Adiponectin Influences FGF2 in the Developing Porcine Corpus Luteum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CL Collection
2.3. Immunohistochemistry
2.4. Gene Expression
2.5. Dispersed Corpora Lutea Cell Cultures
2.6. Radioimmunoassay
2.7. Statistical Analyses
3. Results
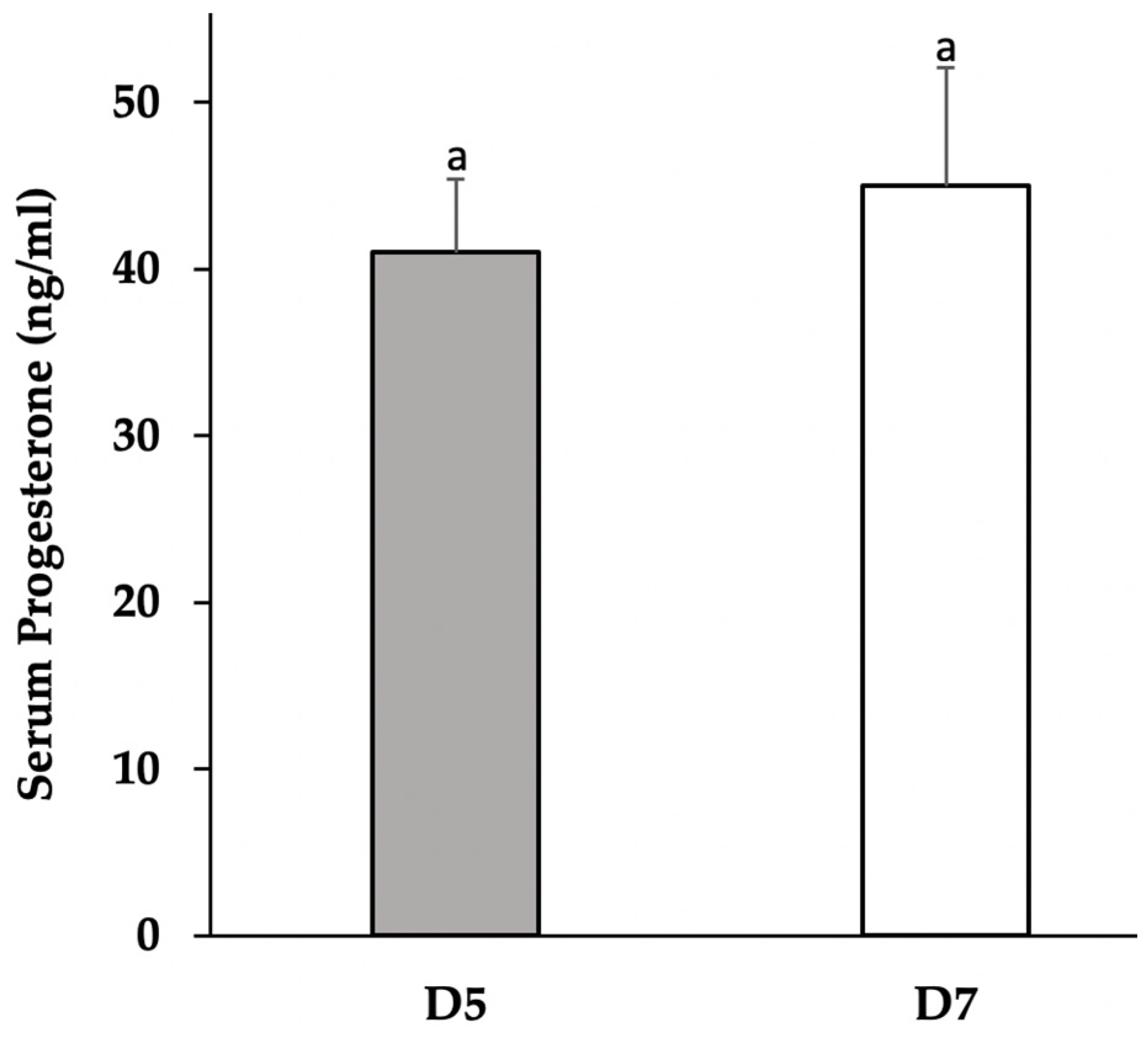
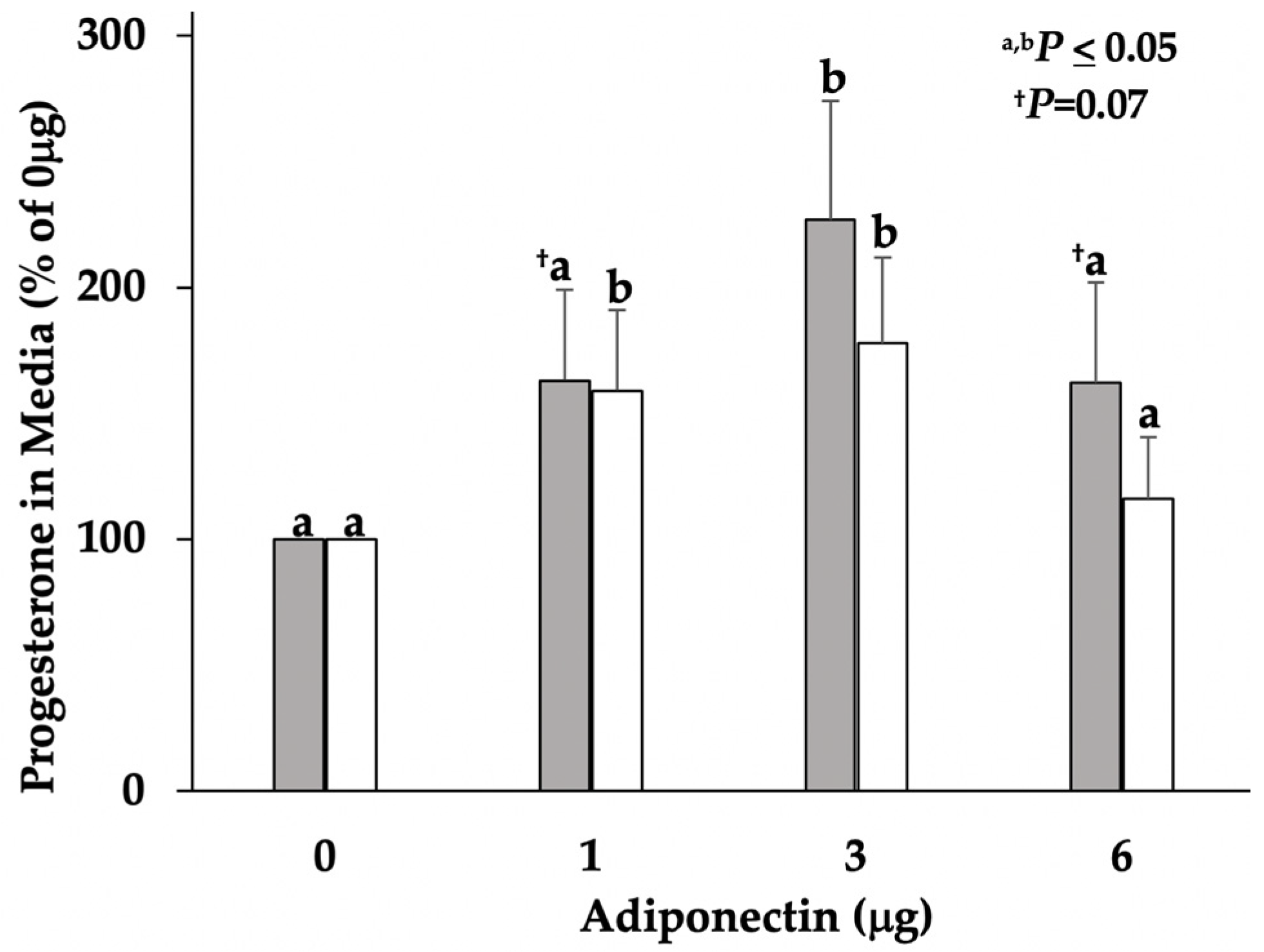
3.1. Progesterone Concentrations in Serum and Cultured Media
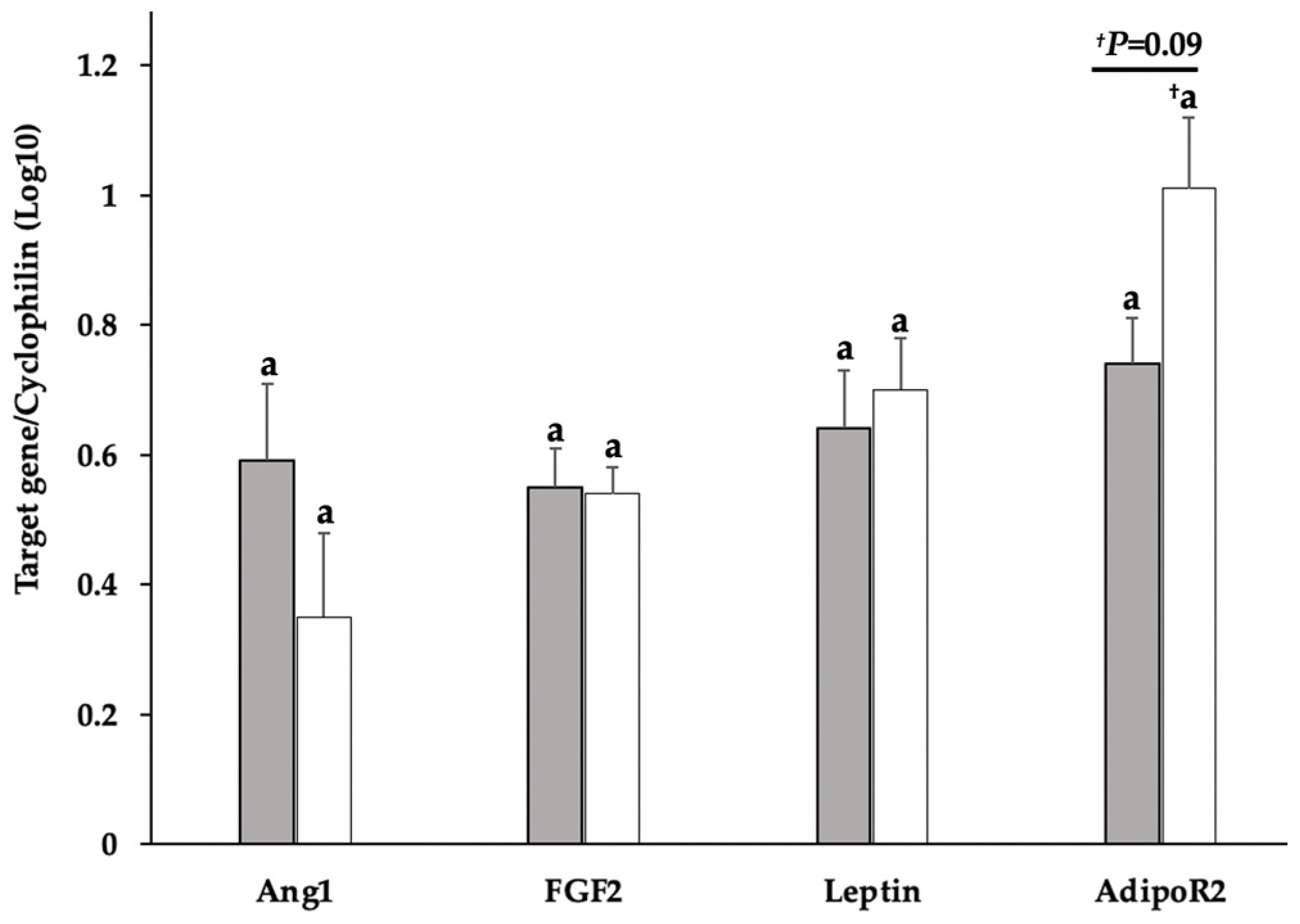
3.2. Gene Expression of FGF2, Ang1, Leptin, and AdipoR2 in Tissue and Cell Cultures
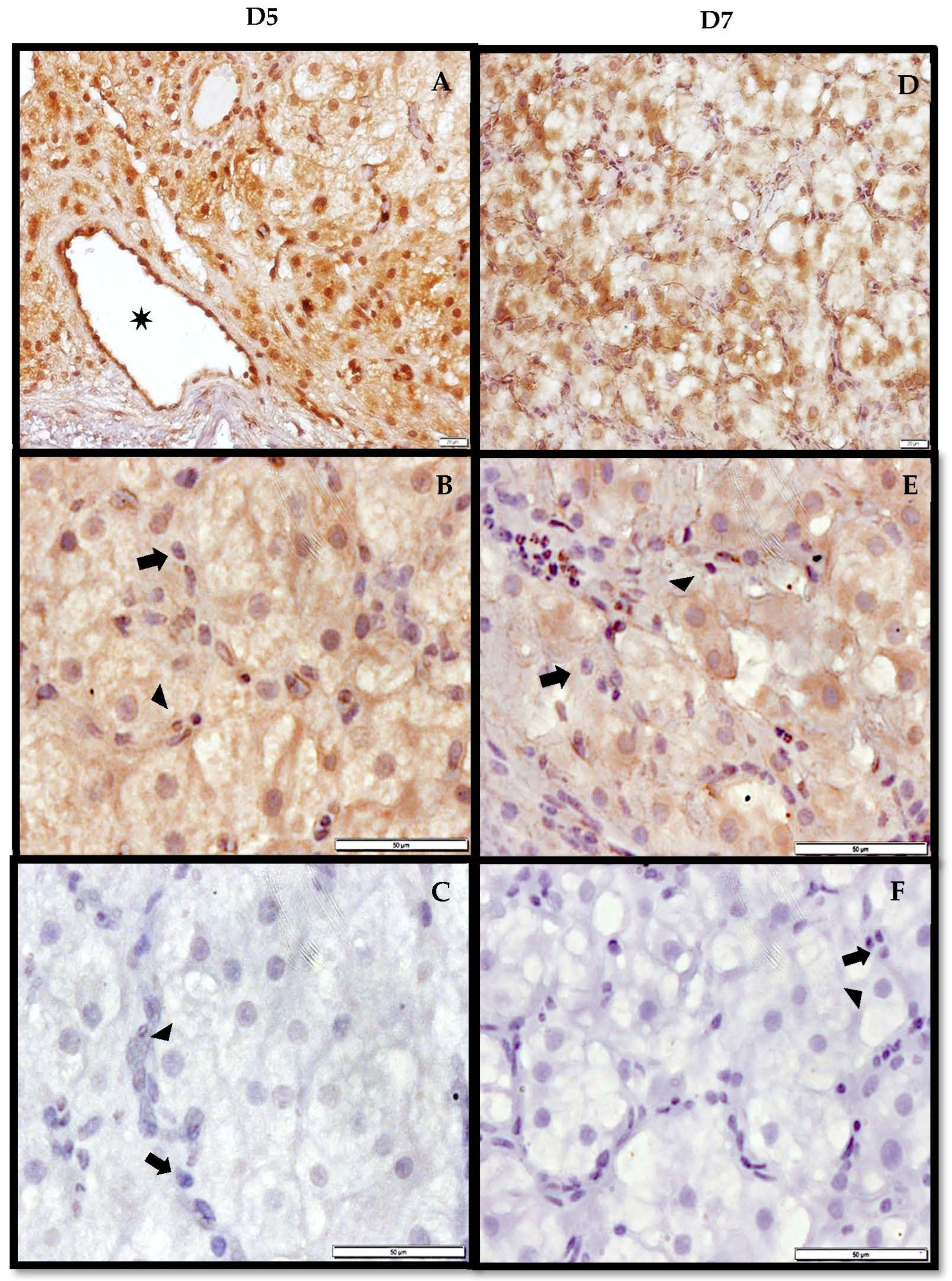
3.3. Immunohistochemistry of AdipoR2 in the CL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, R.S.; Woad, K.J.; Hunter, M.G.; Sinclair, K.D.; Laird, M.; Joseph, C.; Hammond, A.J.; Mann, G.E. Corpus Luteum Development and Angiogenesis. Biosci. Proc. 2019, 8, 327–343. [Google Scholar] [CrossRef]
- Mishra, S.; Parmar, M.; Yadav, V.; Reshma, R.; Bharati, J.; Bharti, M.; Paul, A.; Chouhan, V.; Taru Sharma, G.; Singh, G.; et al. Expression and Localization of Angiopoietin Family in Corpus Luteum During Different Stages of Oestrous Cycle and Modulatory Role of Angiopoietins on Steroidogenesis, Angiogenesis and Survivability of Cultured Buffalo Luteal Cells. Reprod. Domest. Anim. 2016, 51, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Zalman, Y.; Klipper, E.; Farberov, S.; Mondal, M.; Wee, G.; Folger, J.K.; Smith, G.W.; Meidan, R. Regulation of Angiogenesis-Related Prostaglandin F2alpha-Induced Genes in the Bovine Corpus Luteum. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef]
- Katchko, R.A.; Wiles, J.R.; Ramirez, M.A.; Ayala, L.; Xie, F.; O’Gorman, C.W.; Keisler, D.H.; Stanko, R.L.; Garcia, M.R.; Benavides, E.A. The Effect of Leptin on Luteal Angiogenic Factors in the Developing Porcine Corpus Luteum. JSM Invit. Fertil. 2017, 2, 1020. [Google Scholar]
- Petrik, J.J.; Gentry, P.A.; Feige, J.-J.; LaMarre, J. Expression and Localization of Thrombospondin-1 and -2 and Their Cell-Surface Receptor, CD36, During Rat Follicular Development and Formation of the Corpus Luteum. Biol. Reprod. 2002, 67, 1522–1531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, F.; Fu, R.; Liu, L.; Wang, X.; Wu, T.; Shen, W.; Gui, Z.; Mo, X.; Fang, B.; Xia, L. Leptin-Induced Angiogenesis of EA.Hy926 Endothelial Cells via the Akt and Wnt Signaling Pathways In Vitro and In Vivo. Front. Pharmacol. 2019, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Wiles, J.R.; Katchko, R.A.; Benavides, E.A.; O’Gorman, C.W.; Escudero, J.M.; Keisler, D.H.; Stanko, R.L.; Garcia, M.R. The Effect of Leptin on Luteal Angiogenic Factors during the Luteal Phase of the Estrous Cycle in Goats. Anim. Reprod. Sci. 2014, 148, 121–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Micklin, L.T.; Robinson, R.S.; Marsters, P.; Campbell, B.K.; Mann, G.E.; Hunter, M.G. Leptin in the Bovine Corpus Luteum: Receptor Expression and Effects on Progesterone Production. Mol. Reprod. Devel. 2007, 74, 724–729. [Google Scholar] [CrossRef]
- Ramirez, M.A.; Arellano, A.A.; Xie, F.; Benavides, E.A.; Katchko, R.A.; Ayala, L.; Calderon, A.; Flores, R.A.; Escudero, J.M.; Keisler, D.H.; et al. The Role of Leptin in the Development of the Corpus Luteum. In Leptin; Gilles, E., Michael, D., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 73–106. ISBN 978-1-53612-548-1. [Google Scholar]
- Maleszka, A.; Smolinska, N.; Nitkiewicz, A.; Kiezun, M.; Chojnowska, K.; Kamil Dobrzyn, K.; Szwaczek, H.; Kaminski, T. Adiponectin Expression in the Porcine Ovary During the Oestrous Cycle and its Effect on Ovarian Steroidogenesis. Int. J. Endocrinol. 2014, 2014, 957076. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Du, X.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-Function Studies of the Adipocyte-Secreted Hormone Acrp30/Adiponectin. Implications for Metabolic Regulation and Bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the Past Two Decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; Cammarata, R.; Masullo, M.; Nerone, G.; Finamore, F.; D’Andrea, M.; Pilla, F.; Oriani, G. Analysis of Adiponectin Gene and Comparison of its Expression in Two Different Pig Breeds. Obesity 2012, 16, 1869–1874. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Bråkenhielm, E.; Veitonmaki, N.; Cao, R.; Kihara, S.; Matsuzawa, Y.; Zhivotovsky, B.; Funahashi, T.; Cao, Y. Adiponectin-Induced Antiangiogenesis and Antitumor Activity Involve Caspase-Mediated Endothelial Cell Apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Correnti, J.M.; Cook, D.; Aksamitiene, E.; Swarup, A.; Ogunnaike, B.; Vadigepalli, R.; Hoek, J.B. Adiponectin Fine-Tuning of Liver Regeneration Dynamics Revealed Through Cellular Network Modelling. J. Physiol. 2015, 593, 365–383. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Pierre, P.; Froment, P.; Nègre, D.; Ramé, C.; Barateau, V.; Chabrolle, C.; Lecomte, P.; Dupont, J. Role of Adiponectin Receptors, AdipoR1 and AdipoR2, in the Steroidogenesis of the Human Granulosa Tumor Cell Line, KGN. Hum. Reprod. 2009, 24, 2890–2901. [Google Scholar] [CrossRef]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Zuriaga, M.A.; MacLauchlan, S.; Aprahamian, T.R.; Walsh, K. Divergent Roles for Adiponectin Receptor 1 (AdipoR1) and AdipoR2 in Mediating Revascularization and Metabolic Dysfunction in Vivo. J. Biol. Chem. 2014, 289, 16200–16213. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin Receptor Signaling: A New Layer to the Current Model. Cell Metab. 2011, 13, 123–124. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Uchiyama, M.; Nakano, Y.; Nagasaka, S.; Kang, D.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Alpha Agonist Suppresses Neovascularization by Reducing Both Vascular Endothelial Growth Factor and Angiopoietin-2 in Corneal Alkali Burn. Sci. Rep. 2017, 7, 17763. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.W.; Liang, Y.Y.; Yang, F.; Barron, D.A.; Ressler, S.J.; Schauer, I.G.; Feng, X.H.; Rowley, D.R. TGF-β Induction of FGF-2 Expression in Stromal Cells Requires Integrated Smad3 and MAPK Pathways. Am. J. Clin. Exp. Urol. 2014, 2, 239–248. [Google Scholar] [PubMed]
- Maleszka, A.; Smolinska, N.; Nitkiewicz, A.; Kiezun, M.; Dobrzyń, K.; Czerwińska, J.; Szeszko, K.; Kaminski, T. Expression of Adiponectin Receptors 1 and 2 in the Ovary and Concentration of Plasma Adiponectin During the Oestrous Cycle of the Pig. Acta Vet. Hung. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- Gupta, M.; Thakre, A.; Bahiram, K.B.; Sardar, V.M.; Dudhe, S.D.; Korde, J.P.; Bonde, S.W.; Kurkure, N.V. Abundance of Adiponectin mRNA Transcript in the Buffalo Corpus Luteum during the Estrous Cycle and Effects on Progesterone Secretion In Vitro. Anim. Reprod. Sci. 2019, 208, 106110. [Google Scholar] [CrossRef] [PubMed]
- Szeszko, K.; Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Maleszka, A.; Kaminski, T. The Influence of Adiponectin on the Transcriptomic Profile of Porcine Luteal Cells. Funct. Integr. Genom. 2016, 16, 101–114. [Google Scholar] [CrossRef]
- O’Gorman, C.W.; Stanko, R.L.; Keisler, D.H.; Garcia, M.R. Effects of Acute Fasting and Age on Leptin and Peroxisome Proliferator-Activated Receptor Gamma Production Relative to Fat Depot in Immature and Mature Pigs. J. Anim. Physiol. Anim. Nutr. 2010, 94, e266–e276. [Google Scholar] [CrossRef]
- Kalender, H.; Arikan, S. Size Distribution of Dispersed Luteal Cells during Oestrous Cycle in Angora Goats. Reprod. Domest. Anim. 2007, 42, 457–460. [Google Scholar] [CrossRef]
- Garcia, M.R.; Amstalden, M.; Williams, S.W.; Stanko, R.L.; Morrison, C.D.; Keisler, D.H.; Nizielski, S.E.; Williams, G.L. Serum Leptin and Its Adipose Gene Expression During Pubertal Development, the Estrous Cycle, and Different Seasons in Cattle. J. Anim. Sci. 2002, 80, 2158–2167. [Google Scholar] [CrossRef]
- Bustin, S.A. Absolute Quantification of mRNA Using Real-Time Reverse Transcription Polymerase Chain Reaction Assays. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Dai, M.H.; Xia, T.; Zhang, D.G.; Chen, D.X.; Gan, L.; Feng, Q.S.; Qiu, H.; Peng, Y.; Yang, Q.Z. Cloning, Expression and Chromosome Localization of Porcine Adiponectin and Adiponectin Receptors Genes. Dom. Anim. Endocrinol. 2006, 30, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Welter, H.; Wollenhaupt, K.; Einspanier, R. Developmental and Hormonal Regulated Gene Expression of Fibroblast Growth Factor 2 (FGF-2) and its Receptors in Porcine Endometrium. J. Steroid Biochem. Mol. Biol. 2004, 88, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Barb, C.R.; Kraeling, R.R.; Rampacek, G.B. Developmental Changes in the Long Form Leptin Receptor and Related Neuropeptide Gene Expression in the Pig Brain. Biol. Reprod. 1998, 64, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Dozois, C.M.; Oswald, E.; Gautier, N.; Serthelon, J.P.; Fairbrother, J.M.; Oswald, I.P. A Reverse Transcription-Polymerase Chain Reaction Method to Analyze Porcine Cytokine Gene Expression. Vet. Immunol. Immunopathol. 1997, 58, 287–300. [Google Scholar] [CrossRef]
- Merhi, Z.; Bazzi, A.A.; Bonney, E.A.; Buyuk, E. Role of Adiponectin in Ovarian Follicular Development and Ovarian Reserve. Biomed. Rep. 2019, 10, 337–342. [Google Scholar] [CrossRef]
- Goodwin, A.M. In Vitro Assays of Angiogenesis for Assessment of Angiogenic and Anti-Angiogenic Agents. Microvasc. Res. 2007, 74, 172–183. [Google Scholar] [CrossRef]
- Mao, J.; Treacy, B.K.; Almeida, F.R.C.L.; Novak, S.; Dixon, W.T.; Foxcroft, G.R. Feed Restriction and Insulin Treatment Affect Subsequent Luteal Function in the Immediate Postovulatory Period in Pigs: Progesterone Production In Vitro and Messenger Ribonucleic Acid Expression for Key Steroidogenic Enzymes. Biol. Reprod. 2001, 64, 359–367. [Google Scholar] [CrossRef][Green Version]
- Novak, S.; Almeida, F.R.C.L.; Cosgrove, J.R.; Dixon, W.T.; Foxcroft, G.R. Effect of Pre- and Postmating Nutritional Manipulation on Plasma Progesterone, Blastocyst Development, and the Oviductal Environment During Early Pregnancy in Gilts. J. Anim. Sci. 2003, 81, 772–783. [Google Scholar] [CrossRef]
- de las Heras-Saldana, S.; Chung, K.Y.; Lee, S.H.; Lee, S.H. Gene Expression of Hanwoo Satellite Cell Differentiation in Longissimus Dorsi and Semimembranosus. BMC Genom. 2019, 20, 156–171. [Google Scholar] [CrossRef]
- Perkel, K.J.; Madan, P. Spent Culture Medium Analysis from Individually Cultured Bovine Embryos Demonstrates Metabolomic Differences. Zygote 2017, 25, 662–674. [Google Scholar] [CrossRef]
- Lavoie, V.; Kernaleguen, A.-E.; Charron, G.; Farhat, N.; Cossette, M.; Mamarbachi, A.M.; Allen, B.F.; Rheaume, E.; Tardif, J.-C. Functional Effects of Adiponectin on Endothelial Progenitor Cells. Obesity 2011, 19, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.; Fan, Y.; Tang, Z.; Wang, N. Overexpression of Adiponectin Receptors Potentiates the Antiinflammatory Action of Subeffective Dose of Globular Adiponectin in Vascular Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, N.; Dobrzyn, K.; Kiezun, M.; Szeszko, K.; Maleszka, A.; Kaminski, T. Effect of Adiponectin on the Steroidogenic Acute Regulatory Protein, P450 Side Chain Cleavage Enzyme and 3β-Hydroxysteroid Dehydrogenase Gene Expression, Progesterone and Androstenedione Production by the Porcine Uterus During Early Pregnancy. J. Physiol. Pharmacol. 2016, 67, 443–456. [Google Scholar] [PubMed]
- Tu, C.; Fiandalo, M.V.; Pop, E.; Stocking, J.J.; Azabdaftari, G.; Li, J.; Wei, H.; Ma, D.; Qu, J.; Mohler, J.L.; et al. Proteomic Analysis of Charcoal-Stripped Fetal Bovine Serum Reveals Changes in the Insulin-like Growth Factor Signaling Pathway. J. Proteome Res. 2018, 17, 2963–2977. [Google Scholar] [CrossRef]
- Chabrolle, C.; Tosca, L.; Ramé, C.; Lecomte, P.; Royère, D.; Dupont, J. Adiponectin Increases Insulin-Like Growth Factor I-Induced Progesterone and Estradiol Secretion in Human Granulosa Cells. Fertil. Steril. 2009, 92, 1988–1996. [Google Scholar] [CrossRef]
- Maillard, V.; Uzbekova, S.; Guignot, F.; Perreau, C.; Ramé, C.; Coyral-Castel, S.; Dupont, J. Effect of Adiponectin on Bovine Granulosa Cell Steroidogenesis, Oocyte Maturation and Embryo Development. Reprod. Biol. Endocrinol. 2010, 8, 23. [Google Scholar] [CrossRef]
- Woad, J.K.; Hunter, G.M.; Mann, E.G.; Laird, M.; Hammond, J.A.; Robinson, S.R. Fibroblast Growth Factor 2 is a Key Determinant of Vascular Sprouting During Bovine Luteal Angiogenesis. J. Reprod. 2012, 143, 35–43. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, L.; Zhou, J.; Chen, H.; Zhang, L. Sequential Delivery of VEGF, FGF-2 and PDGF from the Polymeric System Enhance HUVECs Angiogenesis In Vitro and CAM Angiogenesis. Cell Immunol. 2018, 323, 19–32. [Google Scholar] [CrossRef]
- Tian, L.; Luo, N.; Zhu, X.; Chung, B.H.; Garvey, W.T.; Fu, Y. Adiponectin-AdipoR1/2-APPL1 Signaling Axis Suppresses Human Foam Cell Formation: Differential Ability of AdipoR1 and AdipoR2 to Regulate Inflammatory Cytokine Responses. Atherosclerosis 2012, 221, 66–75. [Google Scholar] [CrossRef]
- McAinch, A.J.; Steinberg, G.R.; Mollica, J.; O’Brian, P.E.; Dixon, J.B.; Macaulay, S.L.; Kemp, B.E.; Cameron-Smith, D. Differential Regulation of Adiponectin Receptor Gene Expression by Adiponectin and Leptin in Myotubes Derived from Obese and Diabetic Individuals. Obesity 2006, 14, 1898–1904. [Google Scholar] [CrossRef]
- Mistry, T.; Digby, J.E.; Chen, J.; Desai, K.M.; Randeva, H.S. The Regulation of Adiponectin Receptors in Human Prostate Cancer Cell Lines. Biochem. Biophys. Res. Comm. 2006, 348, 832–838. [Google Scholar] [CrossRef]
- Chen, H.; Gao, X.; He, C. Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1044. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lau, W.B.; Yuan, Y.; Booth, D.; Li, J.J.; Scalia, R.; Preston, K.; Gao, E.; Koch, W.; et al. Adiponectin Inhibits Tumor Necrosis Factor-α-Induced Vascular Inflammatory Response Via Caveolin-Mediated Ceramidase Recruitment and Activation. Circ. Res. 2014, 114, 792–805. [Google Scholar] [CrossRef]
- Nigro, E.; Orlandella, F.M.; Polito, R.; Mariniello, R.M.; Monaco, M.L.; Mallardo, M.; De Stefano, A.E.; Iervolino, P.L.C.; Salvatore, G.; Daniele, A. Adiponectin and Leptin Exert Antagonizing Effects on Proliferation and Motility of Papillary Thyroid Cancer Cell Lines. J. Physiol. Biochem. 2021, 77, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Olorunseun, O.O.; Beales, I.L.P. Globular Adiponectin, Acting Via Adiponectin Receptor-1, Inhibits Leptin-Stimulated Oesophageal Adenocarcinoma Cell Proliferation. Mol. Cell Endocrinol. 2008, 285, 43–50. [Google Scholar] [CrossRef]
- Handy, J.A.; Fu, P.P.; Kumar, P.; Mells, J.E.; Sharma, S.; Saxena, N.K.; Anania, F.A. Adiponectin Inhibits Leptin Signalling Via Multiple Mechanisms to Exert Protective Effects Against Hepatic Fibrosis. Biochem. J. 2011, 440, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Wang, J.; Fu, P.P.; Sharma, S.; Nagalingam, A.; Mells, J.; Handy, J.; Page, A.J.; Cohen, C.; Anania, R.A.; et al. Adiponectin Antagonizes the Oncogenic Actions of Leptin in Hepatocellular Carcinogenesis. Hepatology 2010, 52, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Jardé, T.; Caldefie-Chézet, F.; Goncalves-Mendes, N.; Mishellany, F.; Buechler, C.; Penault-Llorca, F.; Vasson, M.P. Involvement of Adiponectin and Leptin in Breast Cancer: Clinical and In Vitro Studies. Endocr. Relat. Cancer 2009, 16, 1197–1210. Available online: https://erc.bioscientifica.com/view/journals/erc/16/4/1197.xml (accessed on 16 December 2021). [CrossRef]
- Tao, T.; Wang, Y.; Xu, B.; Mao, X.; Sun, Y.; Liu, W. Role of Adiponectin/Peroxisome Proliferator-Activated Receptor Alpha Signaling in Human Chorionic Gonadotropin-Induced Estradiol Synthesis in Human Luteinized Granulosa Cells. Mol. Cell. Endocrinol. 2019, 493, 110450. [Google Scholar] [CrossRef]

| Gene | Forward 5′→3′ | Reverse 5′→3′ | Anneal | Accession No. | Ref. |
|---|---|---|---|---|---|
| AdipoR2 | CCATTCTCTGCCTTTCTTTTTCG | CTGCTCTTACTCCCCGATACTGA | 58 °C | AY452711 | [32] |
| Ang1 | GAGCAGCCTGATCTTACATG | GCATTCTCTGTAGTCAAGCC | 53 °C | NM213959 | [7] |
| FGF2 | TCAAAGGAGTGTGTGCGAAC | CAGGGCCACATACCAACTG | 55 °C | AJ577089 | [33] |
| Leptin | ACAGAGGGTCACCGGTTTGG | TAGAGGGAGGCTTCCAGGAC | 61 °C | AF0226976 | [34] |
| Cyclophilin | TGCCATCCAACCACTCAG | TAACCCCACCGTCTTCTT | 52 °C | AF14571 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, R.; Ramirez, M.; Ayala, L.; Benavides, E.A.; Xie, F.; Arellano, A.A.; Stanko, R.L.; Garcia, M.R. Adiponectin Influences FGF2 in the Developing Porcine Corpus Luteum. Vet. Sci. 2022, 9, 77. https://doi.org/10.3390/vetsci9020077
Flores R, Ramirez M, Ayala L, Benavides EA, Xie F, Arellano AA, Stanko RL, Garcia MR. Adiponectin Influences FGF2 in the Developing Porcine Corpus Luteum. Veterinary Sciences. 2022; 9(2):77. https://doi.org/10.3390/vetsci9020077
Chicago/Turabian StyleFlores, Rita, Martha Ramirez, Luis Ayala, Elizabeth A. Benavides, Fang Xie, Adrian Aaron Arellano, Randy Louis Stanko, and Michelle Renee Garcia. 2022. "Adiponectin Influences FGF2 in the Developing Porcine Corpus Luteum" Veterinary Sciences 9, no. 2: 77. https://doi.org/10.3390/vetsci9020077
APA StyleFlores, R., Ramirez, M., Ayala, L., Benavides, E. A., Xie, F., Arellano, A. A., Stanko, R. L., & Garcia, M. R. (2022). Adiponectin Influences FGF2 in the Developing Porcine Corpus Luteum. Veterinary Sciences, 9(2), 77. https://doi.org/10.3390/vetsci9020077






