Mixed Infection of Mycobacterium szulgai, M. lentiflavum, and Gram-Negative Bacteria as a Cause of Death in a Brown Caiman Caiman crocodylus: A Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Description
2.2. Postmortem Examination
2.3. Histological and Microbiological Examination
3. Results
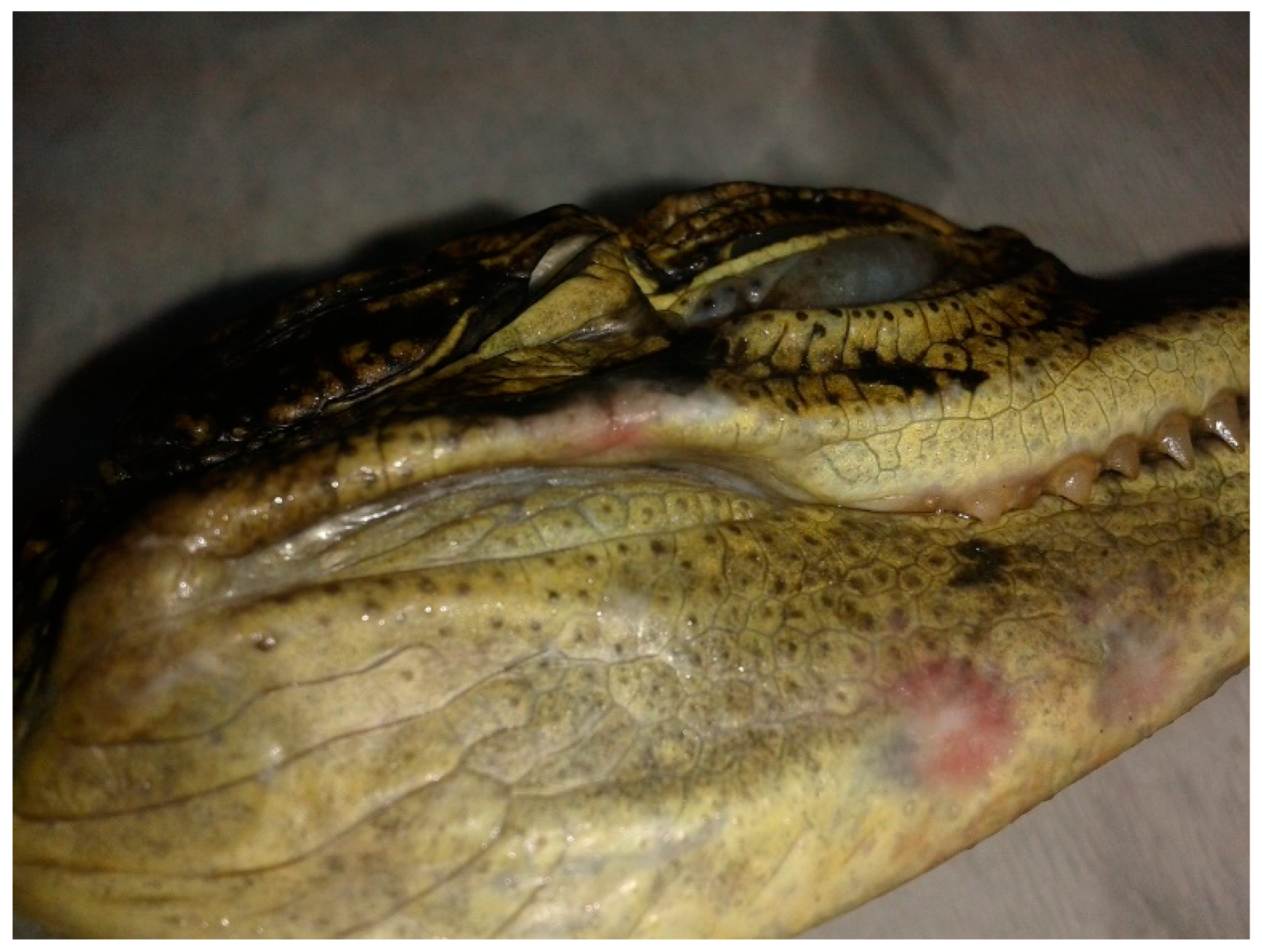
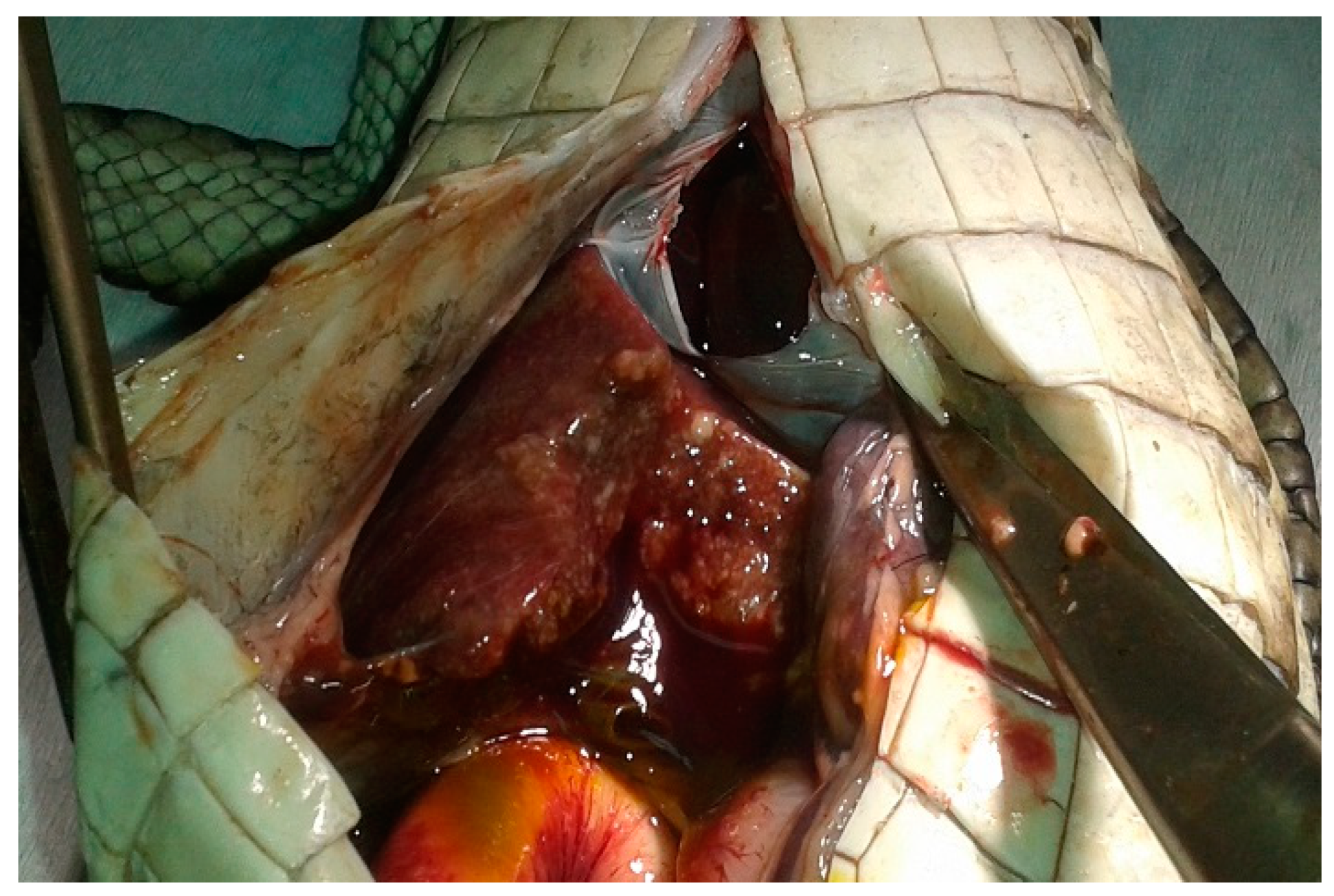
3.1. Postmortem Examination
3.2. Histological and Microbiological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, E.R.; Mecsas, J. Bacterial secretion systems: An overview. Microbiol. Spectr. 2017, 5, 4–10. [Google Scholar] [CrossRef]
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections—A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarkowska, A.; Didkowska, A.; Kwiecień, E.; Stefańska, I.; Rzewuska, M.; Anusz, K. The Mycobacterium avium complex—An underestimated threat to humans and animals. Ann. Agric. Environ. Med. 2021. [Google Scholar] [CrossRef]
- Huchzermeyer, F.W. Crocodiles: Biology, Husbandry and Diseases; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Soldati, G.; Lu, Z.H.; Vaughan, L.; Polkinghorne, A.; Zimmermann, D.R.; Huder, J.B.; Pospischil, A. Detection of Myco-bacteria and Chlamydiae in granulomatous inflammation of reptiles: A retrospective study. Vet. Pathol. 2004, 41, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Girling, S.; Fraser, M. Systemic mycobacteriosis in an inland bearded dragon (Pogona vitticeps). Vet. Rec. 2007, 160, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Divers, S.; Shearer, D. Pulmonary mycobacteriosis caused by Mycobacterium haemophilum and M. marinum in a royal python. J. Am. Veter. Med. Assoc. 2002, 220, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Waliszewski, N.T.; Garner, M.M.; Tseng, F.S. Sepsis and Disseminated Intravascular Coagulation in an Eastern Spiny Softshell Turtle (Apalone spinifera spinifera) with Acute Mycobacteriosis. J. Zoo Wildl. Med. 2009, 40, 572–575. [Google Scholar] [CrossRef]
- Kik, M. Disseminated Mycobacterium intracellulare infection in a broad-snouted caiman Caiman latirostris. Dis. Aquat. Org. 2013, 107, 83–86. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Stacy, B.A.; Ladner, J.T.; Lorch, J.M.; Balazs, G.H.; Barquero-Calvo, E.; Berlowski-Zier, B.M.; Breeden, R.; Corrales-Gómez, N.; et al. A novel host-adapted strain of Salmonella Typhimurium causes renal disease in olive ridley turtles (Lepidochelys olivacea) in the Pacific. Sci. Rep. 2019, 9, 9313. [Google Scholar] [CrossRef] [PubMed]
- Finlay, F.; Furnell, C.; Ridley, P. Salmonella in pets: The risk to children. Community Pract. 2015, 88, 27–28. [Google Scholar]
- Robinson, J.L. Salmonella infections in Canadian children. Paediatr. Child Health 2019, 24, 50–51. [Google Scholar] [CrossRef]
- Dróżdż, M.; Małaszczuk, M.; Paluch, E.; Pawlak, A. Zoonotic potential and prevalence of Salmonella serovars isolated from pets. Infect. Ecol. Epidemiol. 2021, 11, 1975530. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M. Prevalence and serovar distribution of Salmonella in fresh and frozen meat from captive Nile crocodiles (Crocodylus niloticus). Int. J. Food Microbiol. 1996, 29, 111–118. [Google Scholar] [CrossRef]
- Pozio, E.; Foggin, C.M.; Gelanew, T.; Marucci, G.; Hailu, A.; Rossi, P.; Morales, M.A.G. Trichinella zimbabwensis in wild reptiles of Zimbabwe and Mozambique and farmed reptiles of Ethiopia. Veter. Parasitol. 2007, 143, 305–310. [Google Scholar] [CrossRef]
- Schneider, L.; Peleja, R.P.; Jr, A.K.; Freire, G.M.; Marioni, B.; Vogt, R.C.; Da Silveira, R. Mercury Concentration in the Spectacled Caiman and Black Caiman (Alligatoridae) of the Amazon: Implications for Human Health. Arch. Environ. Contam. Toxicol. 2012, 63, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, N.; Nopp, A.; Hamsten, C.; Vetander, M.; Melén, E.; Nilsson, C.; Ollert, M.; Flohr, C.; Kuehn, A.; van Hage, M. Anaphylactic Reactions to Novel Foods: Case Report of a Child with Severe Crocodile Meat Allergy. Pediatrics 2017, 139, e20161404. [Google Scholar] [CrossRef] [PubMed]
- Instrukcja Głównego Lekarza Weterynarii nr GIWpr-02010-8/2016 z dnia 8 lutego 2016. Available online: http://www.ostrowmaz.piwet.net/instrukcje/instrukcja_gruzlica.pdf (accessed on 19 December 2021). (in Polish).
- Tortoli, E.; Cichero, P.; Piersimoni, C.; Simonetti, M.T.; Gesu, G.; Nista, D. Use of BACTEC MGIT 960 for recovery of my-cobacteria from clinical specimens: Multicenter study. J. Clin. Microbiol. 1999, 37, 3578–3582. [Google Scholar] [CrossRef] [PubMed]
- Grimont, P.A.D.; Weill, F.X. Antigenic Formulae of Salmonella serovars, 9th ed.; WHO Collaborating Centre for Research on Salmonella, Institute Pasteur: Paris, France, 2007. [Google Scholar]
- Slany, M.; Knotek, Z.; Skoric, M.; Knotková, Z.; Svobodová, J.; Mrlík, V.; Moravkova, M.; Pavlík, I. Systemic mixed infection in a brown caiman (Caiman crocodilus fuscus) caused by Mycobacterium szulgai and M. chelonae: A case report. Veterinární Med. 2010, 55, 91–96. [Google Scholar] [CrossRef]
- Springer, B.; Wu, W.K.; Bodmer, T.; Haase, G.; Pfyffer, G.E.; Kroppenstedt, R.M.; Schröder, K.H.; Emler, S.; Kilburn, J.O.; Kirschner, P.; et al. Isolation and characterization of a unique group of slowly growing mycobacteria: Description of Mycobacterium lentiflavum sp. nov. J. Clin. Microbiol. 1996, 34, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; Gazzola, L.; Cesari, M.; Lombardi, A.; Salerno, F.; Tortoli, E.; Codecasa, L.; Penati, V.; Franzetti, F.; Gori, A. Mycobacterium lentiflavum Infection in Immunocompetent Patient. Emerg. Infect. Dis. 2005, 11, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Makovcova, J.; Slany, M.; Babak, V.; Slana, I.; Kralik, P. The water environment as a source of potentially pathogenic mycobacteria. J. Water Health 2013, 12, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Fratini, F.; Bertelloni, F.; Cerri, D.; Tortoli, E. Isolation and identification of mycobacteria from captive reptiles. Res. Veter. Sci. 2012, 93, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Agyare, E.O.; Vagvolgyi, A.E.; Halpern, M. Aerobic bacterial oral flora of garter snakes: Development of normal flora and pathogenic potential for snakes and humans. J. Clin. Microbiol. 1981, 13, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.J.; Lovely, C.J.; Pittman, J.M. A preliminary disease survey in the wild Nile crocodile (Crocodylus niloticus) population in the Okavango Delta, Botswana. J. S. Afr. Vet. Assoc. 2011, 82, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.A.; Mota, R.A.; Pinheiro, J.W.; Almeida, M.C.S.; Silva, D.R.; Ferreira, D.R.A.; Azevedo, J.C.N. Aerobic Bacterial Microflora of Broad-Snouted Caiman (Caiman latirostris) Oral Cavity and Cloaca, Originating from Parque Zoologico Arruda Camara, Paraiba, Brazil. Braz. J. Microbiol. 2009, 40, 194–198. [Google Scholar] [CrossRef]
- Cushing, A.; Pinborough, M.; Stanford, M. Review of bacterial and fungal culture and sensitivity results from reptilian samples submitted to a UK laboratory. Veter. Rec. 2011, 169, 390. [Google Scholar] [CrossRef]
- Jho, Y.-S.; Park, D.-H.; Lee, J.-H.; Cha, S.-Y.; Han, J.S. Identification of bacteria from the oral cavity and cloaca of snakes imported from Vietnam. Lab. Anim. Res. 2011, 27, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Maluta, A.; Wasyl, D.; Skarżyńska, M.; Lalak, A.; Samcik, I.; Kwit, R.; Szulowski, K. Genetic relationship of Salmonella isolates found in subcutaneous abscesses in leopard geckos (Eublepharis macularius). J. Veter. Res. 2020, 64, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Puk, K.; Guz, L. Occurrence of Mycobacterium spp. in ornamental fish. Ann. Agric. Environ. Med. 2020, 27, 535–539. [Google Scholar] [CrossRef]
- Delghandi, M.R.; El-Matbouli, M.; Menanteau-Ledouble, S. Mycobacteriosis and Infections with Non-tuberculous Mycobacteria in Aquatic Organisms: A Review. Microorganisms 2020, 8, 1368. [Google Scholar] [CrossRef] [PubMed]
- Guz, L.; Grądzki, Z.; Krajewska, M.; Lipiec, M.; Zabost, A.; Augustynowicz-Kopeć, E.; Zwolska, Z.; Szulowski, K. Occurrence and antimicrobial susceptibility of Mycobacterium peregrinum in ornamental fish. Bul. Vet. Inst. Pulawy. 2013, 57, 489–492. [Google Scholar] [CrossRef]
- Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R.; Adjemian, J. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Porvaznik, I.; Solovič, I.; Mokrý, J. Non-Tuberculous Mycobacteria: Classification, Diagnostics, and Therapy. Adv. Exp. Med. Biol. 2017, 944, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Krajewska-Wędzina, M.; Dąbrowska, A.; Augustynowicz-Kopeć, E.; Weiner, M.; Szulowski, K. Nontuberculous mycobacterial skin disease in cat; diagnosis and treatment—Case report. Ann. Agric. Environ. Med. 2019, 26, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Fratini, F. Bacterial zoonoses among domestic reptiles. Annali. Fac. Med. Vet. 2005, 26, 85–91. [Google Scholar]
- Golawska, O.; Demkowska-Kutrzepa, M.; Borzym, E.; Rozanski, P.; Zajac, M.; Rzezutka, A.; Wasyl, D. Microflora and para-sitofauna of alien and invasive turtle species. Postepy Mikrobiol. 2017, 56, 2. [Google Scholar]
- Golawska, O.; Zajac, M.; Maluta, A.; Pristasc, P.; Hamarova, E.; Wasyl, D. Complex bacterial flora of imported pet tortoises deceased during quarantine: Another zoonotic threat? Comp. Immunol. 2019, 65, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Skarżyńska, M.; Lalak, A.; Kwit, R.; Śmiałowska-Węglińska, A.; Pasim, P.; Szulowski, K.; Wasyl, D. Salmonella in Captive Reptiles and Their Environment—Can We Tame the Dragon? Microorganisms 2021, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Lorenzo-Rebenaque, L.; Laso, O.; Villora-Gonzalez, J.; Vega, S. Pet Reptiles: A Potential Source of Transmission of Multidrug-Resistant Salmonella. Front. Veter. Sci. 2021, 7, 613718. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Reptile-associated salmonellosis-selected states, 1998–2002. Morb. Mortal. Wkly. Rep. 2003, 52, 1206–1209. [Google Scholar]
- Murphy, D.; Oshin, F. Reptile-associated salmonellosis in children aged under 5 years in South West England. Arch. Dis. Child. 2015, 100, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Uhart, M.; Ferreyra, H.; Mattiello, R.; Caffer, M.I.; Terragno, R.; Schettino, A.; Prado, W. Isolation of Salmonella spp. from yacare caiman (Caiman yacare) and broad-snouted caiman (Caiman latirostris) from the argentine chaco. J. Wildl. Dis. 2011, 47, 271–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EFSA; ECDC. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 286. [Google Scholar] [CrossRef]
- Paz, L.N.; Hamond, C.; Dias, C.S.; Curvelo, V.P.; Medeiros, M.A.; Oriá, A.P.; Pinna, M.H. Detection of Leptospira spp. in Captive Broad-Snouted Caiman (Caiman latirostris). EcoHealth 2019, 16, 694–700. [Google Scholar] [CrossRef]
- Bauso, J.; Simoncini, M.S.; Chiani, Y.; Schmeling, M.F.; Larriera, A.; Vanasco, N.B.; Piña, C.I. Presence of Leptospira spp. in Caiman latirostris (Crocodylia, Alligatoridae) populations in Santa Fe, Argentina. Heliyon 2020, 6, e03837. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.G.X.; Pressinotti, L.N.; Carvalho, G.S.; Oliveira, M.C.V.; Moreno, L.; Matajira, C.E.C.; Bergamo, A.S.; Aleixo, V.M.; Veiga, A.C.; Corsino, E.D.S.; et al. Arcobacter spp. in fecal samples from Brazilian farmed caimans (Caiman yacare, Daudin 1802). Trop. Anim. Health Prod. 2017, 189, 777–782. [Google Scholar] [CrossRef]
- Ellerd, R.; Saleh, M.N.; Luksovsky, J.L.; Verocai, G.G. Endoparasites of pet reptiles and amphibians from exotic pet shows in Texas, United States. Vet. Parasitol. Reg. Stud. Rep. 2022, 27, 100671. [Google Scholar] [CrossRef] [PubMed]
- Hertner, G. Caiman bite. Wilderness Environ. Med. 2006, 17, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Abrahamian, F.M.; Goldstein, E.J.C. Microbiology of Animal Bite Wound Infections. Clin. Microbiol. Rev. 2011, 24, 231–246. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maluta, A.; Zając, M.; Krajewska-Wędzina, M.; Wasyl, D.; Heckers, K.; Didkowska, A.; Anusz, K. Mixed Infection of Mycobacterium szulgai, M. lentiflavum, and Gram-Negative Bacteria as a Cause of Death in a Brown Caiman Caiman crocodylus: A Case Report. Vet. Sci. 2022, 9, 133. https://doi.org/10.3390/vetsci9030133
Maluta A, Zając M, Krajewska-Wędzina M, Wasyl D, Heckers K, Didkowska A, Anusz K. Mixed Infection of Mycobacterium szulgai, M. lentiflavum, and Gram-Negative Bacteria as a Cause of Death in a Brown Caiman Caiman crocodylus: A Case Report. Veterinary Sciences. 2022; 9(3):133. https://doi.org/10.3390/vetsci9030133
Chicago/Turabian StyleMaluta, Aleksandra, Magdalena Zając, Monika Krajewska-Wędzina, Dariusz Wasyl, Kim Heckers, Anna Didkowska, and Krzysztof Anusz. 2022. "Mixed Infection of Mycobacterium szulgai, M. lentiflavum, and Gram-Negative Bacteria as a Cause of Death in a Brown Caiman Caiman crocodylus: A Case Report" Veterinary Sciences 9, no. 3: 133. https://doi.org/10.3390/vetsci9030133
APA StyleMaluta, A., Zając, M., Krajewska-Wędzina, M., Wasyl, D., Heckers, K., Didkowska, A., & Anusz, K. (2022). Mixed Infection of Mycobacterium szulgai, M. lentiflavum, and Gram-Negative Bacteria as a Cause of Death in a Brown Caiman Caiman crocodylus: A Case Report. Veterinary Sciences, 9(3), 133. https://doi.org/10.3390/vetsci9030133





