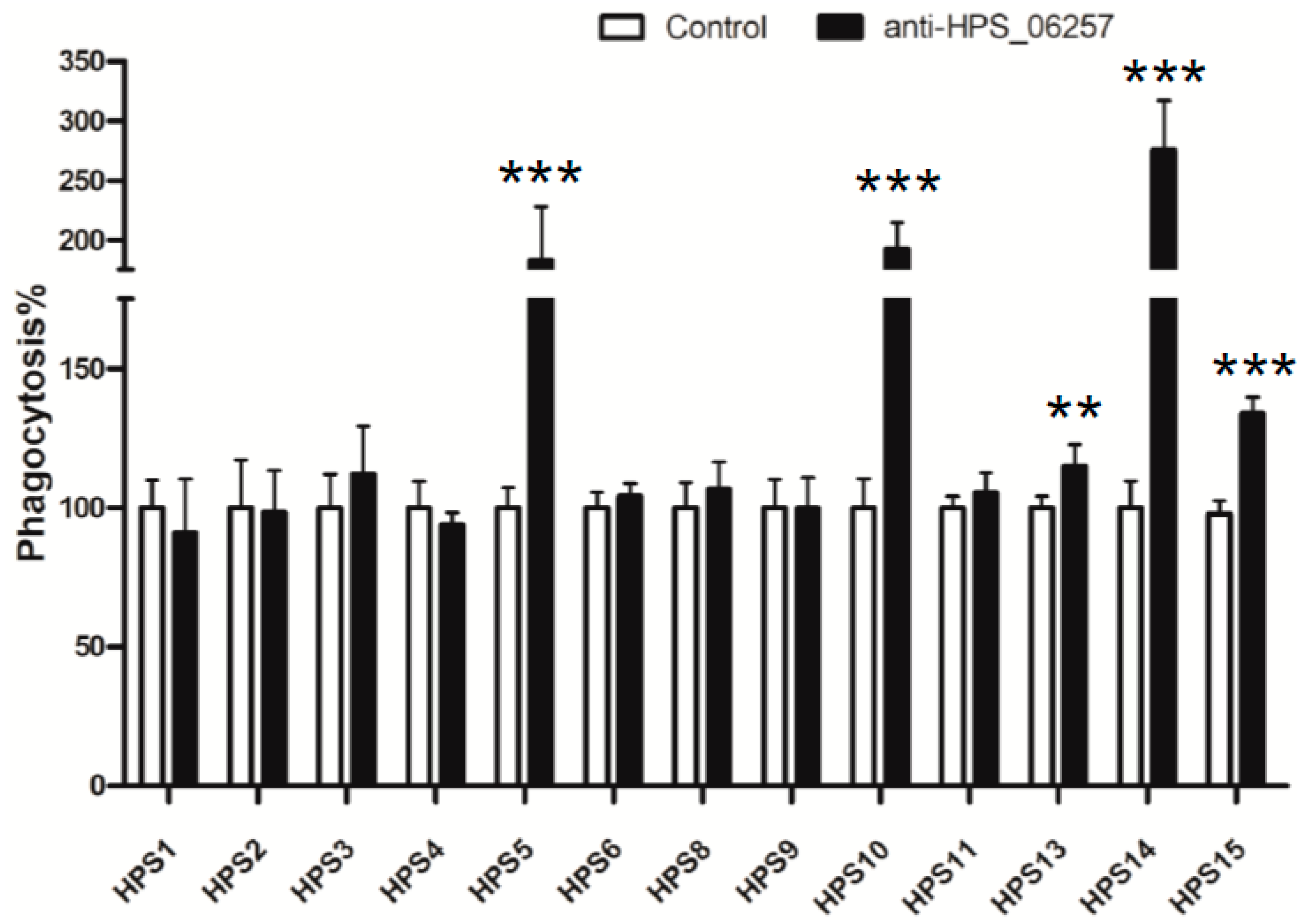
3.4. Antibody-Dependent Phagocytosis Is Associated with the Immunoprotective Effects of HPS_06257 against HPS_06257-Expressing Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, S.; Pijoan, C. Haemophilus parasuis: New trends on diagnosis, epidemiology and control. Vet. Microbiol. 2004, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nedbalcova, K.; Kucerova, Z.; Krejci, J.; Tesarik, R.; Gopfert, E.; Kummer, V.; Leva, L.; Kudlackova, H.; Ondriasova, R.; Faldyna, M. Passive immunisation of post-weaned piglets using Hyperimmune serum against experimental Haemophilus parasuis infection. Res. Vet. Sci. 2011, 91, 225–229. [Google Scholar] [CrossRef]
- Olvera, A.; Ballester, M.; Nofrarías, M.; Sibila, M.; Aragon, V. Differences in phagocytosis susceptibility in Haemophilus parasuis strains. Vet. Res. 2009, 40, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xue, Q.; Zeng, Q.; Zhao, Z. Haemophilus parasuis vaccines. Vet. Immunol. Immunopathol. 2016, 180, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, Y.; Zhao, J.; Hu, Q.; Hu, Y.; Zhang, A.; Chen, H.; Jin, M. Identification and characterization of novel immunogenic outer membrane proteins of Haemophilus parasuis serovar 5. Vaccine 2009, 27, 5271–5277. [Google Scholar] [CrossRef]
- Yuan, F.; Fu, S.; Hu, J.; Li, J.; Chang, H.; Hu, L.; Chen, H.; Tian, Y.; Bei, W. Evaluation of recombinant proteins of Haemophilus parasuis strain SH0165 as vaccine candidates in a mouse model. Res. Vet. Sci. 2012, 93, 51–56. [Google Scholar] [CrossRef]
- Li, M.; Song, S.; Yang, D.; Li, C.; Li, G. Identification of secreted proteins as novel antigenic vaccine candidates of Haemophilus parasuis serovar 5. Vaccine 2015, 33, 1695–1701. [Google Scholar] [CrossRef]
- Wen, Y.; Yan, X.; Wen, Y.; Cao, S.; He, L.; Ding, L.; Zhang, L.; Zhou, P.; Huang, X.; Wu, R.; et al. Immunogenicity of the recombinant HxuCBA proteins encoded by hxuCBA gene cluster of Haemophilus parasuis in mice. Gene 2016, 591, 478–483. [Google Scholar] [CrossRef]
- Li, G.; Xie, F.; Li, J.; Liu, J.; Li, D.; Zhang, Y.; Langford, P.R.; Li, Y.; Liu, S.; Wang, C. Identification of novel Haemophilus parasuis serovar 5 vaccine candidates using an immunoproteomic approach. J. Proteomics. 2017, 163, 111–117. [Google Scholar] [CrossRef]
- Barasuol, B.M.; Guizzo, J.A.; Fegan, J.E.; Martínez-Martínez, S.; Rodríguez-Ferri, E.F.; Gutiérrez-Martín, C.B.; Kreutz, L.C.; Schryvers, A.B.; Frandoloso, R. New insights about functional and cross-reactive properties of antibodies generated against recombinant TbpBs of Haemophilus parasuis. Sci. Rep. 2017, 7, 10377. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, M.; Xu, J.; Ou, J.; Wang, Y.; Liu, H.; Liu, J.; Chen, H.; Bei, W. Immunogenicity and protective efficacy of recombinant Haemophilus parasuis SH0165 putative outer membrane proteins. Vaccine 2013, 31, 347–353. [Google Scholar] [CrossRef] [PubMed]
- McCaig, W.D.; Loving, C.L.; Hughes, H.R.; Brockmeier, S.L. Characterization and Vaccine Potential of Outer Membrane Vesicles Produced by Haemophilus parasuis. PLoS ONE 2016, 11, e0149132. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yue, M.; Zhou, R.; Jin, Q.; Fan, Y.; Bei, W.; Chen, H. Genomic characterization of Haemophilus parasuis SH0165, a highly virulent strain of serovar 5 prevalent in China. PLoS ONE 2011, 6, e19631. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kwok, A.H.; Jiang, J.; Zou, Y.; Zheng, F.; Chen, P.; Hou, C.; Leung, F.C.; Jiang, P. Complete genome analysis of a Haemophilus parasuis serovar 12 strain from China. PLoS ONE 2013, 8, e68350. [Google Scholar] [CrossRef]
- Nicholson, T.L.; Brunelle, B.W.; Bayles, D.O.; Alt, D.P.; Shore, S.M. Comparative genomic and methylome analysis of non-virulent D74 and virulent Nagasaki Haemophilus parasuis isolates. PLoS ONE 2018, 13, e0205700. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Ning, K.; Shen, L.; Qi, X.; Wang, J. Construction and Application of MALDI-TOF Mass Spectrometry for the Detection of Haemophilus parasuis. Biomed. Res. Int. 2021, 2021, 5588855. [Google Scholar] [CrossRef]
- Howell, K.J.; Peters, S.E.; Wang, J.; Hernandez-Garcia, J.; Weinert, L.A.; Luan, S.L.; Chaudhuri, R.R.; Angen, Ø.; Aragon, V.; Williamson, S.M.; et al. Development of a Multiplex PCR Assay for Rapid Molecular Serotyping of Haemophilus parasuis. J. Clin. Microbiol. 2015, 53, 3812–3821. [Google Scholar] [CrossRef]
- Qiu, Y.; Shen, Y.; Li, X.; Liu, Q.; Ma, Z. Polyclonal antibody to porcine p53 protein: A new tool for studying the p53 pathway in a porcine model. Biochem. Biophys. Res. Commun. 2008, 377, 151–155. [Google Scholar] [CrossRef]
- Huang, H.; Sharma, M.; Zhang, Y.; Li, C.; Liu, K.; Wei, J.; Shao, D.; Li, B.; Ma, Z.; Cao, R.; et al. Expression Profile of Porcine TRIM26 and Its Inhibitory Effect on Interferon-β Production and Antiviral Response. Genes 2020, 11, 1226. [Google Scholar] [CrossRef]
- Aronoff, D.M.; Hao, Y.; Chung, J.; Coleman, N.; Lewis, C.; Peres, C.M.; Serezani, C.H.; Chen, G.H.; Flamand, N.; Brock, T.G.; et al. Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii. J. Immunol. 2008, 180, 222–230. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, Y.; Li, Q.; Wei, J.; Liu, K.; Shao, D.; Li, B.; Olszewski, M.A.; Ma, Z.; Qiu, Y. Expression profile of porcine scavenger receptor A and its role in bacterial phagocytosis by macrophages. Dev. Comp. Immunol. 2020, 104, 103534. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Kinoshita, M.; Inoue, K.; Ohara, J.; Moriyama, K. Immunoglobulin for Treating Bacterial Infections: One More Mechanism of Action. Antibodies 2019, 8, 52. [Google Scholar] [CrossRef] [PubMed]


| Strain | GenBank Accession No. | Assembly Status | Serovar | Location | HPS_06257 | PalA | Omp2 | D15 |
|---|---|---|---|---|---|---|---|---|
| SH0104 | NZ_CP024412 | Whole genome | 5 | China | + | + | + | + |
| YHP170504 | NZ_CP054195 | Whole genome | / | China | ||||
| HPS412 | NZ_CP041334 | whole genome | / | China | + | + | + | + |
| aHPS7 | NZ_CP049090 | Whole genome | 7 | China | + | + | + | |
| SCW0912 | NZ_CP046114 | Whole genome | 5 | China | + | + | + | + |
| HPS-1 | NZ_CP040243 | Whole genome | / | China | + | + | + | + |
| CL120103 | NZ_CP020085 | Whole genome | / | China | + | + | ||
| KL0318 | NZ_CP009237 | Whole genome | / | China | + | + | + | + |
| SC1401 | NZ_CP015099 | Whole genome | / | China | + | + | ||
| sHPS7 | NZ_CP049088 | Whole genome | 7 | China | + | + | + | |
| vHPS7 | NZ_CP049089 | Whole genome | 7 | China | + | + | ||
| GZ20170512 | NZ_CP029150 | Whole genome | / | China | + | + | + | + |
| 29755 | NZ_CP021644 | Whole genome | 5 | USA | + | + | + | + |
| D74 | NZ_CP018032 | Whole genome | 9 | Sweden | + | + | ||
| SH0165 | NC_CP001321 | Whole genome | 5 | China | + | + | + | + |
| ZJ0906 | NC_CP005384 | Whole genome | 12 | China | + | + | + | + |
| Nagasaki | NZ_CP018034 | Whole genome | 5 | Japan | + | + | + | + |
| SH03 | NZ_CP009158 | Whole genome | / | China | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Shi, H.; Cheng, X.; Wang, X.; Li, Z.; Shao, D.; Liu, K.; Wei, J.; Li, B.; Wang, J.; et al. Expression Analysis of Outer Membrane Protein HPS_06257 in Different Strains of Glaesserella parasuis and Its Potential Role in Protective Immune Response against HPS_06257-Expressing Strains via Antibody-Dependent Phagocytosis. Vet. Sci. 2022, 9, 342. https://doi.org/10.3390/vetsci9070342
Chen X, Shi H, Cheng X, Wang X, Li Z, Shao D, Liu K, Wei J, Li B, Wang J, et al. Expression Analysis of Outer Membrane Protein HPS_06257 in Different Strains of Glaesserella parasuis and Its Potential Role in Protective Immune Response against HPS_06257-Expressing Strains via Antibody-Dependent Phagocytosis. Veterinary Sciences. 2022; 9(7):342. https://doi.org/10.3390/vetsci9070342
Chicago/Turabian StyleChen, Xiaojun, Hanye Shi, Xingyu Cheng, Xiaoxu Wang, Zongjie Li, Donghua Shao, Ke Liu, Jianchao Wei, Beibei Li, Jian Wang, and et al. 2022. "Expression Analysis of Outer Membrane Protein HPS_06257 in Different Strains of Glaesserella parasuis and Its Potential Role in Protective Immune Response against HPS_06257-Expressing Strains via Antibody-Dependent Phagocytosis" Veterinary Sciences 9, no. 7: 342. https://doi.org/10.3390/vetsci9070342
APA StyleChen, X., Shi, H., Cheng, X., Wang, X., Li, Z., Shao, D., Liu, K., Wei, J., Li, B., Wang, J., Zhou, B., Ma, Z., & Qiu, Y. (2022). Expression Analysis of Outer Membrane Protein HPS_06257 in Different Strains of Glaesserella parasuis and Its Potential Role in Protective Immune Response against HPS_06257-Expressing Strains via Antibody-Dependent Phagocytosis. Veterinary Sciences, 9(7), 342. https://doi.org/10.3390/vetsci9070342








