Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compounds
2.2. Culture of Primary Keratinocytes under Basal Conditions
2.3. Culture of Reconstructed Human Epidermis under Cytokine Mix–Stimulated Conditions
2.4. ELISA Test
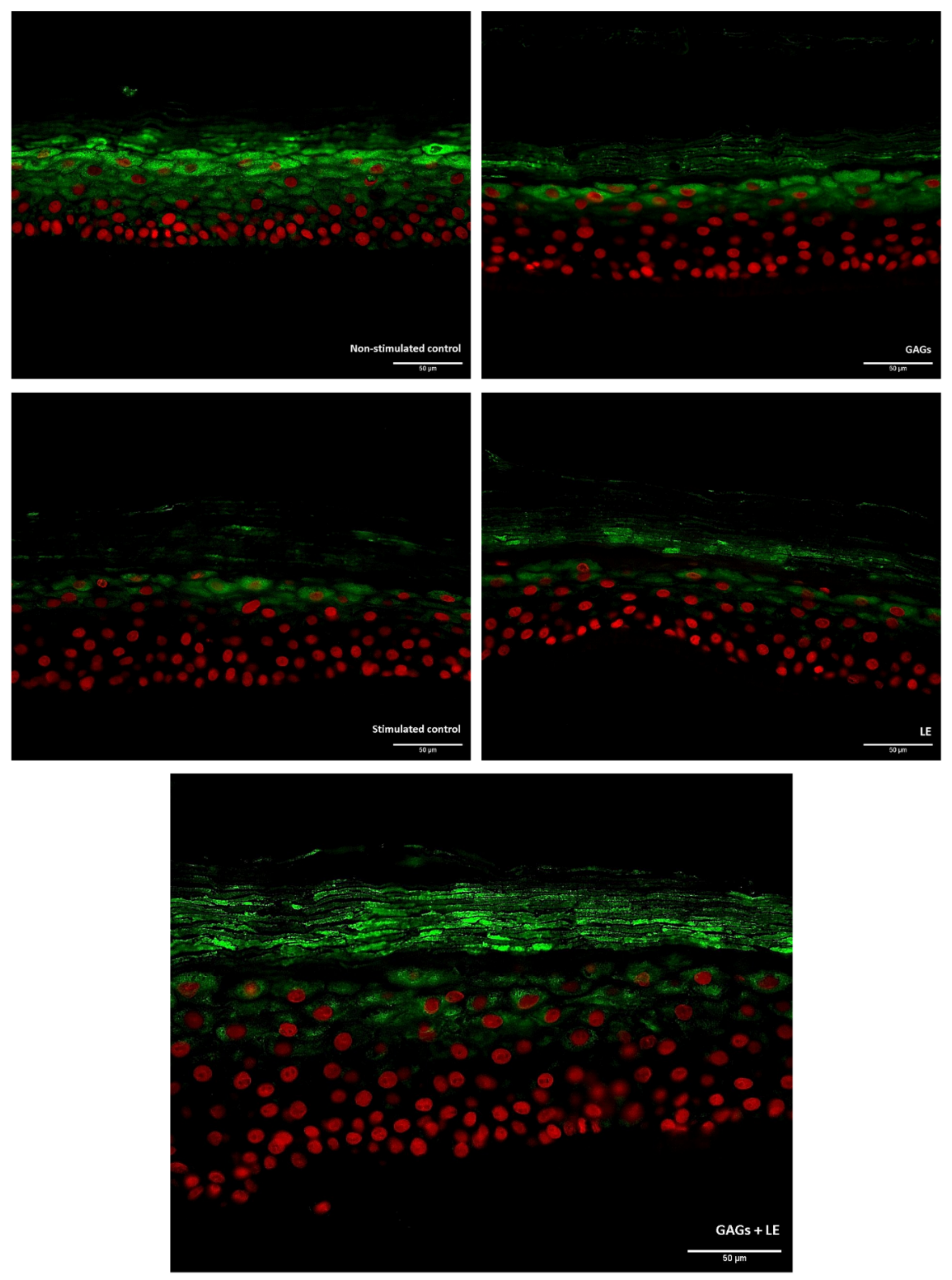
2.5. Immunofluorescence Labeling
2.6. Statistical Methods
3. Results
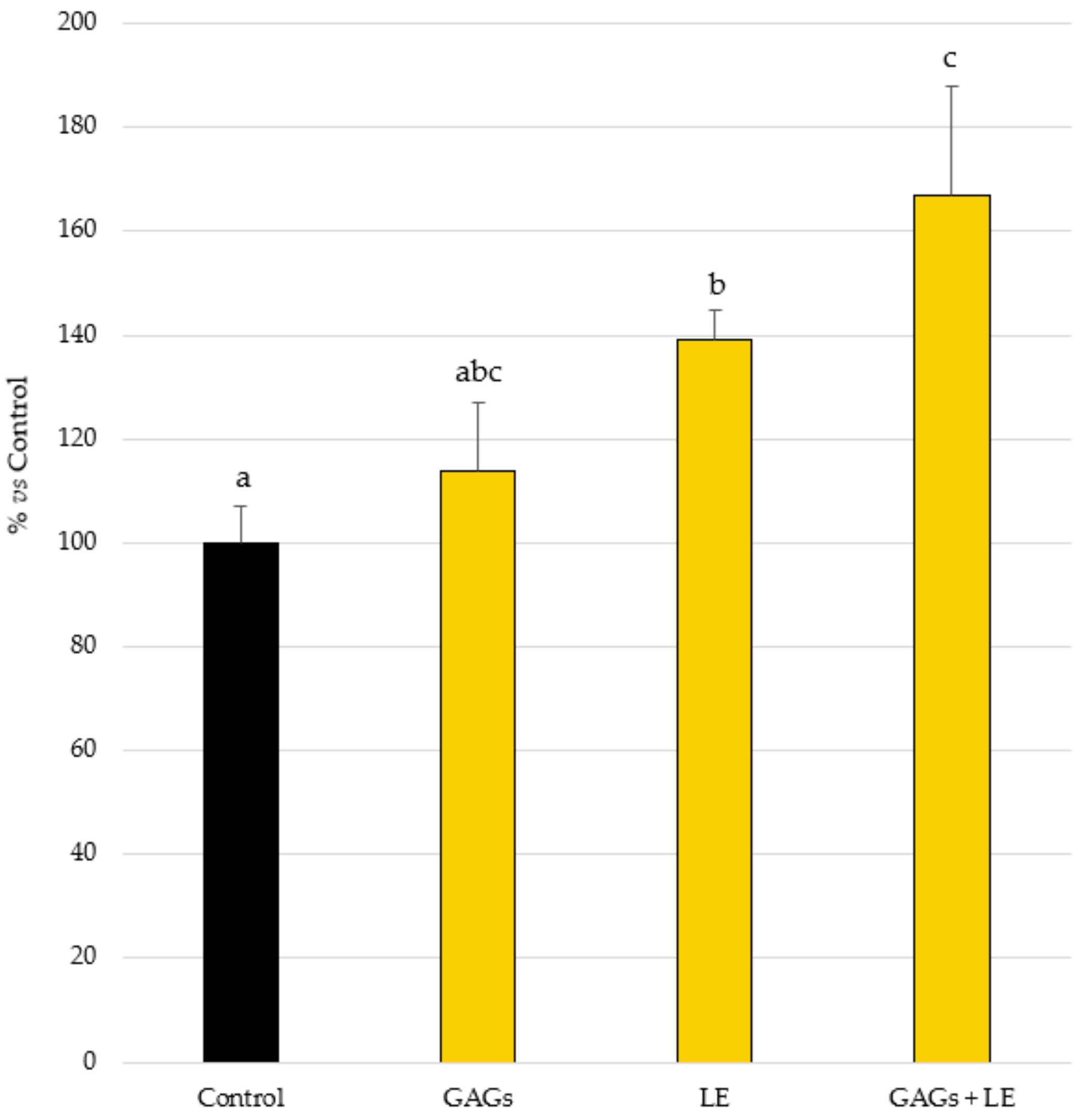
3.1. Antimicrobial Peptide Expression
3.2. Filaggrin Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef]
- Halliwell, R. Revised Nomenclature for Veterinary Allergy. Vet. Immunol. Immunopathol. 2006, 114, 207–208. [Google Scholar] [CrossRef]
- Santoro, D.; Marsella, R.; Pucheu-Haston, C.M.; Eisenschenk, M.N.C.; Nuttall, T.; Bizikova, P. Review: Pathogenesis of Canine Atopic Dermatitis: Skin Barrier and Host-Micro-Organism Interaction. Vet. Dermatol. 2015, 26, 84-e25. [Google Scholar] [CrossRef]
- Marsella, R. Advances in Our Understanding of Canine Atopic Dermatitis. Vet. Dermatol. 2021, 32, 547-e151. [Google Scholar] [CrossRef]
- Combarros, D.; Goudounèche, D.; Cadiergues, M.C.; Simon, M. The Upper Epidermis of Atopic Dogs Is Altered at the Functional and Structural Levels. Vet. Dermatol. 2021, 32, 620-e165. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 531–534. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine Atopic Dermatitis: Detailed Guidelines for Diagnosis and Allergen Identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef]
- Marsella, R.; Olivry, T.; Carlotti, D.-N. Current Evidence of Skin Barrier Dysfunction in Human and Canine Atopic Dermatitis. Vet. Dermatol. 2011, 22, 239–248. [Google Scholar] [CrossRef]
- Olivry, T.; Paps, J.S.; Amalric, N. Transient and Reversible Reduction of Stratum Corneum Filaggrin Degradation Products after Allergen Challenge in Experimentally Mite-Sensitised Atopic Dogs. Vet. Dermatol. 2022, 33, 62-e20. [Google Scholar] [CrossRef]
- Fanton, N.; Santoro, D.; Cornegliani, L.; Marsella, R. Increased Filaggrin-Metabolizing Enzyme Activity in Atopic Skin: A Pilot Study Using a Canine Model of Atopic Dermatitis. Vet. Dermatol. 2017, 28, 111–479. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; Irwin McLean, W.H.; et al. Levels of Filaggrin Degradation Products Are Influenced by Both Filaggrin Genotype and Atopic Dermatitis Severity. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 934–940. [Google Scholar] [CrossRef]
- Santoro, D.; Marsella, R.; Ahrens, K.; Graves, T.K.; Bunick, D. Altered MRNA and Protein Expression of Filaggrin in the Skin of a Canine Animal Model for Atopic Dermatitis. Vet. Dermatol. 2013, 24, 329-e73. [Google Scholar] [CrossRef]
- Asahina, R.; Maeda, S. A Review of the Roles of Keratinocyte-Derived Cytokines and Chemokines in the Pathogenesis of Atopic Dermatitis in Humans and Dogs. Vet. Dermatol. 2017, 28, 16-e5. [Google Scholar] [CrossRef] [PubMed]
- Pin, D.; Pendaries, V.; Keita Alassane, S.; Froment, C.; Amalric, N.; Cadiergues, M.C.; Serre, G.; Haftek, M.; Vidémont, E.; Simon, M. Refined Immunochemical Characterization in Healthy Dog Skin of the Epidermal Cornification Proteins, Filaggrin, and Corneodesmosin. J. Histochem. Cytochem. 2019, 67, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Combarros, D.; Cadiergues, M.C.; Simon, M. Update on Canine Filaggrin: A Review. Vet. Q. 2020, 40, 162–168. [Google Scholar] [CrossRef]
- Marsella, R.; Santoro, D.; Ahrens, K.; Thomas, A.L. Investigation of the Effect of Probiotic Exposure on Filaggrin Expression in an Experimental Model of Canine Atopic Dermatitis. Vet. Dermatol. 2013, 24, 260–266. [Google Scholar] [CrossRef]
- Chervet, L.; Galichet, A.; McLean, W.H.I.; Chen, H.; Suter, M.M.; Roosje, P.J.; Müller, E.J. Missing C-Terminal Filaggrin Expression, NFkappaB Activation and Hyperproliferation Identify the Dog as a Putative Model to Study Epidermal Dysfunction in Atopic Dermatitis. Exp. Dermatol. 2010, 19, 343–346. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U. Reddish, Scaly, and Itchy: How Proteases and Their Inhibitors Contribute to Inflammatory Skin Diseases. Arch. Immunol. Ther. Exp. 2009, 57, 345–354. [Google Scholar] [CrossRef]
- Marsella, R.; Papastavros, V.; Ahrens, K.; Santoro, D. Decreased Expression of Caspase-14 in an Experimental Model of Canine Atopic Dermatitis. Vet. J. 2016, 209, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.C.; Fonseca, R.; Guedes, D.M. Epidermal Dysfunctions in Canine Atopic Dermatitis: Clinical Impacts and Therapies. Rev. Bras. Hig. Sanid. Anim. 2018, 12, 396–406. [Google Scholar] [CrossRef]
- Alexander, H.; Paller, A.S.; Traidl-Hoffmann, C.; Beck, L.A.; De Benedetto, A.; Dhar, S.; Girolomoni, G.; Irvine, A.D.; Spuls, P.; Su, J.; et al. The Role of Bacterial Skin Infections in Atopic Dermatitis: Expert Statement and Review from the International Eczema Council Skin Infection Group. Br. J. Dermatol. 2020, 182, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Marsella, R.; Bunick, D.; Graves, T.K.; Campbell, K.L. Expression and Distribution of Canine Antimicrobial Peptides in the Skin of Healthy and Atopic Beagles. Vet. Immunol. Immunopathol. 2011, 144, 382–388. [Google Scholar] [CrossRef]
- Santoro, D.; Ahrens, K.; Marsella, R.; Segre, M. Evaluation of Antimicrobial Peptides and Cytokine Production in Primary Keratinocyte Cell Culture from Healthy and Atopic Beagles. Exp. Dermatol. 2015, 24, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Pálffy, R.; Gardlík, R.; Behuliak, M.; Kadasi, L.; Turna, J.; Celec, P. On the Physiology and Pathophysiology of Antimicrobial Peptides. Mol. Med. 2009, 15, 51–59. [Google Scholar] [CrossRef]
- Marcinkiewicz, M.; Majewski, S. The Role of Antimicrobial Peptides in Chronic Inflammatory Skin Diseases. Adv. Dermatol. Allergol. 2016, 33, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lohajaroensub, R.; Sawangmake, C.; Rodkhum, C. Expression of Antimicrobial Peptide Genes in the Canine Amniotic Membrane. Vet. Sci. 2022, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y.M. Endogenous Antimicrobial Peptides and Skin Infections in Atopic Dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Lancto, C.A.; Torres, S.M.F.; Hendrickson, J.A.; Martins, K.V.; Rutherford, M.S. Altered Expression of Antimicrobial Peptide Genes in the Skin of Dogs with Atopic Dermatitis and Other Inflammatory Skin Conditions. Vet. Dermatol. 2013, 24, 414-e90. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Mitsutake, S.; Tsuji, K.; Kihara, A.; Igarashi, Y. Ceramide Biosynthesis in Keratinocyte and Its Role in Skin Function. Biochimie 2009, 91, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’être” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T. Is the Skin Barrier Abnormal in Dogs with Atopic Dermatitis? Vet. Immunol. Immunopathol. 2011, 144, 11–16. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Xue, J.F.; Zheng, Z.Y.; Shuhaidi, M.; Thu, H.E.; Hussain, Z. Hyaluronic Acid, an Efficient Biomacromolecule for Treatment of Inflammatory Skin and Joint Diseases: A Review of Recent Developments and Critical Appraisal of Preclinical and Clinical Investigations. Int. J. Biol. Macromol. 2018, 116, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, S.; Ramió-Lluch, L.; Brazís, P.; Fondevila, D.; Segarra, S.; Puigdemont, A. Effects of Sphingolipid Extracts on the Morphological Structure and Lipid Profile in an in Vitro Model of Canine Skin. Vet. J. 2016, 212, 58–64. [Google Scholar] [CrossRef]
- Marsella, R.; Segarra, S.; Ahrens, K.; Alonso, C.; Ferrer, L. Topical Treatment with Sphingolipids and Glycosaminoglycans for Canine Atopic Dermatitis. BMC Vet. Res. 2020, 16, 92. [Google Scholar] [CrossRef]
- Torrent, A.; Ruhí, R.; Martínez, C.; Castells, G.; de Castellarnau-Castellà, C. Anti-Inflammatory Activity and Absorption of a Natural Rooster Comb Extract (Hyal-Joint®). Osteoarthr. Cartil. 2010, 18, S246–S247. [Google Scholar] [CrossRef]
- Torrent, A.; Montell, E.; Verges, J.; Ruhi, R.; Dalmau, P.; Zurbano, M.J.; Romero, J. A New Natural Extract with Anti-Aging and Regenerative Properties for Skin. FASEB J. 2015, 29, 740–743. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Martinez-Puig, D.; Romero-Rueda, J. Comparative in Vitro Efficacy of a Hyaluronic Acid (HA) Matrix Ingredient Containing HA, Dermatan Sulphate and Collagen (Dermial®) versus Pure HA from Extraction or Fermentation Origin Introduction. FASEB J. 2022, 36, 5–7. [Google Scholar] [CrossRef]
- Boniface, K.; Bernard, F.; Garcia, M.; Gurney, A.L.; Lecron, J.; Morel, F.; Boniface, K.; Garcia, M.; Gurney, A.L. IL-22 Inhibits Epidermal Differentiation and Induces Proinflammatory Gene Expression and Migration of Human Keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Guenou, H.; Nissan, X.; Larcher, F.; Feteira, J.; Lemaitre, G. Human Embryonic Stem-Cell Derivatives for Full Reconstruction of the Pluristratified Epidermis: A Preclinical Study. Lancet 2009, 374, 1745–1753. [Google Scholar] [CrossRef]
- Marsella, R. Atopic Dermatitis in Domestic Animals: What Our Current Understanding Is and How This Applies to Clinical Practice. Vet. Sci. 2021, 8, 124. [Google Scholar] [CrossRef]
- Shimada, K.; Yoon, J. Increased Transepidermal Water Loss and Decreased Ceramide Content in Lesional and Non-Lesional Skin of Dogs with Atopic Dermatitis. Vet. Dermatol. 2009, 20, 541–546. [Google Scholar] [CrossRef]
- Marsella, R.; Genovese, D.; Gilmer, L.; Ahrens, K.; Gatto, H.; Navarro, C. Investigations on the Effects of a Topical Ceramides-Containing Emulsion (Allerderm Spot on) on Clinical Signs and Skin Barrier Function in Dogs with Atopic Dermatitis: A Double-Blinded Randomized Controlled Study. Int. J. Appl. Res. Vet. Med. 2013, 11, 110–116. [Google Scholar]
- Blaskovic, M.; Rosenkrantz, W.; Neuber, A.; Sauter-Louis, C.; Mueller, R.S. The Effect of a Spot-on Formulation Containing Polyunsaturated Fatty Acids and Essential Oils on Dogs with Atopic Dermatitis. Vet. J. 2014, 199, 39–43. [Google Scholar] [CrossRef][Green Version]
- Tretter, S.; Mueller, R. The Influence of Topical Unsaturated Fatty Acids and Essential Oils on Normal and Atopic Dogs. J. Am. Anim. Hosp. Assoc. 2011, 47, 236–240. [Google Scholar] [CrossRef]
- Bourdeau, P.; Bruet, V.; Gremillet, C. Evaluation of Phytosphingosine-Containing Shampoo and Microemulsion Spray in the Clinical Control of Allergic Dermatoses in Dogs: Preliminary Results of a Multicentre Study. In Proceedings of the Selected Abstracts from the North American Veterinary Dermatology Forum, Lihue, HI, USA, 18–22 April 2007; pp. 175–195. [Google Scholar]
- Fujimura, M.; Nakatsuji, Y.; Fujiwara, S.; Rème, C.; Gatto, H. Spot-on Skin Lipid Complex as an Adjunct Therapy in Dogs with Atopic Dermatitis: An Open Pilot Study. Vet. Med. Int. 2011, 2011, 281846. [Google Scholar] [CrossRef] [PubMed]
- Hobi, S.; Klinger, C.; Classen, J.; Mueller, R.S. The Effects of a Topical Lipid Complex Therapy on Dogs with Atopic Dermatitis: A Double Blind, Randomized, Placebo-Controlled Study. Vet. Dermatol. 2017, 28, 369-e84. [Google Scholar] [CrossRef]
- Jung, J.; Nam, E.; Park, S.; Han, S.; Hwang, C. Clinical Use of a Ceramide-Based Moisturizer for Treating Dogs with Atopic Dermatitis. J. Vet. Sci. 2013, 14, 199–205. [Google Scholar] [CrossRef]
- Piekutowska, A.; Pin, D.; Rème, C.A.; Gatto, H.; Haftek, M. Effects of a Topically Applied Preparation of Epidermal Lipids on the Stratum Corneum Barrier of Atopic Dogs. J. Comp. Pathol. 2008, 138, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Remoue, N.; Osta, B.; Pin, D.; Gatto, H.; Haftek, M.; Portoukalian, J. The Lipid Alterations in the Stratum Corneum of Dogs with Atopic Dermatitis Are Alleviated by Topical Application of a Sphingolipid-containing Emulsion. Clin. Exp. Dermatol. 2012, 37, 665–671. [Google Scholar] [CrossRef]
- Reme, C.A.; Mondon, A.; Calmon, J.P.; Poisson, L.; Jasmin, P.; Carlotti, D.N. FC-40 Efficacy of Combined Topical Therapy with Antiallergic Shampoo and Lotion for the Control of Signs Associated with Atopic Dermatitis in Dogs. Vet. Dermatol. 2004, 15, 33. [Google Scholar] [CrossRef]
- Chandan, N.; Rajkumar, J.R.; Shi, V.Y.; Lio, P.A. A New Era of Moisturizers. J. Cosmet. Dermatol. 2021, 20, 2425–2430. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segarra, S.; Naiken, T.; Garnier, J.; Hamon, V.; Coussay, N.; Bernard, F.-X. Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract. Vet. Sci. 2022, 9, 323. https://doi.org/10.3390/vetsci9070323
Segarra S, Naiken T, Garnier J, Hamon V, Coussay N, Bernard F-X. Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract. Veterinary Sciences. 2022; 9(7):323. https://doi.org/10.3390/vetsci9070323
Chicago/Turabian StyleSegarra, Sergi, Tanesha Naiken, Julien Garnier, Valérie Hamon, Nathalie Coussay, and François-Xavier Bernard. 2022. "Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract" Veterinary Sciences 9, no. 7: 323. https://doi.org/10.3390/vetsci9070323
APA StyleSegarra, S., Naiken, T., Garnier, J., Hamon, V., Coussay, N., & Bernard, F.-X. (2022). Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract. Veterinary Sciences, 9(7), 323. https://doi.org/10.3390/vetsci9070323






