Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fecal Microbiota Transplantation Preparation
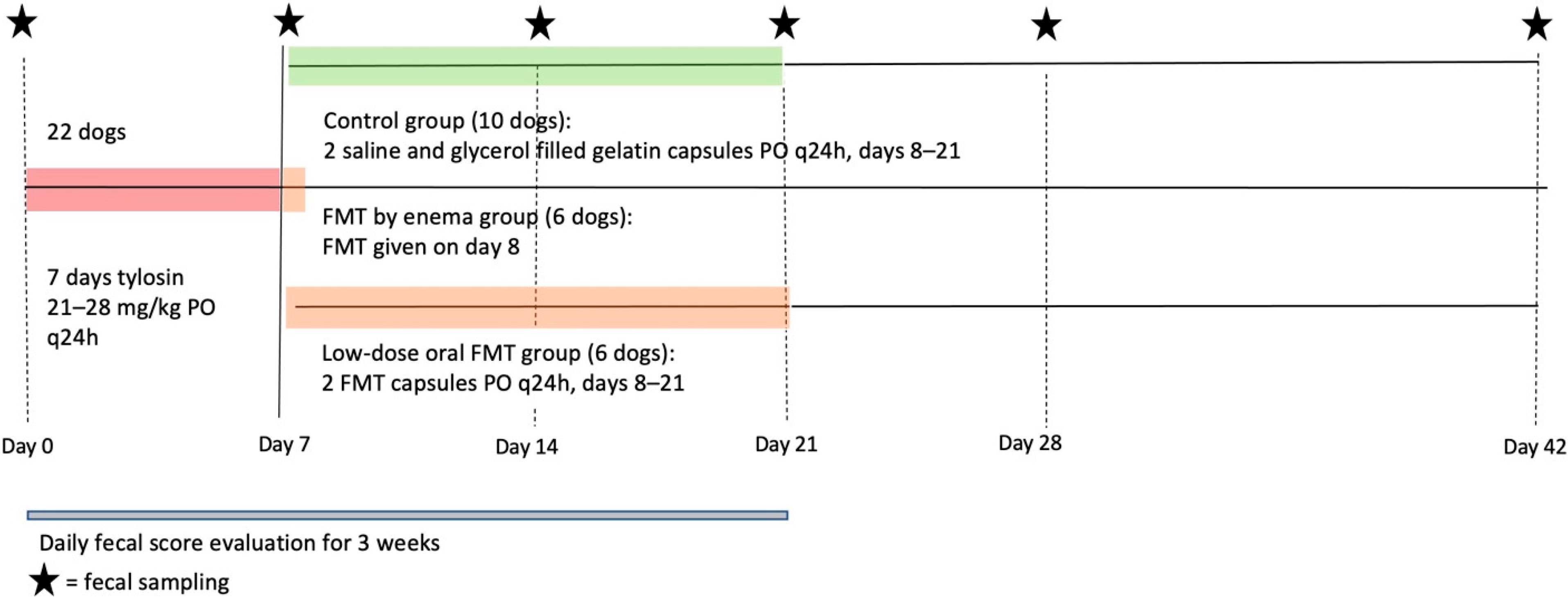
2.3. Study Design
2.4. DNA Extraction and Quantitative PCR
2.5. Fecal Bile Acid Concentrations
2.6. Statistical Analysis
3. Results
3.1. Fecal Scores and Dog Observation
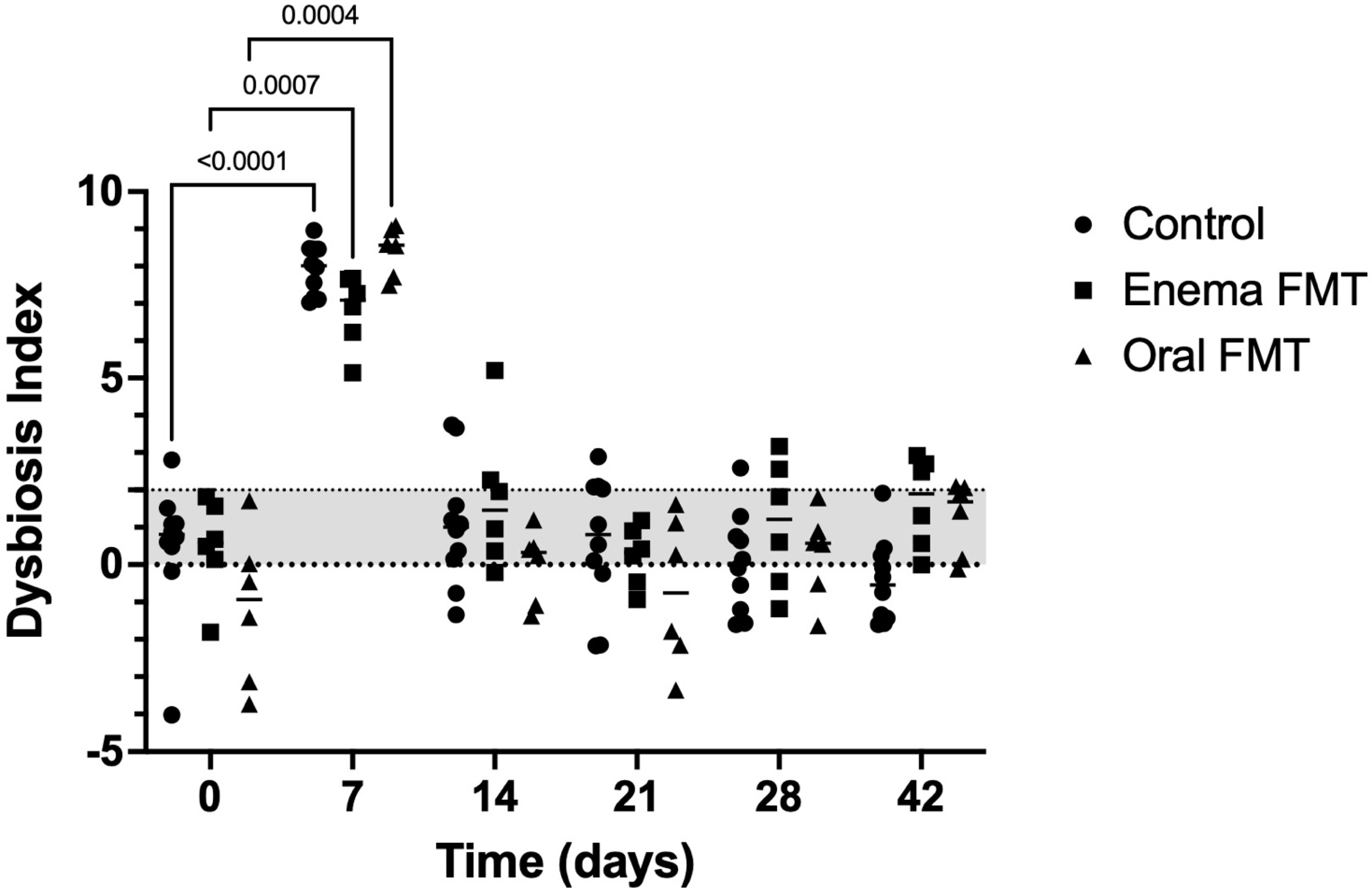
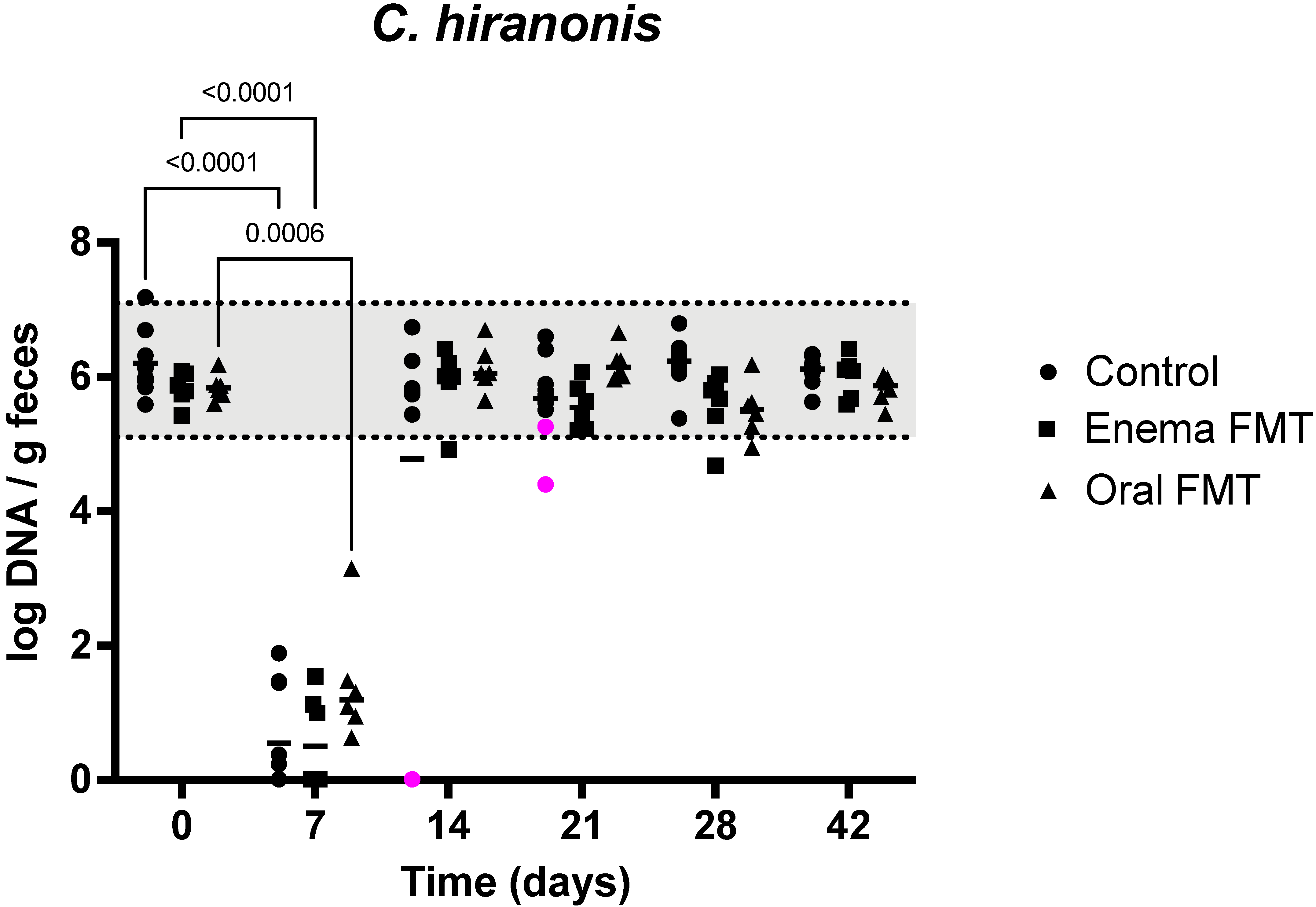
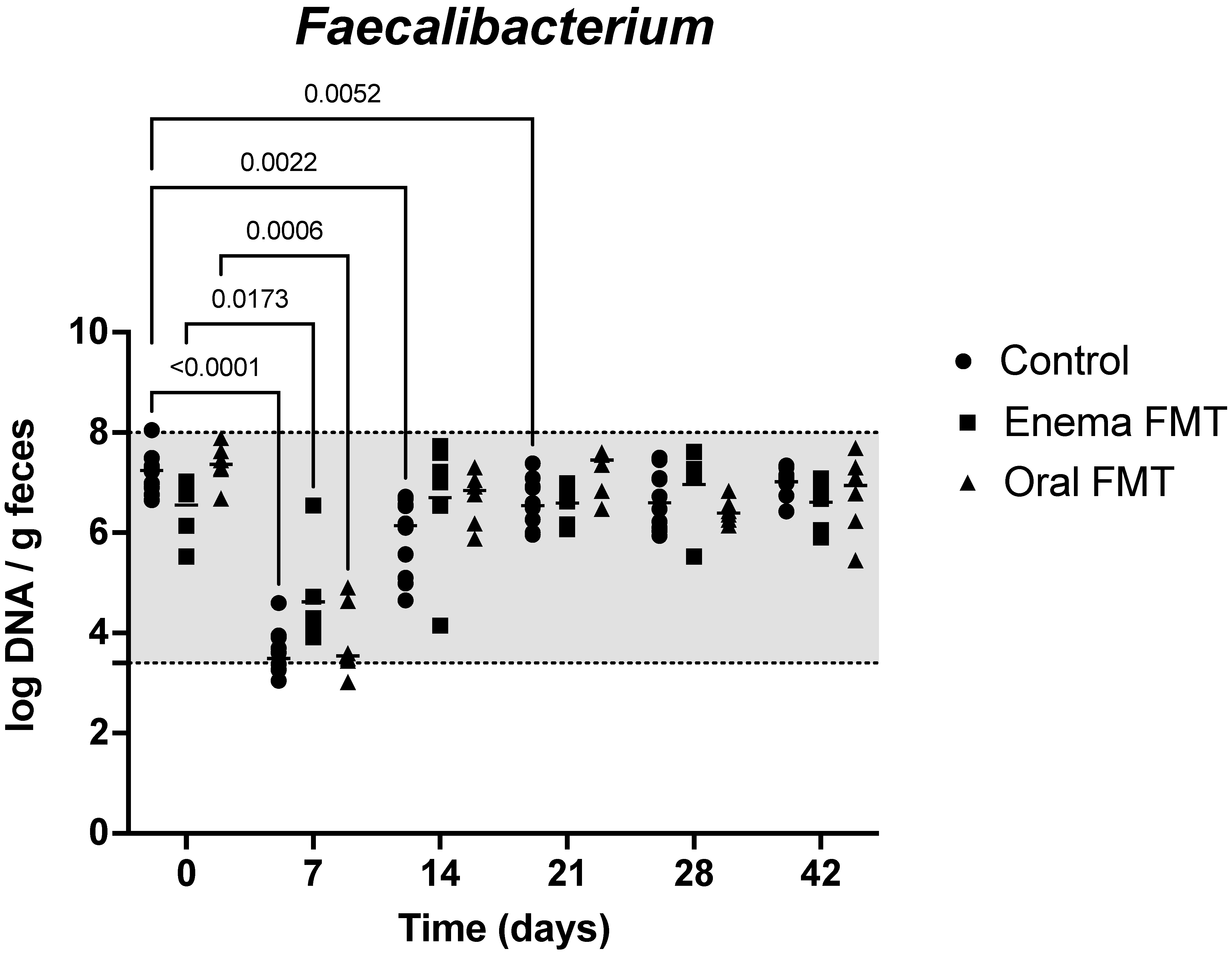
3.2. Abundance of Bacterial Taxa and Fecal Dysbiosis Index
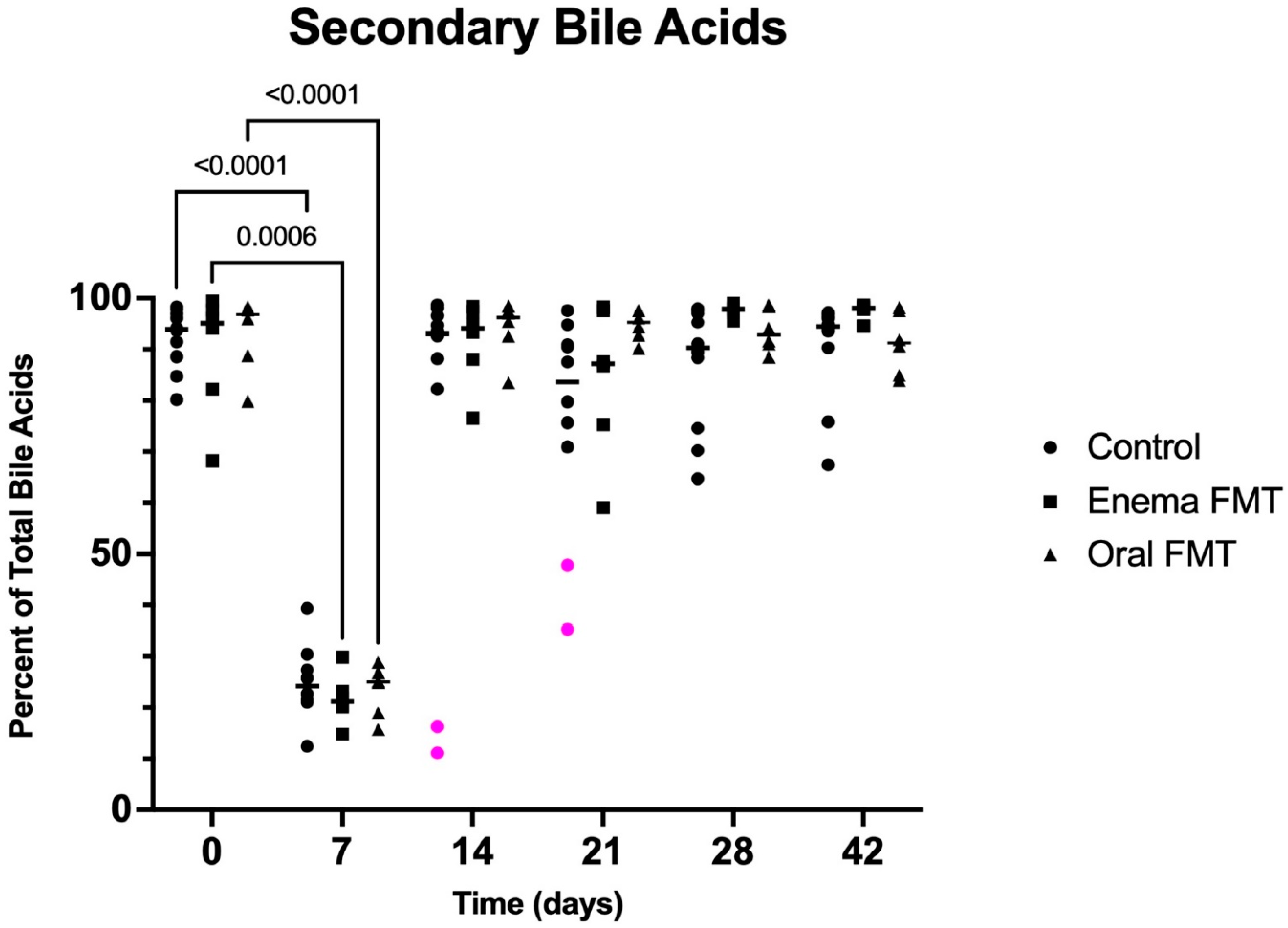
3.3. Fecal Bile Acid Concentrations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2019, 6, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziese, A.L.; Suchodolski, J.S. Impact of Changes in Gastrointestinal Microbiota in Canine and Feline Digestive Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Westermarck, E.; Steiner, J.M.; Wolcott, R.D.; Spillmann, T.; Harmoinen, J.A. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Gronvold, A.M.; L’Abee-Lund, T.M.; Sorum, H.; Skancke, E.; Yannarell, A.C.; Mackie, R.I. Changes in fecal microbiota of healthy dogs administered amoxicillin. Fems. Microbiol. Ecol. 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Igarashi, H.; Maeda, S.; Ohno, K.; Horigome, A.; Odamaki, T.; Tsujimoto, H. Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PLoS ONE 2014, 9, e107909. [Google Scholar] [CrossRef]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs With Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef]
- Espinosa-Gongora, C.; Jessen, L.R.; Kieler, I.N.; Damborg, P.; Bjornvad, C.R.; Gudeta, D.D.; Dos Santos, T.P.; Sablier-Gallis, F.; Sayah-Jeanne, S.; Corbel, T.; et al. Impact of oral amoxicillin and amoxicillin/clavulanic acid treatment on bacterial diversity and beta-lactam resistance in the canine faecal microbiota. J Antimicrob Chemother 2020, 75, 351–361. [Google Scholar] [CrossRef]
- Budde, J.A. Tylosin drug monograph. In Plumb’s Veterinary Drugs Online; Brief Media: Tulsa, OK, USA, 2022; Available online: https://www.plumbsveterinarydrugs.com (accessed on 30 April 2022).
- Cao, X.Y.; Dong, M.; Shen, J.Z.; Wu, B.B.; Wu, C.M.; Du, X.D.; Wang, Z.; Qi, Y.T.; Li, B.Y. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int. J. Antimicrob. Agents 2006, 27, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, A.; Pozzoli, C.; Poli, E.; Lazzaretti, M.; Cantoni, A.; Grandi, D.; Giovannini, E.; Coruzzi, G. Effect of the macrolide antibacterial drug, tylosin, on TNBS-induced colitis in the rat. Pharmacology 2005, 74, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, M.; Takamine, F.; Imamura, T.; Benno, Y. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity. Int. J. Syst. Evol. Microbiol. 2001, 51, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. 1), S21–S26. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef] [Green Version]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Borody, T.J.; Eslick, G.D.; Clancy, R.L. Fecal microbiota transplantation as a new therapy: From Clostridioides difficile infection to inflammatory bowel disease, irritable bowel syndrome, and colon cancer. Curr. Opin. Pharmacol. 2019, 49, 43–51. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Huang, H.; Li, Y.; Chen, H.; He, J.; Du, Y.; Chen, Y.; Zhou, Y.; Nie, Y. Are There Potential Applications of Fecal Microbiota Transplantation beyond Intestinal Disorders? Biomed Res. Int. 2019, 2019, 3469754. [Google Scholar] [CrossRef] [Green Version]
- Chaitman, J.; Gaschen, F. Fecal Microbiota Transplantation in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 219–233. [Google Scholar] [CrossRef]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.F.; Weese, J.S.; Costa, M.C. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef] [PubMed]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. Fems. Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, M.; Suchodolski, J.S.; Straubinger, R.K.; Wolf, G.; Steiner, J.M.; Lidbury, J.A.; Neuerer, F.; Hartmann, K.; Unterer, S. Effect of amoxicillin-clavulanic acid on clinical scores, intestinal microbiome, and amoxicillin-resistant Escherichia coli in dogs with uncomplicated acute diarrhea. J. Vet. Intern. Med. 2020, 34, 1166–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Martins, R.; Sullivan, M.C.; Friedman, E.S.; Misic, A.M.; El-Fahmawi, A.; De Martinis, E.C.P.; O’Brien, K.; Chen, Y.; Bradley, C.; et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Larouche-Lebel, E.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Gut Dysbiosis and Its Associations with Gut Microbiota-Derived Metabolites in Dogs with Myxomatous Mitral Valve Disease. mSystems 2021, 6, e00111-21. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Weisel, M.K.; Powers, J.D.; Powers, T.E.; Baggot, J.D. A pharmacokinetic analysis of tylosin in the normal dog. Am. J. Vet. Res. 1977, 38, 273–275. [Google Scholar]
- Kilpinen, S.; Spillmann, T.; Westermarck, E. Efficacy of two low-dose oral tylosin regimens in controlling the relapse of diarrhea in dogs with tylosin-responsive diarrhea: A prospective, single-blinded, two-arm parallel, clinical field trial. Acta Vet. Scand. 2014, 56, 43. [Google Scholar] [CrossRef] [Green Version]
- Cabral, D.J.; Penumutchu, S.; Reinhart, E.M.; Zhang, C.; Korry, B.J.; Wurster, J.I.; Nilson, R.; Guang, A.; Sano, W.H.; Rowan-Nash, A.D.; et al. Microbial Metabolism Modulates Antibiotic Susceptibility within the Murine Gut Microbiome. Cell Metab. 2019, 30, 800–823.e807. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Bogatyrev, S.R.; Rolando, J.C.; Ismagilov, R.F. Self-reinoculation with fecal flora changes microbiota density and composition leading to an altered bile-acid profile in the mouse small intestine. Microbiome 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, T.-B.; Zhang, X.-Y.; Kohl, K.D.; Wen, J.; Tian, S.-J.; Wang, D.-H. Coprophagy prevention alters microbiome, metabolism, neurochemistry, and cognitive behavior in a small mammal. ISME J. 2020, 14, 2625–2645. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.; Verbrugghe, A.; Gomez, D.E.; Chau, C.; Weese, J.S. The effect of storage at ambient temperature on the feline fecal microbiota. BMC Vet. Res. 2017, 13, 256. [Google Scholar] [CrossRef] [Green Version]
- Tedjo, D.I.; Jonkers, D.M.; Savelkoul, P.H.; Masclee, A.A.; van Best, N.; Pierik, M.J.; Penders, J. The effect of sampling and storage on the fecal microbiota composition in healthy and diseased subjects. PLoS ONE 2015, 10, e0126685. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein fromFaecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [Green Version]
| Universal | Blautia | C. hiranonis | Faecalibacterium | Fusobacterium | E. coli | Streptococcus | Turicibacter | |
|---|---|---|---|---|---|---|---|---|
| Reference interval # | 10.6–11.4 | 9.5–11.0 | 5.1–7.1 | 3.4–8.0 | 7.0–10.3 | 0.9–8.0 | 1.9–8.0 | 4.6–8.1 |
| Control (D0) | 11.2 (11.0–11.4) | 10.4 (10.0–10.9) | 6.1 (5.6–7.2) | 7.2 (6.7–8.1) | 9.1 (8.5–10.1) | 7.3 (5.1–7.6) | 8.2 (4.8–8.5) | 7.4 (6.1–8.2) |
| Control (D7) | 11.1 (10.5–11.4) | 9.8 * (8.7–10.2) | 0.13 * (0.0–1.9) | 3.5 * (3.1–4.6) | 7.6 * (7.0–8.0) | 8.5 * (7.9–8.9) | 7.6 (6.5–8.4) | 5.2 * (4.8–5.6) |
| Control (D14) | 11.0 (10.2–11.4) | 10.2 (9.3–10.6) | 5.8 (0.0–6.7) | 6.1* (4.7–6.7) | 9.4 (8.7–9.7) | 7.1 (6.2–7.7) | 7.3 (5.3–8.3) | 7.2 (5.5–8.1) |
| Enema FMT (D0) | 10.5 (10.4–11.0) | 10.0 (9.8–10.1) | 5.8 (5.4–6.0) | 6.8 (5.5–7.0) | 8.7 (8.4–9.3) | 6.6 (5.6–7.6) | 8.0 (5.7–8.8) | 7.2 (6.7–8.0) |
| Enema FMT (D7) | 10.7 (10.7–10.8) | 10.0 (9.9–10.5) | 0.5 * (0–1.5) | 4.2 * (3.9–6.5) | 7.7 * (7.4–8.2) | 8.0 (7.8–8.8) | 7.9 (7.7–8.6) | 5.2 * (4.8–5.5) |
| Enema FMT (D14) | 11.1 * (11.0–11.3) | 7.5 * (6.4–8.2) | 6.0 (4.9–6.4) | 7.1 (4.2–7.7) | 8.8 (7.8–9.6) | 7.5 (6.4–8.2) | 8.5 (7.7–8.9) | 7.6 (6.0–8.6) |
| Oral FMT (D0) | 11.2 (11.1–11.2) | 10.2 (9.6–10.6) | 5.8 (5.6–6.2) | 7.4 (6.7–7.9) | 9.3 (9.0–9.6) | 5.9 (5.1–6.7) | 6.7 (4.8–7.8) | 7.5 (6.2–8.1) |
| Oral FMT (D7) | 11.1 (11.0–11.3) | 9.5 (6.9–10.3) | 1.2 * (0.6–3.1) | 3.5 * (3.0–4.9) | 7.1 * (6.8–7.5) | 8.2 * (8.0–8.5) | 8.7 * (8.4–8.9) | 5. 2 * (5.1–5.5) |
| Oral FMT (D14) | 11.1 (10.9–11.3) | 10.4 (9.9–11.1) | 6.1 (5.6–6.7) | 6.8 (5.9–7.3) | 9.6 (8.8–10.0) | 6.8 (5.5–7.7) | 7.6 (6.3–8.1) | 7.9 (5.9–8.2) |
| Total UBA Concentration (μg/mg) | Primary UBA Concentration (μg/mg) | Primary UBA Percentage of Total UBA | Secondary UBA Concentration (μg/mg) | Secondary UBA Percentage of Total UBA | |
|---|---|---|---|---|---|
| Control (D0) | 2.65 (1.88–4.27) | 0.16 (0.05–0.72) | 6.1 (1.7–19.8) | 2.51 (0.52–4.00) | 93.4 (80–2-98.3) |
| Control (D7) | 1.79 (1.01–5.03) | 1.40 (0.61–4.41) * | 75.8 (60.7–87.5) * | 0.42 (0.33–0.63) * | 24.2 (12.5–39.3) * |
| Control (D14) | 3.38 (1.34–7.31) | 0.24 (0.07–3.45) | 6.9 (1.3–88.9) | 2.41 (0.43–7.07) | 93.1 (11.1–98.7) |
| Enema FMT (D0) | 2.46 (1.18–5.75) | 0.15 (0.03–0.49) | 4.9 (0.5–31.8) | 2.20 (0.80–5.72) | 95.1 (68.2–99.4) |
| Enema FMT (D7) | 2.02 (1.37–4.01) | 1.56 (1.09–3.37) * | 78.8 (70.1–85.2) * | 0.46 (0.28–0.81) * | 21.2 (14.8–29.9) * |
| Enema FMT (D14) | 4.00 (1.26–4.39) | 0.16 (0.03–0.88) | 5.9 (1.6–23.4) | 3.34 (1.23–4.32) | 94.1 (76.6–98.4) |
| Oral FMT (D0) | 3.15 (1.09–4.01) | 0.11 (0.04–0.79) | 3.1 (1.7–20.1) | 3.02 (0.97–3.92) | 96.9 (79.9–98.9) |
| Oral FMT (D7) | 2.14 (1.44–3.61) | 1.69 (1.08–2.70) * | 74.9 (71.1–84.3) * | 0.53 (0.35–0.91)* | 25.1 (15.7–28.9) * |
| Oral FMT (D14) | 3.33 (2.14–4.81) | 0.16 (0.06–0.35) | 3.7 (1.5–16.6) | 3.19 (1.79–4.70) | 96.3 (83.4–98.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marclay, M.; Dwyer, E.; Suchodolski, J.S.; Lidbury, J.A.; Steiner, J.M.; Gaschen, F.P. Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation. Vet. Sci. 2022, 9, 324. https://doi.org/10.3390/vetsci9070324
Marclay M, Dwyer E, Suchodolski JS, Lidbury JA, Steiner JM, Gaschen FP. Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation. Veterinary Sciences. 2022; 9(7):324. https://doi.org/10.3390/vetsci9070324
Chicago/Turabian StyleMarclay, Margaux, Elizabeth Dwyer, Jan S. Suchodolski, Jonathan A. Lidbury, Joerg M. Steiner, and Frederic P. Gaschen. 2022. "Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation" Veterinary Sciences 9, no. 7: 324. https://doi.org/10.3390/vetsci9070324
APA StyleMarclay, M., Dwyer, E., Suchodolski, J. S., Lidbury, J. A., Steiner, J. M., & Gaschen, F. P. (2022). Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation. Veterinary Sciences, 9(7), 324. https://doi.org/10.3390/vetsci9070324







