Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Kidney Dissection
2.2. qPCR
| bdnf | F: CGAGGAATAGACAAGCGGCA; | R: ATCCGTATAAACCGCCAGCC |
| ngf | F: GAGAAGACTACAAGCGAAT; | R: CGACAACAATAAGGAGGAT |
| nt3 | F: CCCATCAGTGCGCTCATC; | R: TCCGAACTGTCCACCATG |
| nt4 | F: GCTCCTCCTAGAACAGAGGG; | R: CGTCCTGGATGCATCTTCTCTG |
| trka | F: GCATTTACAATGGCAGCCAG; | R: CTTCTTGAGTGGTCACTGTC |
| trkb | F: TCACCTATGGCAAGCAACCC; | R: CTTTGGGGCAAGTACGAGGT |
| trkc | F: CGGAAGTGGATTGGACAGTT; | R: CATGAAGCCGTTATCGTCC |
| ef1a | F: TCAAGGACATCCGTCGTGGTA; | R: ACAGCAAAGCGACCAAGAGG |
2.3. Statistical Analysis
2.4. Chromogenic and Fluorescence In Situ Hybridization (ISH)
| Ngf | GGAGCACAGGAGATCTACGC and CGTGGAAAAACCCAACTCAT |
| bdnf-full (fw) | 5′-ATAGTAACGAACAGGATGG-3′ and 5′-GCTCAGTCATGGGAGTCC-3′ |
| nt3 | ATGGTTACCTTTATTACGATC and CCACCATTTTTCACGTCC |
| nt4 | CAGAGAAGATGCATCCAGGACG and CACTGCCGTTTCCTGACACGCG |
| trka | AGTTGTTGCTTGCAGGGTGG and TGGGTCAATCATGACCTCAG |
| trkb-full | CCAGAGATGTGTACAGCACC and CATTGTTTGAGAGCTGATACC |
| trkc | GGACACTGAGAGCATCTCTATGA and CACGTTGTTCATCCCGACCACATT |
2.5. Immunohistochemistry (IHC)
2.6. Protein Analysis by Western Blot
2.7. Microscopy
3. Results
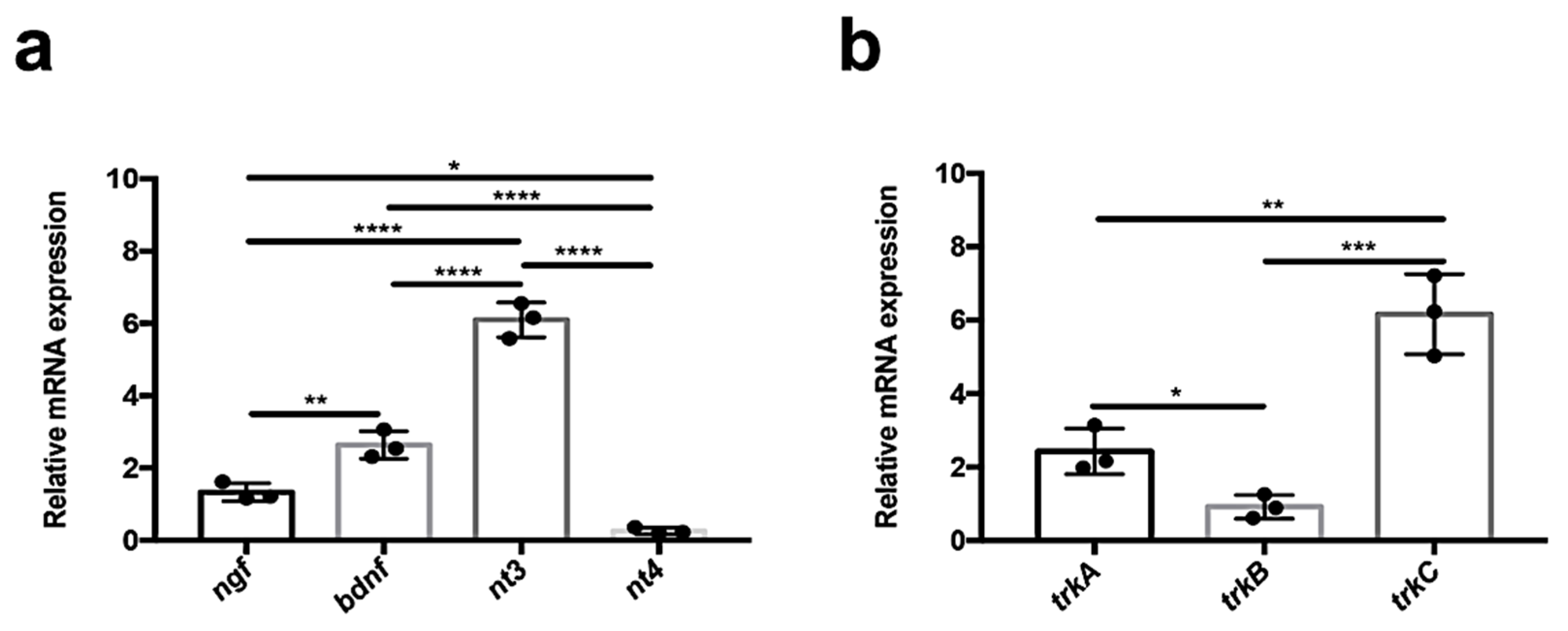
3.1. Quantitative Analysis of Neurotrophin and Receptor Expression in Adult Zebrafish Kidney
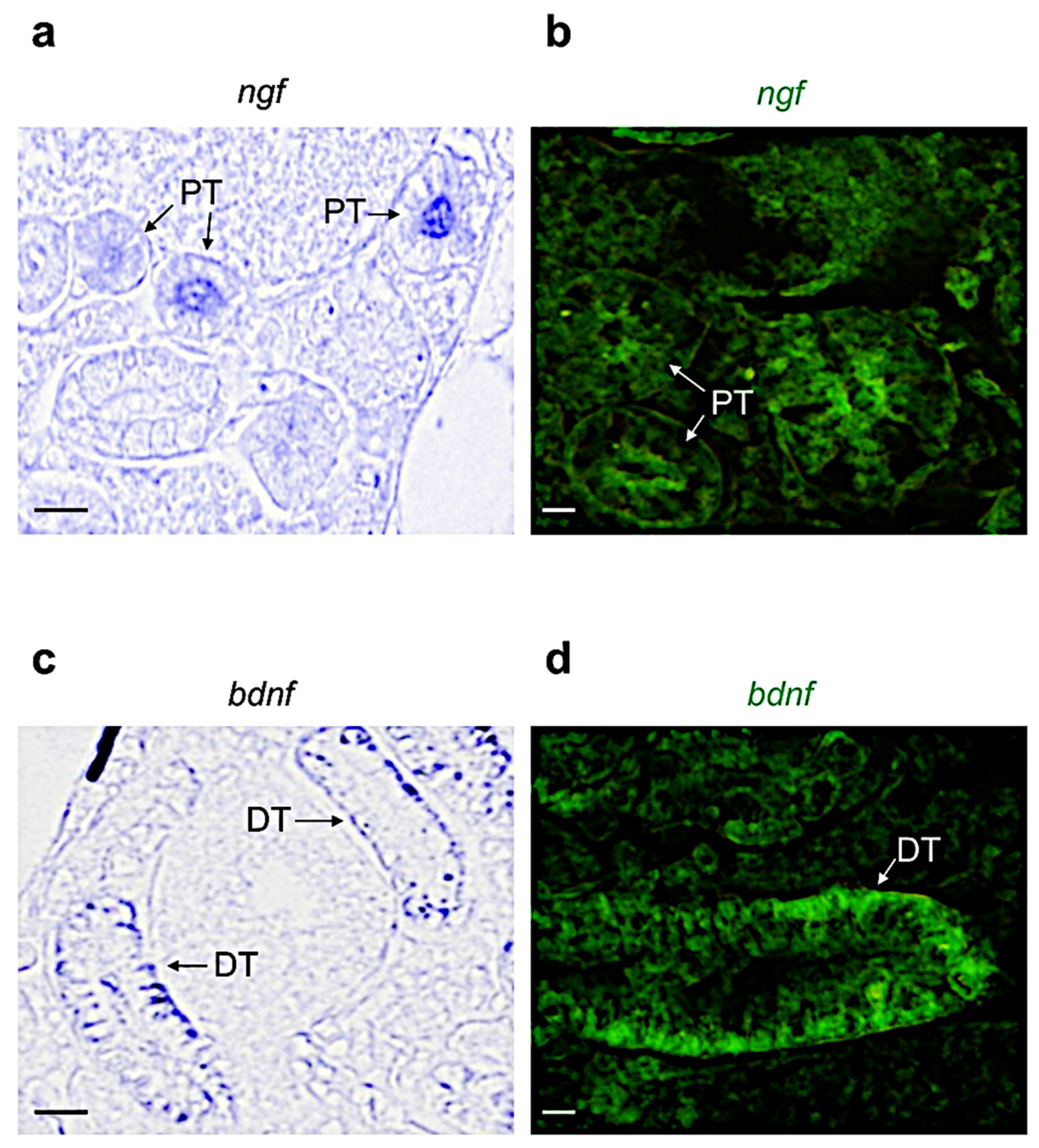
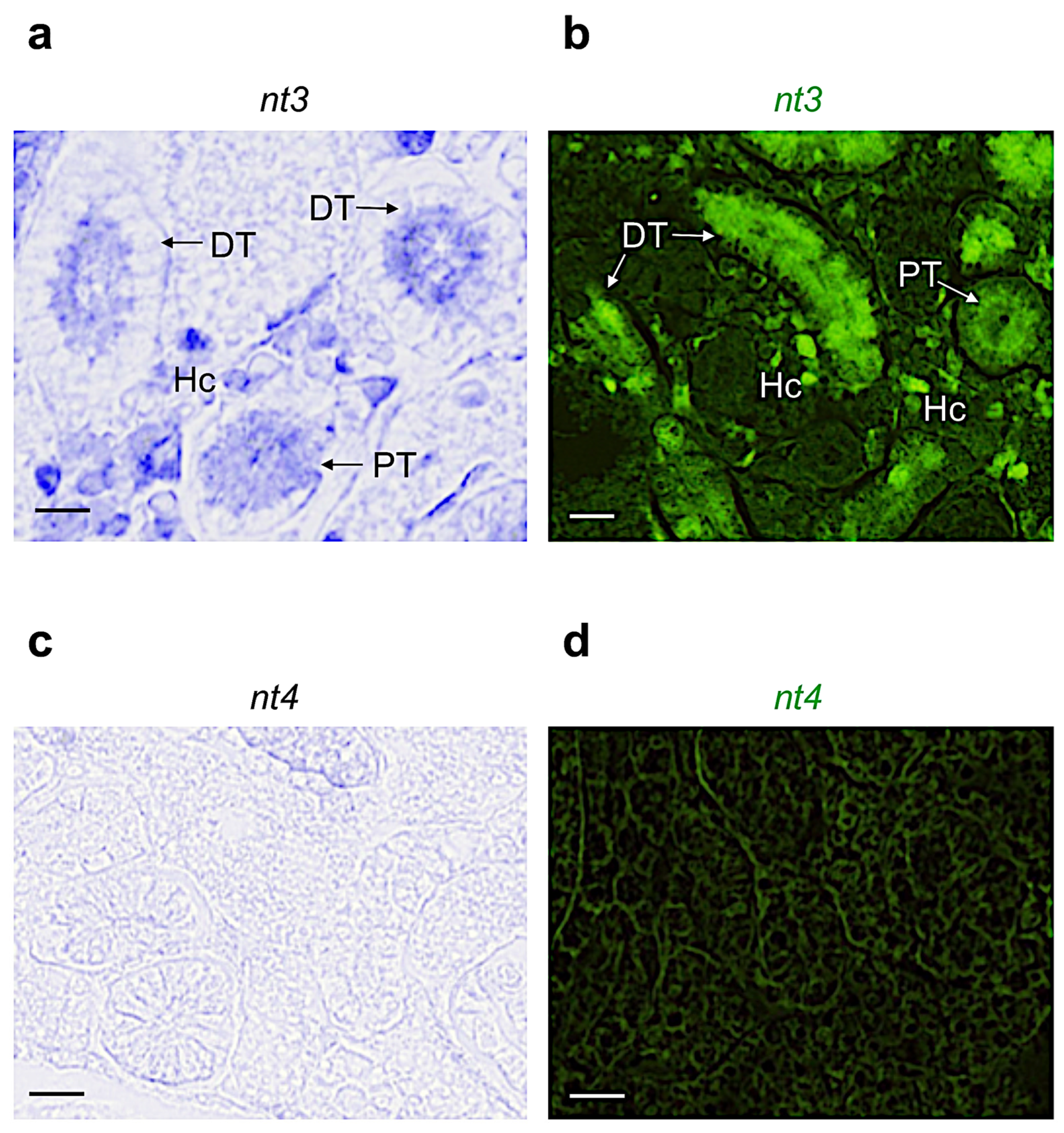
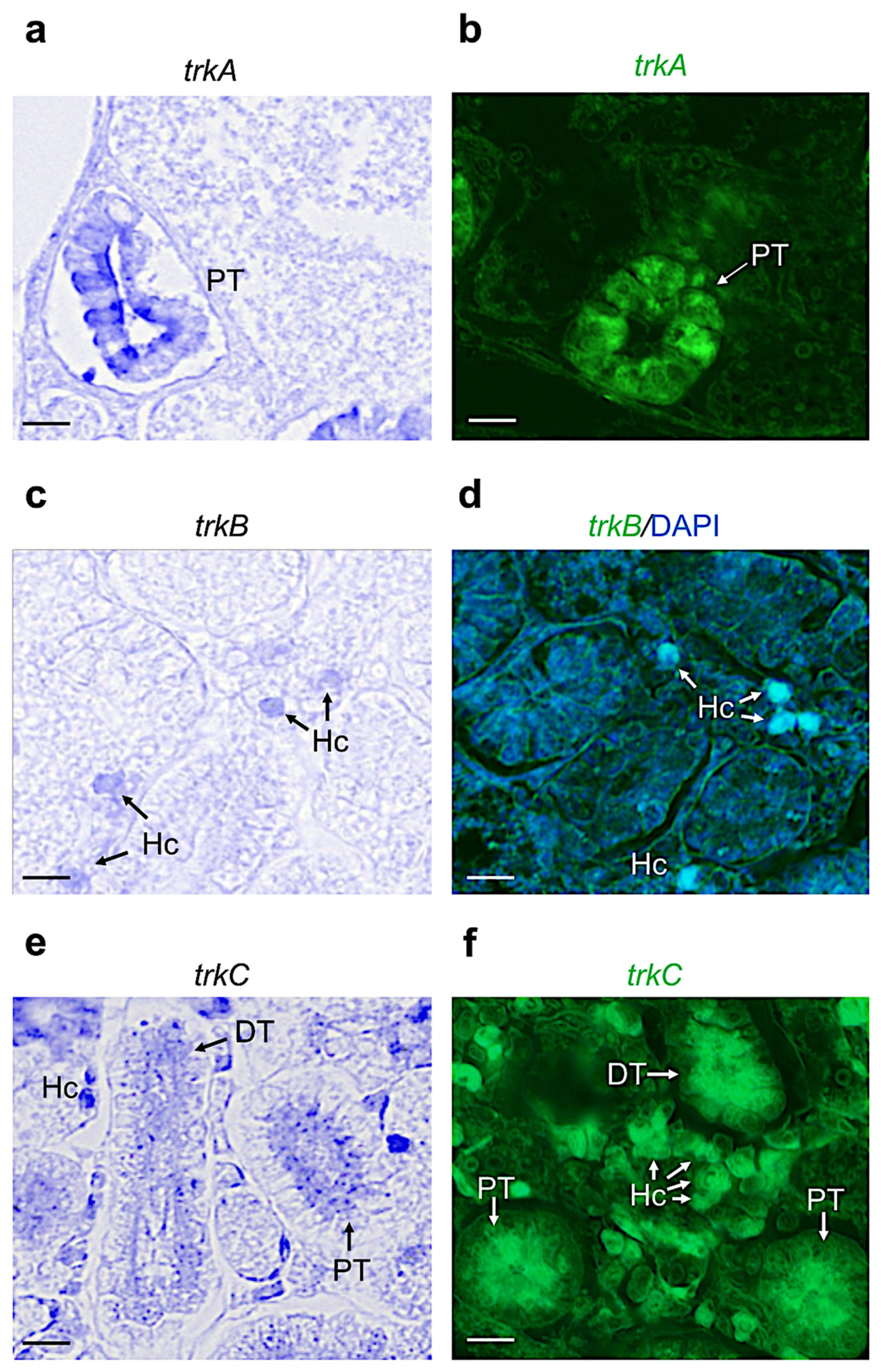
3.2. Distribution of Neurotrophin and Receptor mRNA in Adult Zebrafish Kidney
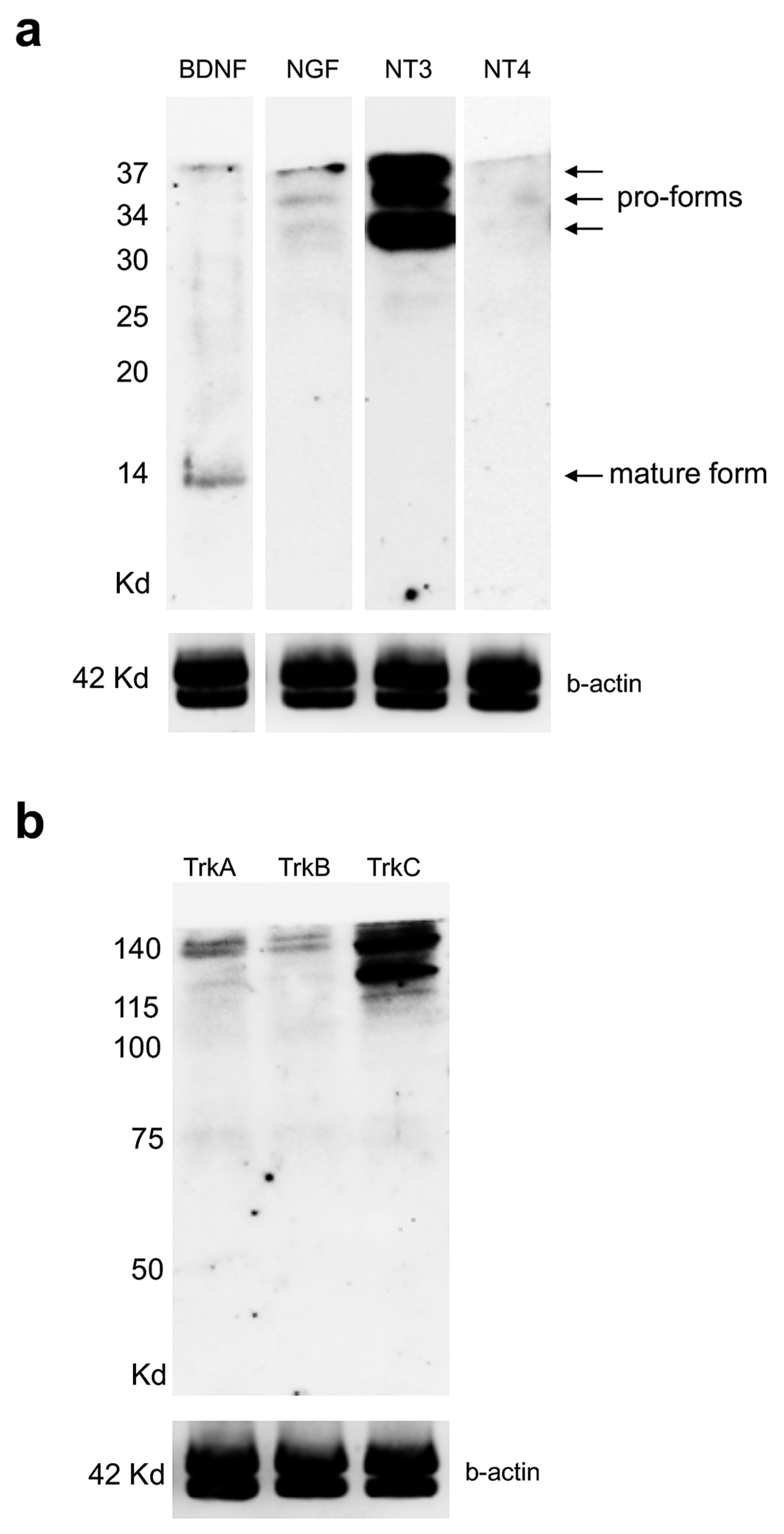
3.3. Expression Levels of Neurotrophin and Receptor Proteins in Adult Zebrafish Kidney
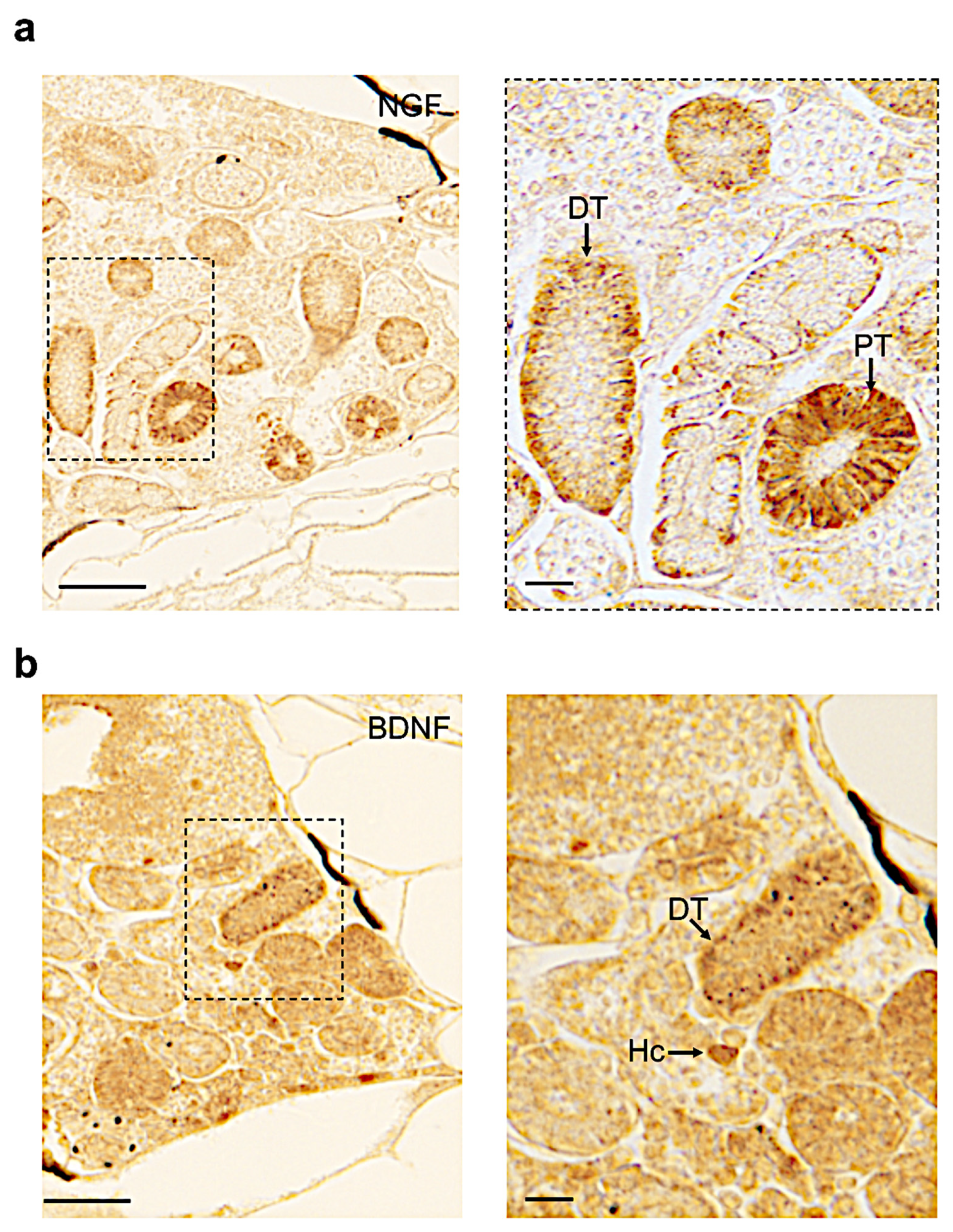
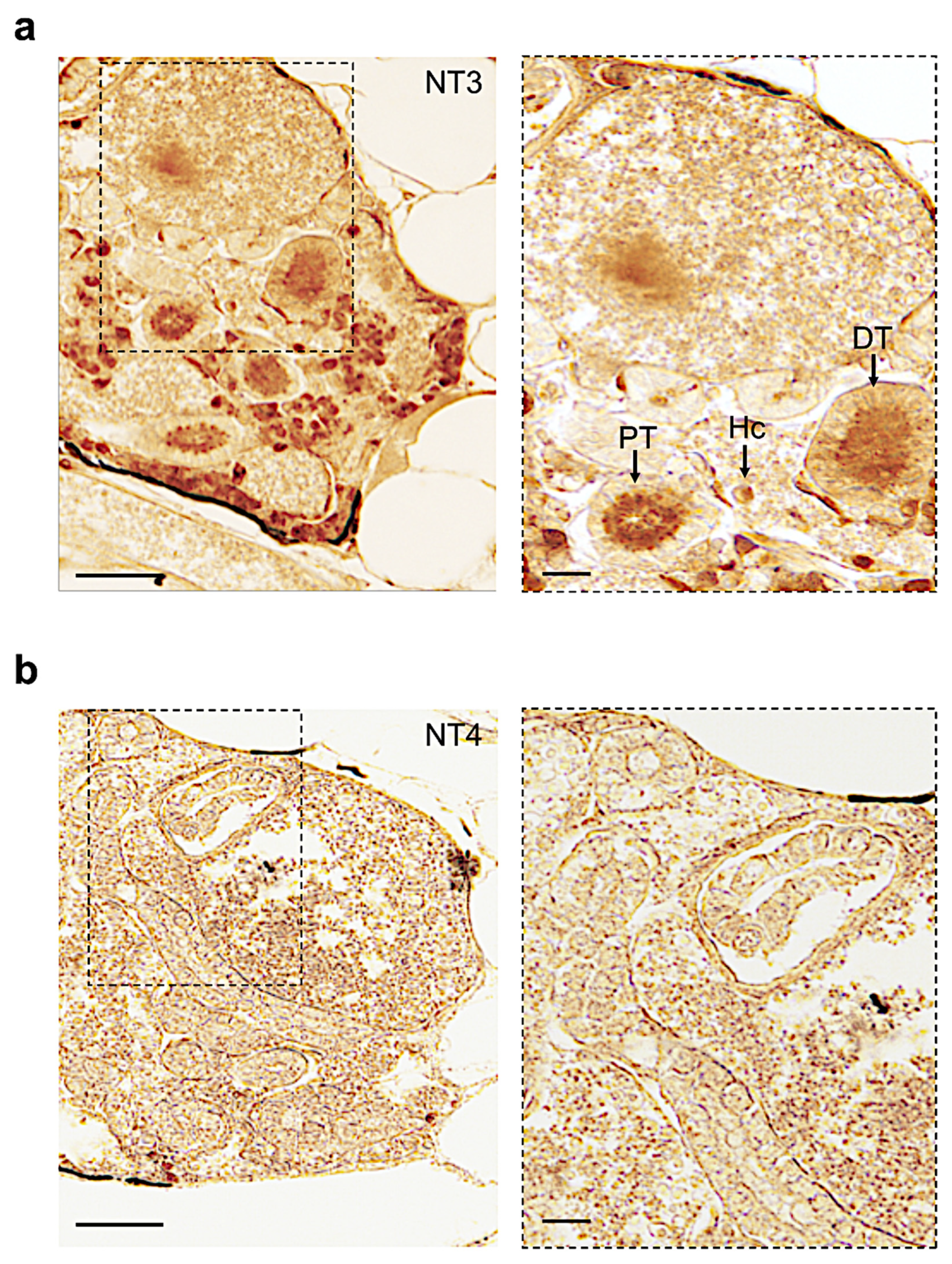
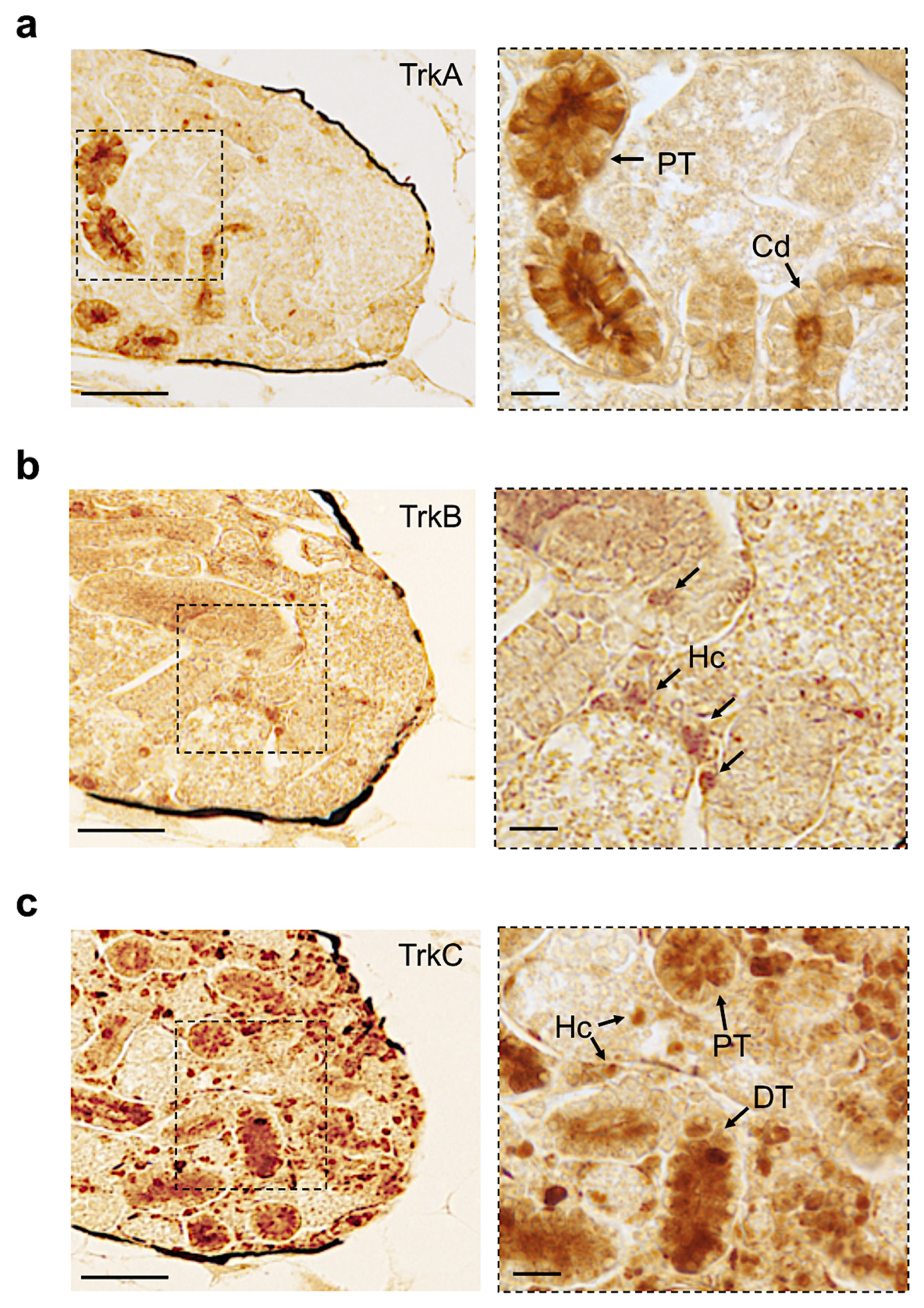
3.4. Distribution of Neurotrophin and Receptor Proteins in Adult Zebrafish Kidney
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chao, M.V. The p75 neurotrophin receptor. J. Neurobiol. 1994, 25, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Chao, M.V. Neurotrophin receptor structure and interactions. Pharm. Acta Helv. 2000, 74, 253–260. [Google Scholar] [CrossRef]
- Lee, F.S.; Rajagopal, R.; Chao, M.V. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002, 13, 11–17. [Google Scholar] [CrossRef]
- Ibanez, C.F.; Hallbook, F.; Godeau, F.; Persson, H. Expression of neurotrophin-4 mRNA during oogenesis in Xenopus laevis. Int. J. Dev. Biol. 1992, 36, 239–245. [Google Scholar] [PubMed]
- Islam, N.; Gagnon, F.; Moss, T. Catalytic and non-catalytic forms of the neurotrophin receptor xTrkB mRNA are expressed in a pseudo-segmental manner within the early Xenopus central nervous system. Int. J. Dev. Biol. 1996, 40, 973–983. [Google Scholar]
- Rodriguez-Tebar, A.; Dechant, G.; Gotz, R.; Barde, Y.A. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992, 11, 917–922. [Google Scholar] [CrossRef]
- Philo, J.; Talvenheimo, J.; Wen, J.; Rosenfeld, R.; Welcher, A.; Arakawa, T. Interactions of neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF), and the NT-3.BDNF heterodimer with the extracellular domains of the TrkB and TrkC receptors. J. Biol. Chem. 1994, 269, 27840–27846. [Google Scholar] [CrossRef]
- Zhang, J.; Geula, C.; Lu, C.; Koziel, H.; Hatcher, L.M.; Roisen, F.J. Neurotrophins regulate proliferation and survival of two microglial cell lines in vitro. Exp. Neurol. 2003, 183, 469–481. [Google Scholar] [CrossRef]
- Hougland, M.T.; Harrison, B.J.; Magnuson, D.S.; Rouchka, E.C.; Petruska, J.C. The Transcriptional Response of Neurotrophins and Their Tyrosine Kinase Receptors in Lumbar Sensorimotor Circuits to Spinal Cord Contusion is Affected by Injury Severity and Survival Time. Front. Physiol. 2012, 3, 478. [Google Scholar] [CrossRef]
- Cacialli, P.; Lucini, C. Adult neurogenesis and regeneration in zebrafish brain: Are the neurotrophins involved in? Neural Regen. Res. 2019, 14, 2067–2068. [Google Scholar] [CrossRef]
- Mizuno, N.; Shiba, H.; Xu, W.P.; Inui, T.; Fujita, T.; Kajiya, M.; Takeda, K.; Hasegawa, N.; Kawaguchi, H.; Kurihara, H. Effect of neurotrophins on differentiation, calcification and proliferation in cultures of human pulp cells. Cell Biol. Int. 2007, 31, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P. Neurotrophins Time Point Intervention after Traumatic Brain Injury: From Zebrafish to Human. Int. J. Mol. Sci. 2021, 22, 1585. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Gatta, C.; D’Angelo, L.; Leggieri, A.; Palladino, A.; de Girolamo, P.; Pellegrini, E.; Lucini, C. Nerve growth factor is expressed and stored in central neurons of adult zebrafish. J. Anat. 2019, 235, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Gueguen, M.M.; Coumailleau, P.; D’Angelo, L.; Kah, O.; Lucini, C.; Pellegrini, E. BDNF Expression in Larval and Adult Zebrafish Brain: Distribution and Cell Identification. PLoS ONE 2016, 11, e0158057. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Lee, F.S.; Chao, M.V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. USA 2001, 98, 3555–3560. [Google Scholar] [CrossRef]
- Johansson, M.; Jonsson, M.; Norrgard, O.; Forsgren, S. New aspects concerning ulcerative colitis and colonic carcinoma: Analysis of levels of neuropeptides, neurotrophins, and TNFalpha/TNF receptor in plasma and mucosa in parallel with histological evaluation of the intestine. Inflamm. Bowel Dis. 2008, 14, 1331–1340. [Google Scholar] [CrossRef]
- Serlin, H.K.; Fox, E.A. Neurotrophin-4 is essential for survival of the majority of vagal afferents to the mucosa of the small intestine, but not the stomach. Auton. Neurosci. 2021, 233, 102811. [Google Scholar] [CrossRef]
- Sariola, H. The neurotrophic factors in non-neuronal tissues. Cell Mol. Life Sci. 2001, 58, 1061–1066. [Google Scholar] [CrossRef]
- Cacialli, P.; D’Angelo, L.; de Girolamo, P.; Avallone, L.; Lucini, C.; Pellegrini, E.; Castaldo, L. Morpho-Functional Features of the Gonads of Danio rerio: The Role of Brain-Derived Neurotrophic Factor. Anat. Rec. 2018, 301, 140–147. [Google Scholar] [CrossRef]
- Gatta, C.; Castaldo, L.; Cellerino, A.; de Girolamo, P.; Lucini, C.; D’Angelo, L. Brain derived neurotrophic factor in the retina of the teleost N. furzeri. Ann. Anat. 2014, 196, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Germana, A.; Guerrera, M.C.; Laura, R.; Levanti, M.; Aragona, M.; Mhalhel, K.; Germana, G.; Montalbano, G.; Abbate, F. Expression and Localization of BDNF/TrkB System in the Zebrafish Inner Ear. Int. J. Mol. Sci. 2020, 21, 5787. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P. Expression of Nerve Growth Factor and Its Receptor TrkA in the Reproductive System of Adult Zebrafish. Vet. Sci. 2022, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Karavanov, A.; Sainio, K.; Palgi, J.; Saarma, M.; Saxen, L.; Sariola, H. Neurotrophin 3 rescues neuronal precursors from apoptosis and promotes neuronal differentiation in the embryonic metanephric kidney. Proc. Natl. Acad. Sci. USA 1995, 92, 11279–11283. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.J.; Hempstead, B.; Donovan, M.J. Neurotrophin and neurotrophin receptors in human fetal kidney. Dev. Biol. 1996, 179, 369–381. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, P.; Arcamone, N.; Lucini, C.; Simeoli, M.P.; Castaldo, L.; Gargiulo, G. TRK neurotrophin receptor-like proteins in the kidney of frog (Rana esculenta) and lizard (Podarcis sicula): An immunohistochemical study. Anat. Embryol. 2004, 207, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.H.; Suh, J.S.; Cho, B.S. Linkage and association study of neurotrophins and their receptors as novel susceptibility genes for childhood IgA nephropathy. Pediatr. Res. 2011, 69, 299–305. [Google Scholar] [CrossRef]
- Tao, Y.S.; Piao, S.G.; Jin, Y.S.; Jin, J.Z.; Zheng, H.L.; Zhao, H.Y.; Lim, S.W.; Yang, C.W.; Li, C. Expression of brain-derived neurotrophic factor in kidneys from normal and cyclosporine-treated rats. BMC Nephrol. 2018, 19, 63. [Google Scholar] [CrossRef]
- Fedou, C.; Lescat, O.; Feuillet, G.; Buleon, M.; Neau, E.; Breuil, B.; Alves, M.; Batut, J.; Blader, P.; Decramer, S.; et al. The low affinity p75 neurotrophin receptor is down-regulated in congenital anomalies of the kidney and the urinary tract: Possible involvement in early nephrogenesis. Biochem. Biophys. Res. Commun. 2020, 533, 786–791. [Google Scholar] [CrossRef]
- D’Angelo, L.; Lossi, L.; Merighi, A.; de Girolamo, P. Anatomical features for the adequate choice of experimental animal models in biomedicine: I. Fishes. Ann. Anat. 2016, 205, 75–84. [Google Scholar] [CrossRef]
- van den Bos, R.; Mes, W.; Galligani, P.; Heil, A.; Zethof, J.; Flik, G.; Gorissen, M. Further characterisation of differences between TL and AB zebrafish (Danio rerio): Gene expression, physiology and behaviour at day 5 of the larval stage. PLoS ONE 2017, 12, e0175420. [Google Scholar] [CrossRef] [PubMed]
- Avendano-Herrera, R.; Benavides, I.; Espina, J.A.; Soto-Comte, D.; Poblete-Morales, M.; Valdes, J.A.; Feijoo, C.G.; Reyes, A.E. Zebrafish (Danio rerio) as an animal model for bath infection by Flavobacterium psychrophilum. J. Fish Dis. 2020, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Dunn, C.; Ramos, M.F. Zebrafish as an Animal Model for Ocular Toxicity Testing: A Review of Ocular Anatomy and Functional Assays. Toxicol. Pathol. 2021, 49, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, L.M.; Duchowicz, P.R. Predicting zebrafish (Danio rerio) embryo developmental toxicity through a non-conformational QSAR approach. Sci. Total Environ. 2021, 796, 148820. [Google Scholar] [CrossRef]
- Sales Cadena, M.R.; Cadena, P.G.; Watson, M.R.; Sarmah, S.; Boehm Ii, S.L.; Marrs, J.A. Zebrafish (Danio rerio) larvae show behavioral and embryonic development defects when exposed to opioids at embryo stage. Neurotoxicol. Teratol. 2021, 85, 106964. [Google Scholar] [CrossRef]
- Baran, A.; Yildirim, S.; Ghosigharehaghaji, A.; Bolat, I.; Sulukan, E.; Ceyhun, S.B. An approach to evaluating the potential teratogenic and neurotoxic mechanism of BHA based on apoptosis induced by oxidative stress in zebrafish embryo (Danio rerio). Hum. Exp. Toxicol. 2021, 40, 425–438. [Google Scholar] [CrossRef]
- Prakash, V.; Jain, V.; Chauhan, S.S.; Parthasarathi, R.; Roy, S.K.; Anbumani, S. Developmental toxicity assessment of 4-MBC in Danio rerio embryo-larval stages. Sci. Total Environ. 2022, 804, 149920. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Kato, Y.; Tonomura, Y.; Hanafusa, H.; Nishimura, K.; Fukushima, T.; Ueno, M. Adult Zebrafish Model for Screening Drug-Induced Kidney Injury. Toxicol. Sci. 2020, 174, 241–253. [Google Scholar] [CrossRef]
- Jin, M.; Wang, D.; Xu, W.; Wang, H.; Cao, Y. Claudin-7b and Claudin-h are required for controlling cilia morphogenesis in the zebrafish kidney. Mech. Dev. 2020, 161, 103595. [Google Scholar] [CrossRef] [PubMed]
- Brilli Skvarca, L.; Han, H.I.; Espiritu, E.B.; Missinato, M.A.; Rochon, E.R.; McDaniels, M.D.; Bais, A.S.; Roman, B.L.; Waxman, J.S.; Watkins, S.C.; et al. Enhancing regeneration after acute kidney injury by promoting cellular dedifferentiation in zebrafish. Dis. Model. Mech. 2019, 12, dmm037390. [Google Scholar] [CrossRef] [PubMed]
- Bonatesta, F.; Emadi, C.; Price, E.R.; Wang, Y.; Greer, J.B.; Xu, E.G.; Schlenk, D.; Grosell, M.; Mager, E.M. The developing zebrafish kidney is impaired by Deepwater Horizon crude oil early-life stage exposure: A molecular to whole-organism perspective. Sci. Total Environ. 2022, 808, 151988. [Google Scholar] [CrossRef] [PubMed]
- Poureetezadi, S.J.; Wingert, R.A. Little fish, big catch: Zebrafish as a model for kidney disease. Kidney Int. 2016, 89, 1204–1210. [Google Scholar] [CrossRef]
- Drummond, I.A.; Davidson, A.J. Zebrafish kidney development. Methods Cell Biol. 2016, 134, 391–429. [Google Scholar] [CrossRef]
- Outtandy, P.; Russell, C.; Kleta, R.; Bockenhauer, D. Zebrafish as a model for kidney function and disease. Pediatr. Nephrol. 2019, 34, 751–762. [Google Scholar] [CrossRef]
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.; Woutersen, R.A. Normal anatomy and histology of the adult zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef]
- Kobayashi, I.; Kondo, M.; Yamamori, S.; Kobayashi-Sun, J.; Taniguchi, M.; Kanemaru, K.; Katakura, F.; Traver, D. Enrichment of hematopoietic stem/progenitor cells in the zebrafish kidney. Sci. Rep. 2019, 9, 14205. [Google Scholar] [CrossRef]
- Drummond, B.E.; Wingert, R.A. Insights into kidney stem cell development and regeneration using zebrafish. World J. Stem Cells 2016, 8, 22–31. [Google Scholar] [CrossRef]
- Perner, B.; Schnerwitzki, D.; Graf, M.; Englert, C. Analysis of Zebrafish Kidney Development with Time-lapse Imaging Using a Dissecting Microscope Equipped for Optical Sectioning. J. Vis. Exp. 2016, 110, e53921. [Google Scholar] [CrossRef]
- Marra, A.N.; Wingert, R.A. Epithelial cell fate in the nephron tubule is mediated by the ETS transcription factors etv5a and etv4 during zebrafish kidney development. Dev. Biol. 2016, 411, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Song, H.D.; Sun, X.J.; Deng, M.; Zhang, G.W.; Zhou, Y.; Wu, X.Y.; Sheng, Y.; Chen, Y.; Ruan, Z.; Jiang, C.L.; et al. Hematopoietic gene expression profile in zebrafish kidney marrow. Proc. Natl. Acad. Sci. USA 2004, 101, 16240–16245. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.; Bowman, T.V.; Zon, L. Transplantation of whole kidney marrow in adult zebrafish. J. Vis. Exp. 2007, 2, 159. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, O.; Kulkeaw, K.; Nakagawa, M.; Sasaki, T.; Mizuochi, C.; Horio, Y.; Ishitani, T.; Sugiyama, D. Characterization of kidney marrow in zebrafish (Danio rerio) by using a new surgical technique. Prilozi 2009, 30, 71–80. [Google Scholar] [PubMed]
- Cacialli, P.; Mahony, C.B.; Petzold, T.; Bordignon, P.; Rougemont, A.L.; Bertrand, J.Y. A connexin/ifi30 pathway bridges HSCs with their niche to dampen oxidative stress. Nat. Commun. 2021, 12, 4484. [Google Scholar] [CrossRef]
- Mahony, C.B.; Cacialli, P.; Pasche, C.; Monteiro, R.; Savvides, S.N.; Bertrand, J.Y. Hapln1b, a central organizer of the ECM, modulates kit signaling to control developmental hematopoiesis in zebrafish. Blood Adv. 2021, 5, 4935–4948. [Google Scholar] [CrossRef]
- Gasanov, E.V.; Rafieva, L.M.; Korzh, V.P. BDNF-TrkB axis regulates migration of the lateral line primordium and modulates the maintenance of mechanoreceptor progenitors. PLoS ONE 2015, 10, e0119711. [Google Scholar] [CrossRef]
- Cacialli, P.; D’Angelo, L.; Kah, O.; Coumailleau, P.; Gueguen, M.M.; Pellegrini, E.; Lucini, C. Neuronal expression of brain derived neurotrophic factor in the injured telencephalon of adult zebrafish. J. Comp. Neurol. 2018, 526, 569–582. [Google Scholar] [CrossRef]
- Sahu, M.P.; Pazos-Boubeta, Y.; Pajanoja, C.; Rozov, S.; Panula, P.; Castren, E. Neurotrophin receptor Ntrk2b function in the maintenance of dopamine and serotonin neurons in zebrafish. Sci. Rep. 2019, 9, 2036. [Google Scholar] [CrossRef]
- Nittoli, V.; Sepe, R.M.; Coppola, U.; D’Agostino, Y.; De Felice, E.; Palladino, A.; Vassalli, Q.A.; Locascio, A.; Ristoratore, F.; Spagnuolo, A.; et al. A comprehensive analysis of neurotrophins and neurotrophin tyrosine kinase receptors expression during development of zebrafish. J. Comp. Neurol. 2018, 526, 1057–1072. [Google Scholar] [CrossRef]
- Gatta, C.; Altamura, G.; Avallone, L.; Castaldo, L.; Corteggio, A.; D’Angelo, L.; de Girolamo, P.; Lucini, C. Neurotrophins and their Trk-receptors in the cerebellum of zebrafish. J. Morphol. 2016, 277, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Squinto, S.; Ip, N.Y.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. Neurotrophin-3: A neurotrophic factor related to NGF and BDNF. Science 1990, 247, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M.; Soderstrom, S.; Ebendal, T.; Birchmeier, C.; Ekblom, P. Differential expression of neurotrophin receptors during renal development. Development 1993, 119, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Good, D.W.; George, T. Neurotrophin-3 inhibits HCO absorption via a cAMP-dependent pathway in renal thick ascending limb. Am. J. Physiol. Cell Physiol. 2001, 281, C1804–C1811. [Google Scholar] [CrossRef]
- Gromnitza, S.; Lepa, C.; Weide, T.; Schwab, A.; Pavenstadt, H.; George, B. Tropomyosin-related kinase C (TrkC) enhances podocyte migration by ERK-mediated WAVE2 activation. FASEB J. 2018, 32, 1665–1676. [Google Scholar] [CrossRef]
- Lepa, C.; Hoppe, S.; Stober, A.; Skryabin, B.V.; Sievers, L.K.; Heitplatz, B.; Ciarimboli, G.; Neugebauer, U.; Lindenmeyer, M.T.; Cohen, C.D.; et al. TrkC Is Essential for Nephron Function and Trans-Activates Igf1R Signaling. J. Am. Soc. Nephrol. 2021, 32, 357–374. [Google Scholar] [CrossRef]
- Arcamone, N.; Lucini, C.; Borzacchiello, G.; Castaldo, L.; Gargiulo, G.; De Girolamo, P. Distribution of NGF and NT-3-like protein immunoreactivity in the teleost kidney. Microsc. Res. Tech. 2005, 66, 17–24. [Google Scholar] [CrossRef]
- Bonofiglio, R.; Antonucci, M.T.; Papalia, T.; Romeo, F.; Capocasale, G.; Caroleo, M.C.; Di Fausto, V.; Aloe, L. Nerve growth factor (NGF) and NGF-receptor expression in diseased human kidneys. J. Nephrol. 2007, 20, 186–195. [Google Scholar]
- Gigliotti, P.; Lofaro, D.; Leone, F.; Perri, A.; Vizza, D.; Papalia, T.; Bonofiglio, R. High nerve growth factor blood concentration in renal transplantation: A new prognostic marker? Transplant. Proc. 2013, 45, 2654–2656. [Google Scholar] [CrossRef]
- Vizza, D.; Perri, A.; Lofaro, D.; Toteda, G.; Lupinacci, S.; Leone, F.; Gigliotti, P.; Papalia, T.; Bonofiglio, R. Exposure to nerve growth factor worsens nephrotoxic effect induced by Cyclosporine A in HK-2 cells. PLoS ONE 2013, 8, e80113. [Google Scholar] [CrossRef][Green Version]
- Hamano, Y.; Ito, F.; Suzuki, O.; Koura, M.; Matsuoka, S.; Kobayashi, T.; Sugitani, Y.; Wali, N.; Koyanagi, A.; Hino, O.; et al. Vasculitis and crescentic glomerulonephritis in a newly established congenic mouse strain derived from ANCA-associated vasculitis-prone SCG/Kj mice. Autoimmunity 2019, 52, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.K.; Petrova, T.; Marchesi, F.; Gierlinski, M.; Razsolkov, M.; Lee, K.L.; Wright, S.W.; Rao, V.R.; Cohen, P.; Arthur, J.S.C. Distinct signals and immune cells drive liver pathology and glomerulonephritis in ABIN1[D485N] mice. Life Sci. Alliance 2019, 2, e201900533. [Google Scholar] [CrossRef] [PubMed]
- Moschovaki-Filippidou, F.; Steiger, S.; Lorenz, G.; Schmaderer, C.; Ribeiro, A.; von Rauchhaupt, E.; Cohen, C.D.; Anders, H.J.; Lindenmeyer, M.; Lech, M. Growth Differentiation Factor 15 Ameliorates Anti-Glomerular Basement Membrane Glomerulonephritis in Mice. Int. J. Mol. Sci. 2020, 21, 6978. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, X.; Lin, Z.; Liu, Y.; Gu, L.; Zhou, H.; Tang, W.; Zuo, J. Reversible SAHH inhibitor protects against glomerulonephritis in lupus-prone mice by downregulating renal alpha-actinin-4 expression and stabilizing integrin-cytoskeleton linkage. Arthritis Res. Ther. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Suarez, O.; Gonzalez-Martinez, T.; Germana, A.; Monjil, D.F.; Torrecilla, J.R.; Laura, R.; Silos-Santiago, I.; Guate, J.L.; Vega, J.A. Expression of TrkB in the murine kidney. Microsc. Res. Tech. 2006, 69, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Catania, S.; Germana, A.; Laura, R.; Gonzalez-Martinez, T.; Ciriaco, E.; Vega, J.A. The crypt neurons in the olfactory epithelium of the adult zebrafish express TrkA-like immunoreactivity. Neurosci. Lett. 2003, 350, 5–8. [Google Scholar] [CrossRef]
- Gezginci-Oktayoglu, S.; Coskun, E.; Ercin, M.; Bolkent, S. 4-Methylcatechol prevents streptozotocin-induced acute kidney injury through modulating NGF/TrkA and ROS-related Akt/GSK3beta/beta-catenin pathways. Int. Immunopharmacol. 2018, 64, 52–59. [Google Scholar] [CrossRef]
- Endlich, N.; Lange, T.; Kuhn, J.; Klemm, P.; Kotb, A.M.; Siegerist, F.; Kindt, F.; Lindenmeyer, M.T.; Cohen, C.D.; Kuss, A.W.; et al. BDNF: mRNA expression in urine cells of patients with chronic kidney disease and its role in kidney function. J. Cell. Mol. Med. 2018, 22, 5265–5277. [Google Scholar] [CrossRef]
| NGF | N-terminus of the mature chain of NGF of human origin | 1:100 | sc-548 and sc-548P S. Cruz Biotechnology, CA |
| BDNF | Internal region of BDNF of human origin aa. 100–150 | 1:100 | sc-546 and sc-546P S. Cruz Biotechnology, CA |
| NT-3 | Internal region of NT-3 of human origin | 1:100 | sc-547 and sc-547P S. Cruz Biotechnology, CA |
| NT-4 | Internal region of NT-4 of human origin | 1:100 | sc-545 and sc-545P S. Cruz Biotechnology, CA |
| TrkA | Human COO-domain 763–777 (intracytoplasmic region) | 1:100 | sc-118 and sc-118P S. Cruz Biotechnology, CA |
| TrkB | Human COO-domain 794–808 (intracytoplasmic region) | 1:100 | sc-12 and sc-12P S. Cruz Biotechnology, CA |
| TrkC | Human COO-domain 798–812 (intracytoplasmic region) | 1:100 | sc-117 and sc-117P S. Cruz Biotechnology, CA |
| Name | Proximal Tubule | Distal Tubule | Collecting Duct | Hematopoietic Cells |
|---|---|---|---|---|
| Ngf | + | + | − | − |
| Bdnf | − | + | − | + |
| Nt3 | + | + | + | + |
| Nt4 | − | − | − | − |
| Trka | + | − | + | − |
| Trkb | − | − | − | + |
| Trkc | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacialli, P.; Lucini, C. Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney. Vet. Sci. 2022, 9, 296. https://doi.org/10.3390/vetsci9060296
Cacialli P, Lucini C. Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney. Veterinary Sciences. 2022; 9(6):296. https://doi.org/10.3390/vetsci9060296
Chicago/Turabian StyleCacialli, Pietro, and Carla Lucini. 2022. "Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney" Veterinary Sciences 9, no. 6: 296. https://doi.org/10.3390/vetsci9060296
APA StyleCacialli, P., & Lucini, C. (2022). Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney. Veterinary Sciences, 9(6), 296. https://doi.org/10.3390/vetsci9060296







