Simple Summary
Chorioptes mites are particularly common in goats, with infestations usually subclinical and often asymptomatic. In the present case, many animals had a severe clinical presentation, possibly due to the associated dermatophyte infection (Trichophyton verrucosum), since a concurrent or underlying disease may exacerbate clinical response. Goats were treated topically with pour-on Eprinomectin (1 mg/kg), while an enilconazole solution was used for environmental disinfection against dermatophyte spores.
Abstract
A concurrent chorioptic mange and dermatophytosis outbreak occurred in a goat flock in northwestern Italy. Sanitation of the flock was obtained following pour-on eprinomectin application at a dose of 1 mg/kg; enilconazole was used for environmental disinfection against dermatophyte spores.
1. Introduction
The surface-living mite, Chorioptes bovis, causes chorioptic mange; the infestation affects livestock species such as cattle, horses, sheep, and alpacas and is also a common problem in goat farming worldwide [1,2,3,4,5,6]. The mites are found principally on the feet of animals, where they cause crust formation (the so-called “foot mange”) [4]. Other clinical presentations are possible, such as small crusts hidden under the hair coat. As the mite feeds on skin debris, there is rarely severe skin damage, resulting in subclinical infection, especially in goats. Chorioptes infection is often observed on farms with poor management and nutrition [4].
Dermatophytosis, or “ringworm”, is a fungal skin infection representing a significant problem in veterinary medicine with substantial consequences on public health [7]. Indeed, all animal-associated dermatophytes can infect humans [8,9,10]. Dermatophytes also include anthropophilic species, strictly human pathogens, and geophilic species. These latter reside in the soil, where they degrade keratinous materials spread in the environment by mammals and birds [7].
Each zoophilic dermatophyte recognises a few animal species as primary hosts. The most important species are Microsporum canis, associated with cats, Trichophyton erinacei with hedgehogs, Trichophyton mentagrophytes with rabbits, and Trichophyton benhamiae with guinea pigs [7,11,12]. Trichophyton verrucosum is largely diffused as the causative agent of cattle ringworm [7,13,14], with enzootic situations commonly occurring in herds worldwide [8,15,16,17]. Infection occurs mainly through direct transmission from animal to animal; therefore, a high prevalence can be reached in overcrowded stables where the fungus can spread quickly among subjects confined to small areas. The environmental fungal propagules also represent a significant infection source [18].
Ringworm in cattle may negatively impact milk and meat production and lead to impairments in the hide industries, as lesion scars can persist on leather following tawing and tanning [7]. Moreover, T. verrucosum has a high zoonotic potential [8,15,19,20]. Farmers, their families, veterinaries and technicians involved in animal management are at higher risk of infection. Lesions are usually highly inflammatory due to an allergic reaction elicited by the fungus in the stratum corneum. This disease is commonly called “barn itch” [20].
Dermatophytosis is considered a more sporadic disease in goats, with fewer cases reported in the literature. T. verrucosum and T. mentagrophytes are the most frequently reported species [14,21,22,23,24,25,26].
This article describes an episode of an unusually concurrent infection in dairy goats caused by T.verrucosum and C. bovis.
2. Materials and Methods
2.1. History and Signalment
The outbreak involved a farm of 152 dairy goats of the Alpine (n = 83), and Saanen (n = 79) breeds in North-Western Italy. The farmer reported that skin lesions, mainly alopecia and crusts, had been present in many Alpine goats for about two months, while Saanen goats did not seem affected. The animals were housed without breed partition, and the management was the same for both breeds (Figure 1).

Figure 1.
Some of the goats housed on the farm where dermatological problems were referred.
None of the animals exhibited itching or other symptoms; appetite and milk production, recorded by automated milk sensors, were not affected. The vaccination and deworming status of the animals (fenbendazole administration in the dry period) were up to date.
2.2. Clinical Examination and Samplings
The individual clinical examination was performed on all animals. Samples, including skin scrapings, scab materials and hair samples, were taken from 10 animals with skin lesions (all Alpine) and 10 randomly selected asymptomatic animals (all Saanen).
2.3. Laboratory Procedures
Samples were digested in 10% sodium hydroxide (NaOH) for three hours at room temperature and centrifuged at 3000 rpm for 10 min. After the supernatant was removed, the remaining material was transferred to slides and observed for mites and fungal elements.
Fungal cultures were performed on a commercial medium for dermatophyte isolation (Mycobios Selective Agar, Biolife, Milan, Italy) and Sabouraud Dextrose agar enriched with thiamine and inositol. The latter medium was employed to facilitate the growth of T. verrucosum. For the same reason, the plates were incubated at 37 °C [27]. The culture media included antibiotics and cycloheximide to limit the growth of contaminant organisms. The fungal identification was obtained by analysing the colonies’ gross and microscopic morphology compared with standard descriptions [28].
3. Results
The clinical examination revealed 45 symptomatic animals belonging to the Alpine breed (54.2%). Lesions included multifocal alopecia on the chest and croup, crusts, thickening, and wrinkling on legs, axillae, inguinal and perineal region (Figure 2).
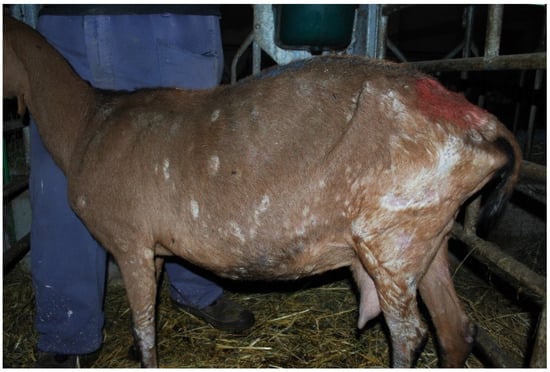
Figure 2.
Extensive exfoliative and crusty dermatitis in a goat infected by C. bovis and T. verrucosum.
None of the Saanen goats exhibited symptoms.
All ten skin samples from symptomatic animals resulted positive for C. bovis.
Moreover, in eight of these samples, hairs were invaded by fungal structures with morphology suggestive of T. verrucosum (hyphae and chains of large ectothrix arthroconidia) (Figure 3 and Figure 4).
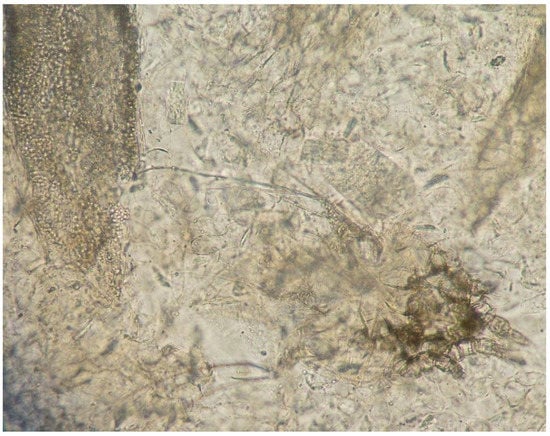
Figure 3.
Direct examination of a skin scraping: hair invaded by fungal structures (arthroconidia) (left) and C. bovis mite (right) (10× magnification).
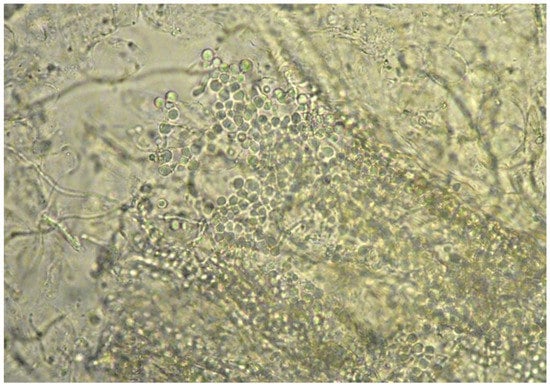
Figure 4.
Direct examination of a skin scraping: hair invaded by fungal structures (arthroconìdia) (40× magnification).
Cultures yielded flat, white/cream colour, with glabrous texture colonies. The growth was slow, with colonies evident only after prolonged incubation (around two weeks). The isolation was difficult due to a large number of contaminant mould colonies. Irregular hyphae were observed to have terminal and intercalary chlamydospores. The chlamydospores were arranged in characteristic long chains. Based on the results of direct examination and cultures, the fungus was identified as T. verrucosum.
No mites or dermatophytes were detected in asymptomatic goats.
All goats were treated topically with pour-on Eprinomectin (EPM) (Eprinex® Multi Pour-On- Boehringer Ingelheim Animal Health) at 1 mg/kg. Enilconazole solution (Clinafarm Spray®, Ely Lilli Italia S.p.A.) was used for environmental disinfection against dermatophyte conìdia.
The response to treatment was evaluated clinically, and skin scraping and fungal culture were performed weekly for up to 2 consecutive negative scrapings.
After two months, all skin samples gave negative results, and all the goats were fully clinically recovered.
4. Discussion
Chorioptes mites are particularly common in goats [2,3], with infestations usually subclinical [4] and often asymptomatic [29]. In the present case, many animals had a severe clinical presentation, possibly due to the associated dermatophyte infection, since clinical response may be exacerbated by a concurrent or underlying disease [5,30]. Lesions on the animals (alopecia with scaling and crusts) were similar to those described elsewhere in goats with dermatophytosis [14,23].
All positive goats were of the Alpine breed; this may be related to the coat’s colour (white in the Saanen breed; brown in the Alpine breed). Indeed, there is evidence that hair colour influences resistance to ectoparasites. Darker individuals appear more attractive to flies [31,32,33], ticks [3,33,34,35] and mites [36] than their paler counterparts.
Concerning ringworm in livestock, cattle are generally considered the more at risk of problems [7], especially in intensive and semi-intensive farming systems. In these situations, infection rates often reach very high values due to a combination of factors, such as the presence of young animals without acquired immunity, overcrowding of animals, and increased environmental humidity and temperature [8,16]. The most frequent dermatophyte species reported in the literature infecting bovines is T. verrucosum [7].
Few studies on goat ringworm are available. The prevalence and importance of this fungal infection in goat farming appear extremely variable from study to study. A retrospective study covering 20 years of medical records at the School of Veterinary Medicine, University of California, Davis, revealed a very low prevalence of animals with dermatophytosis out of a population of goats with skin problems. The only species isolated was T. mentagrophytes [23]. Similarly, in a Pakistan study, only 0.2% of goats proved to be infected (by T. verrucosum) [14]. Moreover, the infection frequency was significantly lower than in cattle [14], reinforcing the idea that cattle are the main reservoir of T. verrucosum. In a study performed in Nigeria, one goat out of 13 examined was positive for a dermatophyte species [24]. The fungus isolated was Trichophyton schoenleinii, an anthropophilic dermatophyte. The explanation provided by the authors for this finding was that humans and sampled animals shared the same environment. Therefore the goat may have acquired the infection following contact with spores derived from infected humans or a contaminated environment. Another study reporting a low prevalence of infection regards goats in India. Two animals out of 28 were positive for T. mentagrophytes and Microsporum gypseum (now called Nannizia gypsea, a geophilic species), respectively [25]. Curiously, two other surveys performed in the same countries mentioned above (Nigeria and India) reported a higher prevalence of infected animals. In Nigeria, Emenuga and Oyeka [26] found 11 (13.8%) and 16 (20%) goats out of 80 sampled positive for T. verrucosum and T. mentagrophytes, respectively. In India, Begum and Kumar [22] reported around half of the goats (52.2%) were positive for various dermatophyte species. T. mentagrophytes and T. verrucosum accounted for the majority of cases.
Despite the zoonotic potential of T. verrucosum, human involvement apparently did not occur in the present case. It is not easy to find an explanation for this lack of contagion since it is known that the infection can easily spread from animals to humans [8,15,19]. Humans contract T. verrucosum by contact with infected animals, contaminated fomites, or soil. The disease often follows local trauma, employed by the fungus as an entry portal [20]. Generally, the condition is highly evident since it presents as a well-circumscribed erythematous and pruriginous plaque covered with vesicles and papules. Clinical findings also include constitutional symptoms, especially fever and regional lymphadenopathy [20].
Because dermatophytosis is infectious and contagious, rapid confirmation of the disease is necessary to limit its spread. Different diagnostic tests can be used for this purpose.
Dermoscopy is a noninvasive point-of-care diagnostic tool that allows for illuminated skin magnification. It is widely used in human medicine to diagnose causes of hair and follicular abnormalities, including dermatophytosis [37]. Concerning veterinary medicine, this tool’s use is limited to canine and feline dermatology [38].
Direct examination of skin samples is instead routinely employed [7,13]. It is a quick and inexpensive test which allows putting in evidence hyphae and conìdia invading hair and scales. When the sample includes abundant and thick material rich in keratin (scales, crusts), as in the present case, it is necessary to digest it in NaOH or potassium hydroxide (KOH) to allow the visualisation of the fungal elements [14]. This method could rapidly detect the organisms (Chorioptes and Trichophyton) responsible for the dermatological problems affecting our goats. Though the definitive fungal identification was achieved later by culture, the morphology of the fungal elements on direct microscopy was already highly suggestive of T. verrucosum. The large (about 10 µm size) arthroconìdia, in chains or groups, were pretty typical of T. verrucosum and allowed differentiation of this fungus from other dermatophytes affecting livestock, e.g., T. mentagrophytes.
Diagnosis based on direct examination has a very high sensitivity concerning dermatophytosis by T. verrucosum in animals [8]. Indeed, infected crusts and scales generally contain infected hair that can be easily identified, provided the sample is adequately cleared in a NaOH or KOH solution.
Culture is a useful diagnostic test since it allows a definitive identification through morphological and molecular analyses [7,13]. In our case, the cultures took several days to be positive since T. verrucosum grows very slowly, even on enriched culture media [14]. The poor growth of T. verrucosum often represents a severe problem for its isolation and identification, mainly due to the rapid development of a variety of non-pathogenic moulds that contaminate the hair of animals [7].
More advanced tests for dermatophytosis diagnosis are based on detecting fungal DNA from hair and scales through various PCR techniques [39]. Though not yet primarily employed in routine practice, these methods have also been proven valid for diagnosing animal infections, including cattle ringworm due to T. verrucosum [40].
Concerning the treatment of goats, we preferred to encompass the asymptomatic ones since we could not completely rule out the possibility of animals with subclinical lesions. Leaving untreated animals could have led to a disease spread, nullifying the efficacy of the EPM treatment. Moreover, EPM administration (with endectocide activity and with zero milk-withdrawal period) substituted the antihelmintic treatment (fenbendazole) routinely applied in the dry period.
Author Contributions
Methodology, A.R.M.M. and M.P.; writing—original draft preparation, L.R. and A.P.; writing—review and editing, L.R., A.P. and P.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colebrook, E.; Wall, R. Ectoparasites of livestock in Europe and the Mediterranean region. Veter. Parasitol. 2004, 120, 251–274. [Google Scholar] [CrossRef]
- Cornall, K.; Wall, R. Ectoparasites of goats in the UK. Veter. Parasitol. 2015, 207, 176–179. [Google Scholar] [CrossRef]
- Lusat, J.; Morgan, E.; Wall, R. Mange in alpacas, llamas and goats in the UK: Incidence and risk. Veter. Parasitol. 2009, 163, 179–184. [Google Scholar] [CrossRef]
- Cremers, H.J.W.M. The incidence ofChorioptes bovis(Acarina: Psoroptidae) on the feet of horses, sheep, and goats in the Netherlands. Veter. Q. 1985, 7, 283–289. [Google Scholar] [CrossRef]
- Foster, A.; Mitchell, S.; Wall, R. Cattle ectoparasites in Great Britain. Cattle Pract. 2015, 23, 280–287. [Google Scholar]
- Castilla-Castaño, E.; Herman, N.; Martinelli, E.; Lecru, L.A.; Pressanti, C.; Schelcher, F.; Cadiergues, M.C. Treatment of sarcoptic and chorioptic mange in an alpaca (Vicugna pacos) herd with a combination of topical amitraz and subcutaneous ivermectin. New Zealand Veter. J. 2020, 69, 121–126. [Google Scholar] [CrossRef]
- Chermette, R.; Ferreiro, L.; Guillot, J. Dermatophytoses in Animals. Mycopathologia 2008, 166, 385–405. [Google Scholar] [CrossRef]
- Agnetti, F.; Righi, C.; Scoccia, E.; Felici, A.; Crotti, S.; Moretta, I.; Moretti, A.; Maresca, C.; Troiani, L.; Papini, M. Trichophyton verrucosum infection in cattle farms of Umbria (Central Italy) and transmission to humans. Mycoses 2014, 57, 400–405. [Google Scholar] [CrossRef]
- Pasquetti, M.; Min, A.R.M.; Scacchetti, S.; Dogliero, A.; Peano, A. Infection by Microsporum canis in Paediatric Patients: A Veterinary Perspective. Veter. Sci. 2017, 4, 46. [Google Scholar] [CrossRef]
- Veraldi, S.; Genovese, G.; Peano, A. Tinea corporis caused by Trichophyton equinum in a rider and review of the literature. Infection 2017, 46, 135–137. [Google Scholar] [CrossRef]
- Čmoková, A.; Kolařík, M.; Dobiáš, R.; Hoyer, L.L.; Janouškovcová, H.; Kano, R.; Kuklová, I.; Lysková, P.; Machová, L.; Maier, T.; et al. Resolving the taxonomy of emerging zoonotic pathogens in the Trichophyton benhamiae complex. Fungal Divers. 2020, 104, 333–387. [Google Scholar] [CrossRef]
- Peano, A.; Hubka, V.; Cavana, P.; Ottino, C.; Blandolino, M.; Molinar Min, A.R.; Pasquetti, M. Cases of dermatophytosis caused by Trichophyton benhamiae var. luteum and T. europaeum, newly described dermatophytes within the T. benhamiae complex. Vet. Dermatol. 2022, 33, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Bond, R. Superficial veterinary mycoses. Clin. Dermatol. 2010, 28, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hameed, K.; Ch, F.R.; Nawaz, M.A.; Naqvi, S.M.S.; Gräser, Y.; Kupsch, C.; Pasquetti, M.; Rossi, L.; Min, A.R.M.; Tizzani, P.; et al. Trichophyton verrucosum infection in livestock in the Chitral district of Pakistan. J. Infect. Dev. Ctries. 2017, 11, 326–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ming, P.X.; Ti, Y.L.X.; Bulmer, G.S. Outbreak of Trichophyton verrucosum in China Transmitted from Cows to Humans. Mycopathologia 2006, 161, 225–228. [Google Scholar] [CrossRef]
- Papini, R.; Nardoni, S.; Fanelli, A.; Mancianti, F. High Infection Rate of Trichophyton verrucosum in Calves from Central Italy. Zoonoses Public Health 2009, 56, 59–64. [Google Scholar] [CrossRef]
- Wabacha, J.K.; Gitau, G.K.; Bebora, L.C.; Bwanga, C.O.; Wamuri, Z.M.; Mbithi, P.M. Occurrence of dermatomycosis (ringworm) due to Trichophyton verrucosum in dairy calves and its spread to animal attendants. J. S. Afr. Vet. Assoc. 1998, 69, 172–173. [Google Scholar] [CrossRef][Green Version]
- Gnat, S.; Łagowski, D.; Dyląg, M.; Osińska, M.; Nowakiewicz, A. Airborne dermatophyte propagules concentration in cowsheds as an underestimated reservoir of potential zoonoses. J. Appl. Microbiol. 2022, 133, 544–554. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Song, Y.; Wang, A. Generalized tinea corporis caused by Trichophyton verrucosum misdiagnosed as allergic dermatitis and erythema annulare in an adult. Int. J. Infect. Dis. 2021, 113, 339–340. [Google Scholar] [CrossRef]
- Silver, S.; Vinh, D.C.; Embil, J.M. The man who got too close to his cows. Diagn. Microbiol. Infect. Dis. 2008, 60, 419–420. [Google Scholar] [CrossRef]
- Shokri, H.; Khosravi, A. An epidemiological study of animals dermatomycoses in Iran. J. De Mycol. Médicale 2016, 26, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Kumar, R. Prevalence of dermatophytosis in animals and antifungal susceptibility testing of isolated Trichophyton and Microsporum species. Trop. Anim. Health Prod. 2020, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- White, S.D.; Affolter, V.K.; Molinaro, A.M.; Depenbrock, S.M.; Chigerwe, M.; Heller, M.C.; Rowe, J.D. Skin disease in goats (Capra aegagrus hircus): A retrospective study of 358 cases at a university veterinary teaching hospital (1988–2020). Veter. Dermatol. 2021, 33, 227. [Google Scholar] [CrossRef] [PubMed]
- Chah, K.F.; Majiagbe, K.A.; Kazeem, H.M.; Ezeanyika, O.; Agbo, I.C. Dermatophytes from skin lesions of domestic animals in Nsukka, Enugu State, Nigeria. Veter. Dermatol. 2012, 23, 522-e104. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Sikdar, A.; Das, P. Dermatophytes isolated from selected ruminants in India. Mycopathologia 1998, 142, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Emenuga, V.N.; Oyeka, C.A. Epidemiology, Health Effects and Treatment of Cutaneous Mycoses of Goat and Sheep from Some Eastern States of Nigeria. Am. J. Infect. Dis. Microbiol. 2013, 1, 106–110. [Google Scholar] [CrossRef]
- Rebell and Taplin. Dermatophytes: Their Recognition and Identification, 2nd ed.; Miami Press Publisher: Coral Gables, FL, USA, 1970. [Google Scholar]
- De Hoog, G.S.; Guarro, J.; Gene, J.F.M. Atlas of Clinical Fungi, 2nd ed.; Virgili, U.R.I., Reus, S., Eds.; Schimmel, Centralbureau Voorcultures: Utrecht, The Netherlands, 2000. [Google Scholar]
- Heath, A.; Bishop, D.M.; Tenquist, J. The prevalence and pathogenicity of Chorioptes bovis (Hering, 1845) and Psoroptes cuniculi (Delafond, 1859) (Acari: Psoroptidae) infestations in feral goats in New Zealand. Veter. Parasitol. 1983, 13, 159–169. [Google Scholar] [CrossRef]
- Alogninouwa, T.; Parent, R. Traitement par l’ivermectine d’une gale mixte (Sarcoptes scabiei et Chorioptes caprae) chez la chèvre au Senegal. Observations cliniques. Bull. Mens. Soc. Vet. Prat. Fr. 1986, 70, 399–403. [Google Scholar]
- Steelman, C.D.; Brown, M.A.; Gbur, E.E.; Tolley, G. The effects of hair density of beef cattle on Haematobia irritans horn fly populations. Med Veter. Èntomol. 1997, 11, 257–264. [Google Scholar] [CrossRef]
- Steelman, C.D.; Brown, A.H.; Gbur, E.E.; Tolley, G. Interactive Response of the Horn Fly (Diptera: Muscidae) and Selected Breeds of Beef Cattle. J. Econ. Èntomol. 1991, 84, 1275–1282. [Google Scholar] [CrossRef]
- Herrera, M.L.; Arguedas, B.E. Comparison of two racial groups of cattle in terms of incidence of ticks (acari: Ixodidae) and torsos (diptera: Ooestridae). Nutr. Anim. Trop. 2014, 8, 1–9. [Google Scholar]
- Perito Cardoso, C.; Da Silva, B.F.; Gonçalves, D.S.; Tagliari, N.J.; Saito, M.E.; Amarane, A.F.T. Resistance against ectoparasites in Crioulo Lageano and crossbred Angus cattle in southern Brazil under natural conditions. Pesqui. Vet. Bras. 2014, 34, 141–146. [Google Scholar]
- Da Silva, A.M.; Alencar, M.; Regitano, L.; Oliveira, M.; Júnior, W.B. Artificial infestation of Boophilus microplus in beef cattle heifers of four genetic groups. Genet. Mol. Biol. 2007, 30, 1150–1155. [Google Scholar] [CrossRef]
- Bemji, M.N.; Ogundiyi, A.I.; Adebambo, O.A.; Dipeolu, M.A.; Onagbesan, O.M.; James, I.J.; Osinowo, O. Prevalence of coat colour phenotypes and its influence on mange infestation of West African Dwarf goat. Bull. Anim. Health Prod. Africa. 2012, 60, 427. [Google Scholar]
- Durdu, M.; Errichetti, E.; Eskiocak, A.H.; Ilkit, M. High accuracy of recognition of common forms of folliculitis by dermoscopy: An observational study. J. Am. Acad. Dermatol. 2019, 81, 463–471. [Google Scholar] [CrossRef]
- Scarampella, F.; Zanna, G.; Peano, A. Dermoscopic features in canine dermatophytosis: Some preliminary observations. Veter. Dermatol. 2016, 28, 255–256. [Google Scholar] [CrossRef]
- Bin Lin, B.; Pattle, N.; Kelley, P.; Jaksic, A.S. Multiplex RT-PCR provides improved diagnosis of skin and nail dermatophyte infections compared to microscopy and culture: A laboratory study and review of the literature. Diagn. Microbiol. Infect. Dis. 2021, 101, 115413. [Google Scholar] [CrossRef]
- Łagowski, D.; Gnat, S.; Nowakiewicz, A.; Trościańczyk, A. Real-Time PCR as an Alternative Technique for Detection of Dermatophytes in Cattle Herds. Animals 2021, 11, 1662. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).