Microscopical Evaluation of Smears of the Leptomeninges to Predict Meningitis in Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Necropsy and Sampling
2.3. Bacterial Culture
2.4. Cytological Examination of Smears of the Leptomeninges
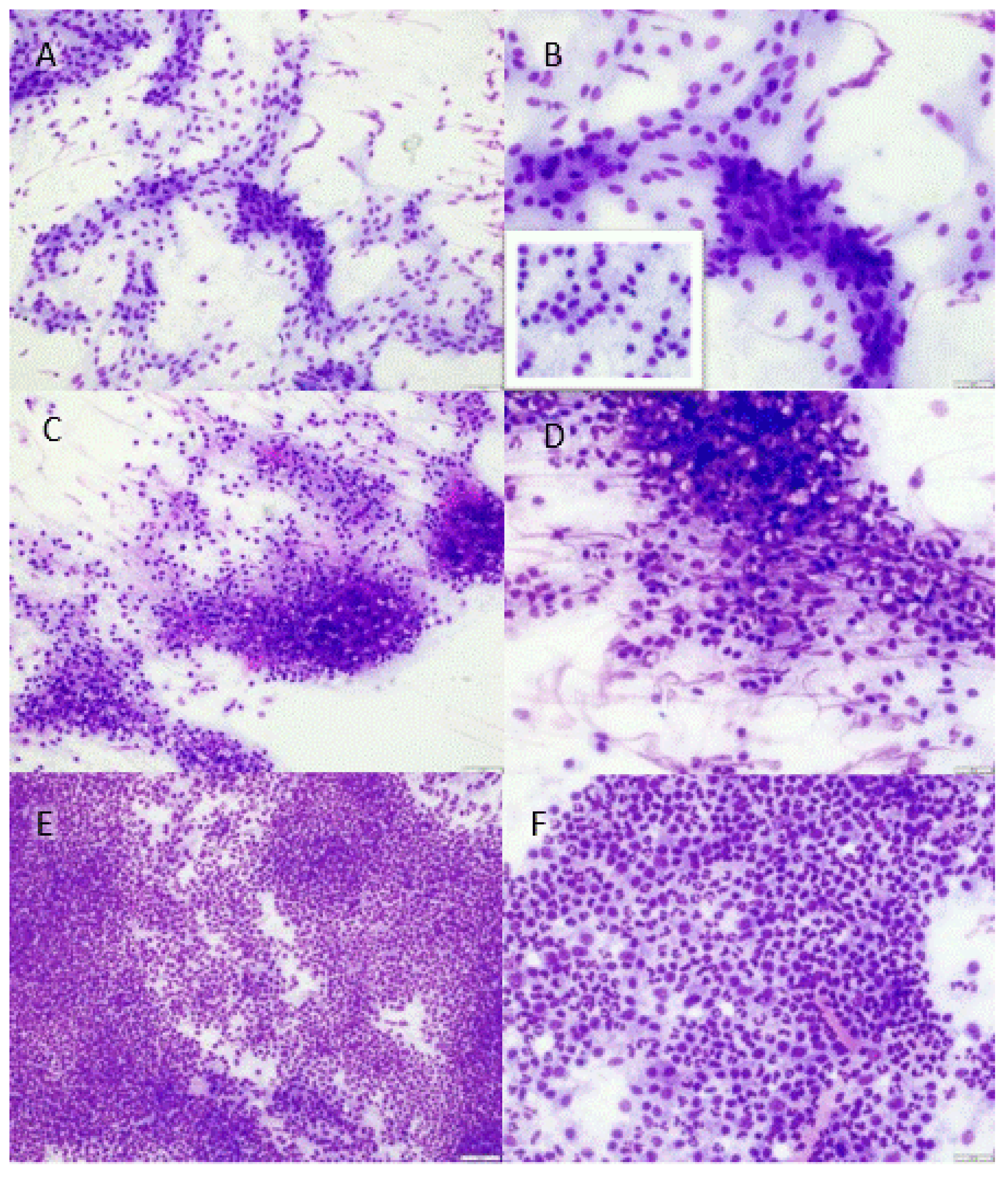
- Low: maximum of 5 neutrophils per 400× magnification field, only found after thorough searching. Other visible cells only comprised monomorphous, flat spindeloid to sometimes slightly cuboidal cells which were regularly surrounded by some faint fibrillar extracellular matrix (fibroblasts with collagen) (Figure 1a,b). No other clusters of potential mononuclear inflammatory cells were visible.
- Moderate: amid areas with fibroblasts and collagen, small focal to multifocal clusters of neutrophils (5–100 per 400× magnification field), mostly only spotted after some searching through the slide. Sometimes, clusters of heterogeneous round cells were observed (Figure 1c,d) that did not resemble the monomorphous flat to cuboidal fibroblastic cells (likely mononuclear inflammatory cells).
- High: large numbers (>100, ‘uncountable’) of neutrophils already visible with low magnification (40–100×) in almost every frame viewed (Figure 1e,f). The presence of other cell types (mononuclear inflammatory cells, fibroblasts) was generally obscured due to the large numbers of neutrophils.
2.5. Data Analysis
3. Results
3.1. Bacterial Cultures of the Leptomeninges
3.2. Presence of Neutrophilic Granulocytes in the Cytologic Impression Smears
3.3. Presence of Bacteria in the Cytology Smears
3.4. Sensitivity, Specificity, and Predictive Values of Microscopic Evaluation of Smears of the Leptomeninges (Abundance of Neutrophils, Presence of Short Chains of Coccoid Bacteria)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottschalk, M.; Segura, M. Streptococcosis. In Diseases of Swine, 11th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 934–950. [Google Scholar]
- Geudeke, T.; Duinhof, T.; Heuvelink, A. Monitoring Diergezondheid Varkens—Rapportage Tweede Halfjaar 2019; Royal GD: Deventer, The Netherlands, 2019. [Google Scholar]
- Bonten, M.J.M.; Geijlswijk, I.M.; Heederik, D.J.J.; Mevius, D.J.; Wagenaar, J.A.; Sanders, P. Het Gebruik van Antibiotica Bij Landbouwhuisdieren in 2020; SDa, Autoriteit Diergeneesmiddelen: Utrecht, The Netherlands, 2020. [Google Scholar]
- Derk, J.; Jones, H.E.; Como, C.; Pawlikowski, B.; Siegenthaler, J.A. Living on the Edge of the CNS: Meninges Cell Diversity in Health and Disease. Front. Cell. Neurosci. 2021, 15, 703944. [Google Scholar] [CrossRef]
- Sanford, S.E. Gross and Histopathological Findings in Unusual Lesions Caused by Streptococcus Suis in Pigs. I. Cardiac Lesions. Can. J. Vet. Res. 1987, 51, 481–485. [Google Scholar] [PubMed]
- Dunbar, S.A.; Eason, R.A.; Musher, D.M.; Clarridge Iii, J.E. Microscopic Examination and Broth Culture of Cerebrospinal Fluid in Diagnosis of Meningitis. J. Clin. Microbiol. 1998, 36, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, C.; Feng, Y.; Yang, W.; Song, H.; Chen, Z.; Yu, H.; Pan, X.; Zhou, X.; Wang, H.; et al. Streptococcal Toxic Shock Syndrome Caused by Streptococcus Suis Serotype 2. PLoS Med. 2006, 3, 668–676. [Google Scholar] [CrossRef]
- Huh, H.J.; Park, K.J.; Jang, J.H.; Lee, M.; Lee, J.H.; Ahn, Y.H.; Kang, C.I.; Ki, C.S.; Lee, N.Y. Streptococcus Suis Meningitis with Bilateral Sensorineural Hearing Loss. Korean J. Lab. Med. 2011, 31, 205–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barenfanger, J.; Drake, C.A. Interpretation of Gram Stains for the Nonmicrobiologist. Lab. Med. 2001, 32, 368–375. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Erickson, G.A.; Hesse, R.A.; Nelson, E.A.; Rossow, S.; Scaria, J.; Slavic, D. Diagnostic Tests, Test Performance, and Consideration for Interpretation. In Diseases of Swine, 11th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 75–97. [Google Scholar]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Bleuzé, M.; Gottschalk, M.; Segura, M. Neutrophils in Streptococcus Suis Infection: From Host Defense to Pathology. Microorganisms 2021, 9, 2392. [Google Scholar] [CrossRef] [PubMed]
- Varela, N.P.; Gadbois, P.; Thibault, C.; Gottschalk, M.; Dick, P.; Wilson, J. Antimicrobial Resistance and Prudent Drug Use for Streptococcus Suis. Anim. Health Res. Rev. 2013, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É. Colibacillosis. In Diseases of Swine; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 807–834. [Google Scholar]
- Cantile, C.; Youssef, S. Chapter 4. Nervous System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 1, pp. 353–361. ISBN 9780702053177. [Google Scholar]
- Beineke, A.; Bennecke, K.; Neis, C.; Schröder, C.; Waldmann, K.H.; Baumgärtner, W.; Valentin-Weigand, P.; Baums, C.G. Comparative Evaluation of Virulence and Pathology of Streptococcus Suis Serotypes 2 and 9 in Experimentally Infected Growers. Vet. Microbiol. 2008, 128, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, N.; Ventrella, D.; Giunti, M.; Dondi, F.; Sorrentino, N.C.; Fraldi, A.; Surace, E.M.; Bacci, M.L. Access to Cerebrospinal Fluid in Piglets via the Cisterna Magna: Optimization and Description of the Technique. Lab. Anim. 2014, 48, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Meurer, M.; Öhlmann, S.; Bonilla, M.C.; Valentin-Weigand, P.; Beineke, A.; Hennig-Pauka, I.; Schwerk, C.; Schroten, H.; Baums, C.G.; von Köckritz-Blickwede, M.; et al. Role of Bacterial and Host DNases on Host-Pathogen Interaction during Streptococcus Suis Meningitis. Int. J. Mol. Sci. 2020, 21, 5289. [Google Scholar] [CrossRef] [PubMed]
- McGavin, M.D.; Zachary, J.F. Section II Pathology of Organs: Nervous System. In Pathology basis of Veterinary Disease; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; pp. 833–972. [Google Scholar]
- USDA. Swine 2012 Part III: Changes in the U.S. Swine Industry, 1995−2012; USDA: Washington, DC, USA, 2015. [Google Scholar]

| Abundance of Neutrophils in Smear | |||||
|---|---|---|---|---|---|
| Bacterial Culture | High (n = 18) | Moderate (n = 17) | Low (n = 19) | Total | |
| Negative | 1 | 3 | 14 | 18 | |
| Positive | Streptococcus spp. | 10 | 12 | 2 | 24 |
| E. coli | 1 | 0 | 1 | 2 | |
| G. parasuis | 1 | 0 | 0 | 1 | |
| E. coli and Streptococcus spp. | 2 | 0 | 0 | 2 | |
| Mixed culture and Streptococcus spp. | 1 | 1 | 0 | 2 | |
| Mixed culture | 2 | 1 | 2 | 5 | |
| Short Chains of Coccoid Shaped Bacteria in Smear | ||||
|---|---|---|---|---|
| Bacterial Culture | Present (n = 12) | Absent (n = 42) | Total | |
| Negative | 0 | 18 | 18 | |
| Positive | Streptococcus spp. | 10 | 14 | 24 |
| E. coli | 0 | 2 | 2 | |
| G. parasuis | 0 | 1 | 1 | |
| E. coli and Streptococcus spp. | 0 | 2 | 2 | |
| Mixed culture and Streptococcus spp. | 2 | 0 | 2 | |
| Mixed culture | 0 | 5 | 5 | |
| Bacterial Culture of Leptomeninges | |||
|---|---|---|---|
| Abundance of Neutrophils in Smear of the Leptomeninges | Positive | Negative | Total |
| High and moderate | 31 | 4 | 35 |
| Low | 5 | 14 | 19 |
| Total | 36 | 18 | 54 |
| Streptococcus spp. Found in Bacterial Culture | |||
|---|---|---|---|
| Presence of Short Chains of Coccoid Shapes | Yes | No | Total |
| Yes | 12 | 0 | 12 |
| No | 16 | 26 | 42 |
| Total | 28 | 26 | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schyns, M.; Maes, D.; Kuller, W.; Weerts, E. Microscopical Evaluation of Smears of the Leptomeninges to Predict Meningitis in Piglets. Vet. Sci. 2022, 9, 341. https://doi.org/10.3390/vetsci9070341
Schyns M, Maes D, Kuller W, Weerts E. Microscopical Evaluation of Smears of the Leptomeninges to Predict Meningitis in Piglets. Veterinary Sciences. 2022; 9(7):341. https://doi.org/10.3390/vetsci9070341
Chicago/Turabian StyleSchyns, Marc, Dominiek Maes, Wikke Kuller, and Erik Weerts. 2022. "Microscopical Evaluation of Smears of the Leptomeninges to Predict Meningitis in Piglets" Veterinary Sciences 9, no. 7: 341. https://doi.org/10.3390/vetsci9070341
APA StyleSchyns, M., Maes, D., Kuller, W., & Weerts, E. (2022). Microscopical Evaluation of Smears of the Leptomeninges to Predict Meningitis in Piglets. Veterinary Sciences, 9(7), 341. https://doi.org/10.3390/vetsci9070341







