Application of ORF3 Subunit Vaccine for Avian Hepatitis E Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Chicks
2.2. Viruses and Plasmids
2.3. Primer Designs and Synthesis
2.4. Amplification of ORF3 Genes and Plasmids Construction
2.5. Protein-Induced Expression and Western Blot Identification
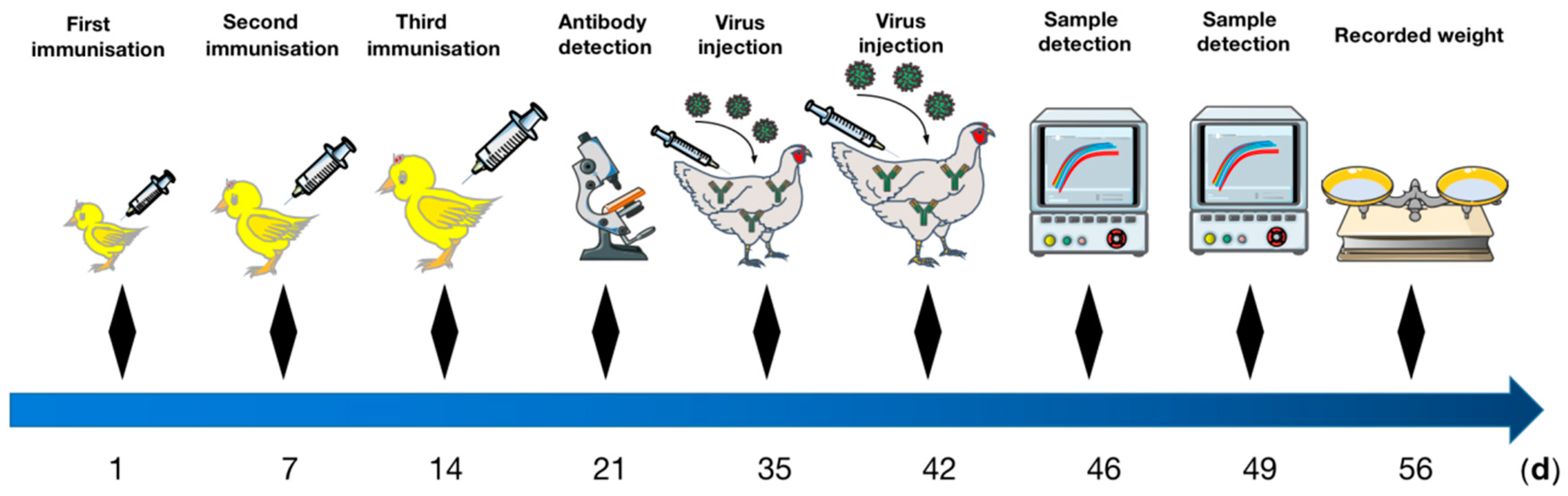
2.6. Vaccine Immunization
2.7. IFA Detections of HEV Antibodies
2.8. Artificial Challenge Test
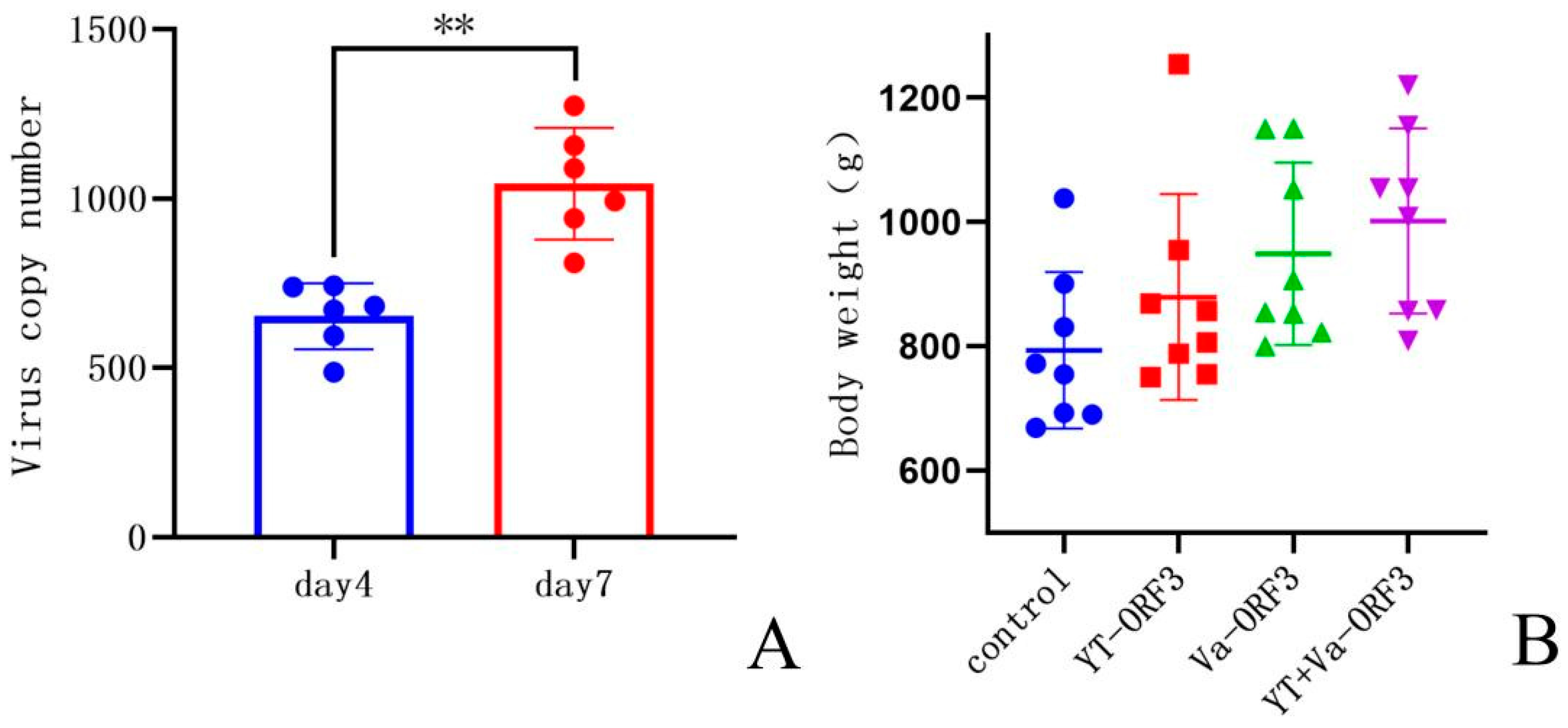
3. Results
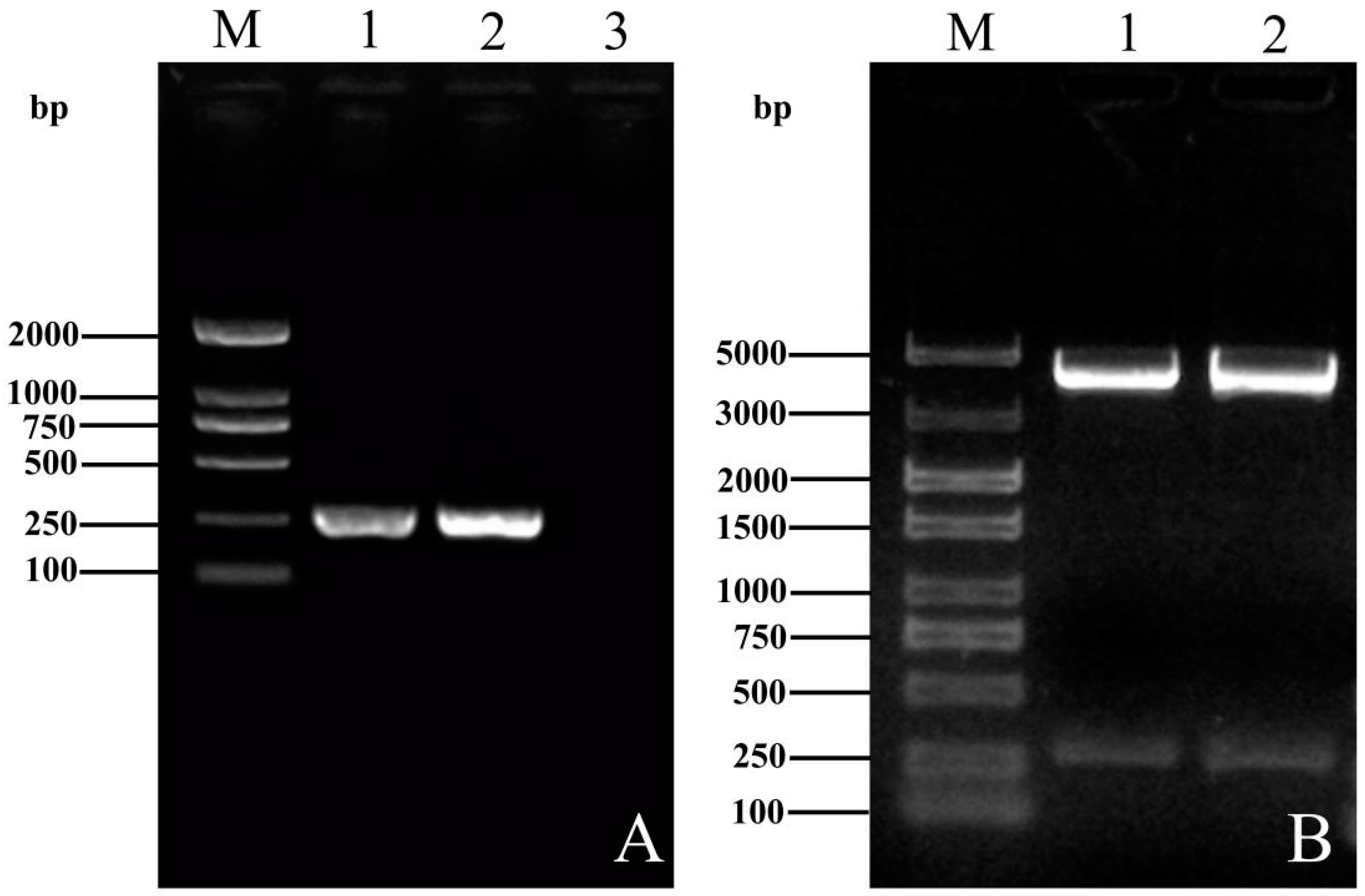
3.1. Construction and Homology Comparison of the ORF3 Recombinant Plasmids of YT-aHEV and VaHEV Strains
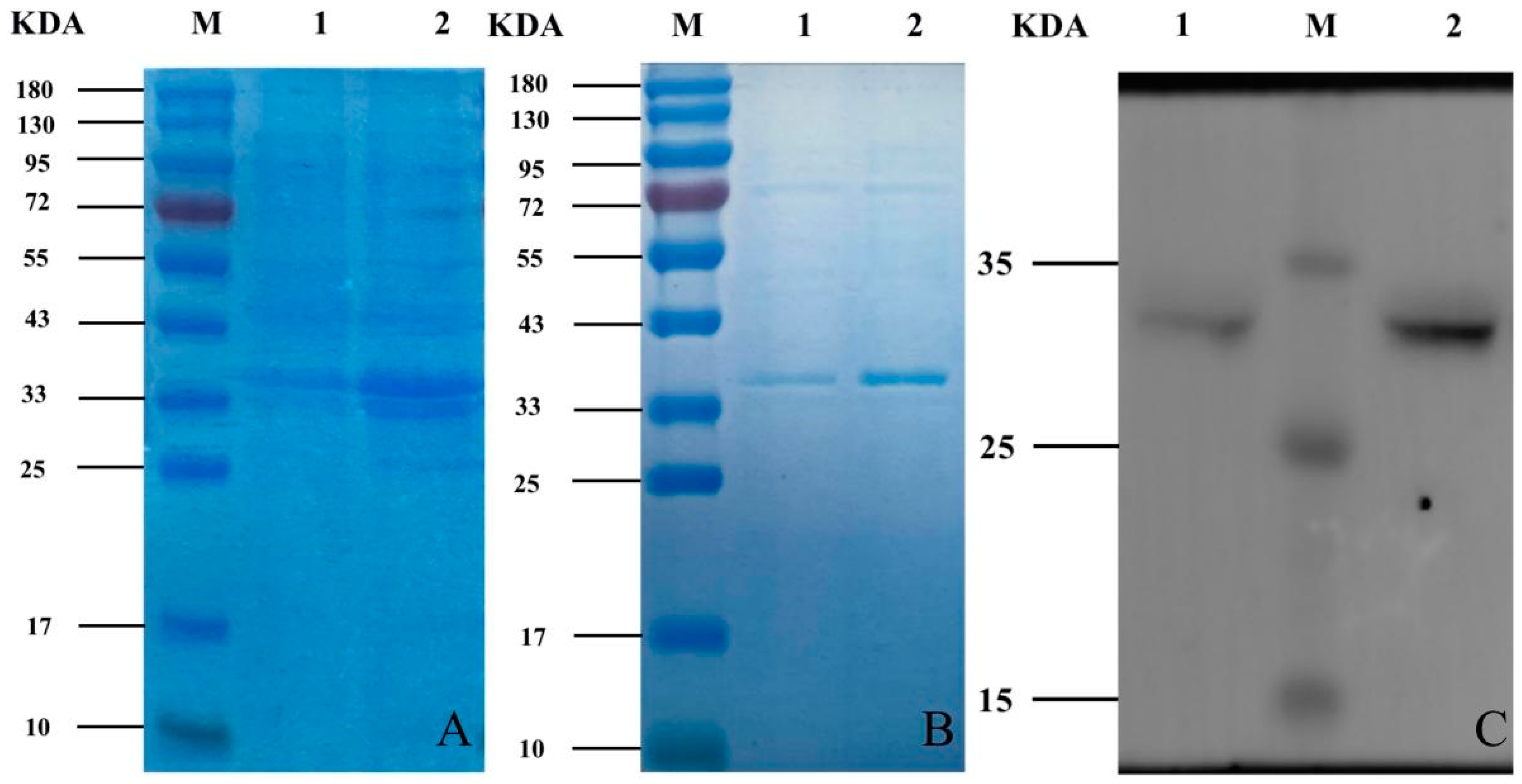
3.2. Induced Expression, Protein Purification and Western Blot Assay of Recombinant Bacteria
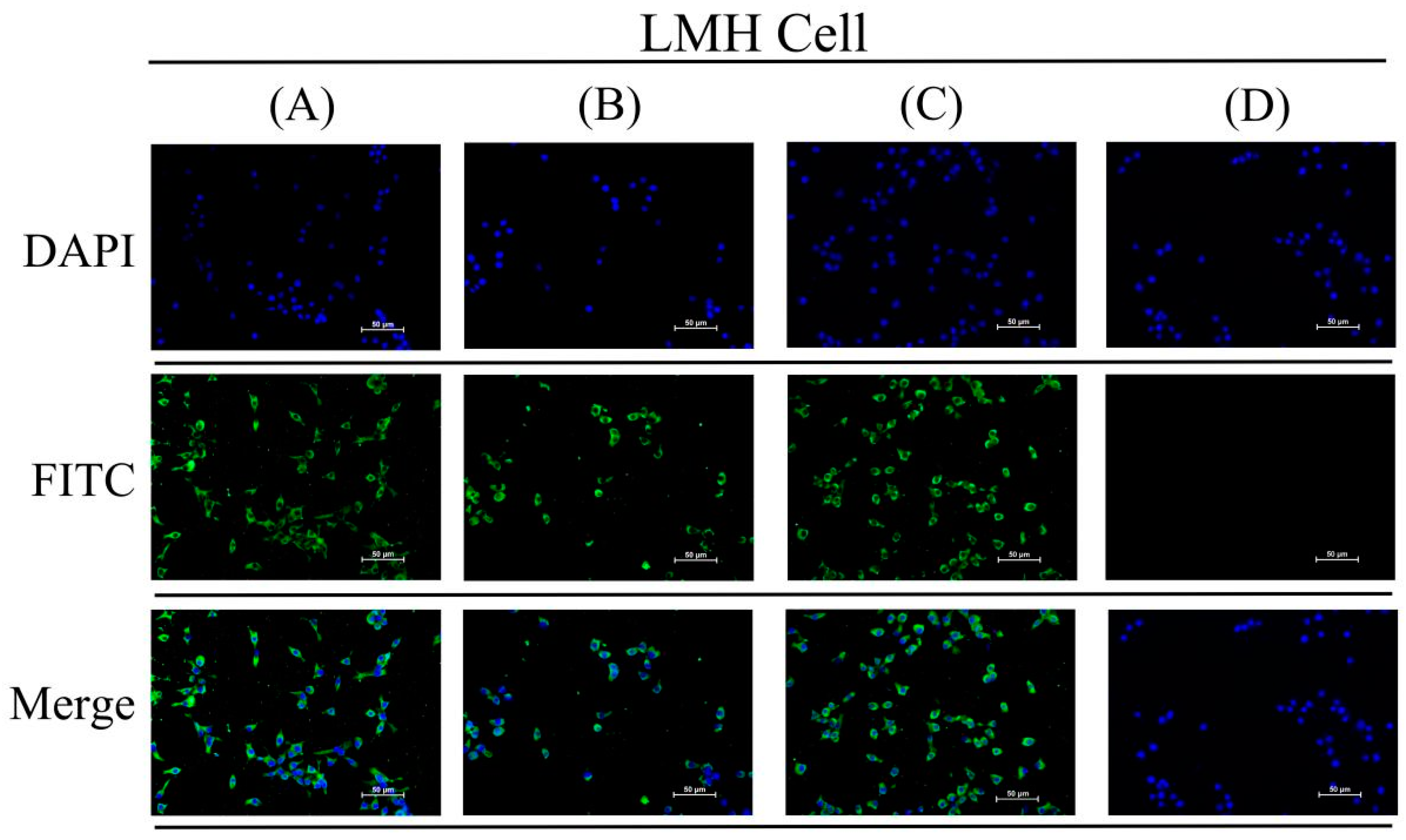
3.3. HEV Antibodies Detection by IFA
3.4. Results of the Challenge Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Q.; Zhou, E.M.; Dong, S.W.; Qiu, H.K.; Zhang, L.; Hu, S.B.; Zhao, F.F.; Jiang, S.J.; Sun, Y.N. Analysis of avian hepatitis E virus from chickens, China. Emerg. Infect. Dis. 2010, 16, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, Y.; Zhang, Y.W.; Zhang, Z.H.; Meng, F.F.; Cui, Z.Z.; Chang, S.; Zhao, P. Characterization of the novel genotype avian hepatitis E viruses from outbreaks of hepatic rupture haemorrhage syndrome in different geographical regions of China. Transbound. Emerg. Dis. 2018, 65, 2017–2026. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Meng, F.F.; Cui, Z.Z.; Chang, S.; Zhao, P. Hepatic rupture hemorrhage syndrome in chickens caused by a novel genotype avian hepatitis E virus. Vet. Microbiol. 2018, 222, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Y.Q.; Cui, Z.Z.; Chang, S.; Zhao, P. Genetic diversity of avian hepatitis E virus in China, 2018–2019. Transbound. Emerg. Dis. 2020, 67, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, B.Y.; Sun, Y.N.; Du, T.F.; Chen, Y.Y.; Wang, X.J.; Li, H.X.; Nan, Y.C.; Zhang, G.P.; Zhou, E.M. Decreased egg production in laying hens associated with infection with genotype 3 avian hepatitis E virus strain from China. Vet. Microbiol. 2017, 203, 174–180. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Zhao, H.; Chi, Z.N.; Cui, Z.Z.; Chang, S.; Wang, Y.X.; Zhao, P. Isolation, identification and genome analysis of an avian hepatitis E virus from white-feathered broilers in China. Poult. Sci. 2022, 101, 101633–101641. [Google Scholar] [CrossRef]
- Liu, B.Y.; Fan, M.N.; Zhang, B.B.; Chen, Y.Y.; Sun, Y.N.; Du, T.F.; Nan, Y.C.; Zhou, E.M.; Zhao, Q. Avian hepatitis E virus infection of duck, goose, and rabbit in northwest China. Emerg. Microbes Infect. 2018, 7, 76. [Google Scholar] [CrossRef]
- Li, H.Q.; Zhang, F.F.; Tan, M.F.; Zeng, Y.B.; Yang, Q.; Tan, J.; Huang, J.N.; Huang, Y.; Kang, Z.F. A putative novel subtype of the avian hepatitis E virus of genotype 3, Jiangxi province, China. Poult. Sci. 2020, 99, 6657–6663. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Ma, C.L.; Ma, D.X.; Zhao, Q.; Wang, F.; Huang, Y.C.; Li, J.; Zhang, L.L.; Zhou, E.M. Live recombinant Lactococcuslactis expressing avian hepatitis virus ORF2 protein: Immunoprotection against homologous virus challenge in chickens. Vaccine 2018, 36, 1108–1115. [Google Scholar] [CrossRef]
- Kabrane-Lazizi, Y.; Meng, X.J.; Purcell, R.H.; Emerson, S.U. Evidence that the genomic RNA of hepatitis E virus is capped. J. Virol. 1999, 73, 8848–8850. [Google Scholar] [CrossRef]
- Jiang, F.L. Immunoprotective Analysis of the ORF3 Protein of Avian Hepatitis E Virus. Master’s Thesis, North West Agriculture and Forestry University, Shanxi, China, 2014. [Google Scholar]
- Syed, S.F.; Sun, Y.N.; Du, T.F.; Chen, Y.Y.; Liu, B.Y.; Wang, X.J.; Li, H.X.; Nan, Y.C.; Zhou, E.M.; Zhao, Q. Evaluation of recombinant Chinese avian hepatitis E virus (CaHEV) ORF2 and ORF3 proteins for protection of chickens against CaHEV infection. Vaccine 2017, 35, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q. Development and Application of an RT-PCR Coupled with Nucleic Acid Spot Hybridization Method for the Detection of Avian Hepatitis E Virus. Master’s Thesis, Shandong Agricultural University, Shandong, China, 2020. [Google Scholar]
- Sun, Y.N.; Lu, Q.Z.; Zhang, J.F.; Li, X.X.; Zhao, J.K.; Fan, W.Q.; Ji, P.P.; Wang, K.; Zhou, E.M.; Zhao, Q. Co-infection with avian hepatitis E virus and avian leukosis virus subgroup J as the cause of an outbreak of hepatitis and liver hemorrhagic syndromes in a brown layer chicken flock in China. Poult. Sci. 2020, 99, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Osamudiamen, F.T.; Akanbi, O.A.; Zander, S.; Oluwayelu, D.O.; Bock, C.T.; Klink, P. Identification of a Putative Novel Genotype of Avian Hepatitis E Virus from Apparently Healthy Chickens in Southwestern Nigeria. Viruses 2021, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Rashid, U.; Idrees, M.; Afroz, A.; Kamili, S.; Purdy, M.A. A novel avian isolate of hepatitis E virus from Pakistan. Virol. J. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Razmyar, J.; Abbasi, M.; Mirsalimi, S.M.; Baghkheirati, A.A.; Ahmadian, G.; Yazdani, A. Serologic and Molecular Evidence of Widespread Infection of Avian Hepatitis E Virus in Poultry Farms of Iran. Avian Dis. 2021, 65, 572–577. [Google Scholar] [CrossRef]
- Siedlecka, M.; Kublicka, A.; Wieliczko, A.; Matczuk, A.K. Molecular detection of avian hepatitis E virus (Orthohepevirus B) in chickens, ducks, geese, and western capercaillies in Poland. PLoS ONE 2022, 17, e0269854. [Google Scholar] [CrossRef]
- Peralta, B.; Biarnés, M.; Ordóñez, G.; Porta, R.; Martín, M.; Mateu, E.; Pina, S.; Meng, X.J. Evidence of widespread infection of avian hepatitis E virus (avian HEV) in chickens from Spain. Vet. Microbiol. 2009, 137, 31–36. [Google Scholar] [CrossRef]
- Morrow, C.J.; Samu, G.; Mátrai, E.; Klausz, A.; Wood, A.M.; Richter, S.; Jaskulska, B.; Hess, M. Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathol. 2008, 37, 527–535. [Google Scholar] [CrossRef]
- Asif, K.; O’Rourke, D.; Sabir, A.J.; Shil, P.; Noormohammadi, A.H.; Marenda, M.S. Characterisation of the whole genome sequence of an avian hepatitis E virus directly from clinical specimens reveals possible recombination events between European and USA strains. Infect. Genet. Evol. 2021, 96, 105095. [Google Scholar] [CrossRef]
- Sprygin, A.V.; Nikonova, Z.B.; Zinyakov, N.G. Avian hepatitis E virus identified in Russian chicken flocks exhibits high genetic divergence based on the ORF2 capsid gene. Avian Pathol. 2012, 41, 459–463. [Google Scholar] [CrossRef]
- Meng, J.; Dai, X.; Chang, J.C.; Lopareva, E.; Pillot, J.; Fields, H.A.; Khudyakov, Y.E. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology 2001, 288, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.J.; Glamann, J.; Emerson, S.U.; Purcell, R.H. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000, 74, 5548–5555. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.F.; Zhao, Q.; Lu, M.Z.; Sun, P.M.; Qiu, H.K.; Zhang, L.; Lv, J.H.; Zhou, E.M. Analysis of epitopes in the capsid protein of avian hepatitis E virus by using monoclonal antibodies. J. Virol. Methods 2011, 171, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, Y.N.; Hu, S.B.; Wang, X.J.; Xiao, Y.H.; Hsu, W.H.; Xiao, S.Q.; Wang, C.B.; Mu, Y.; Hiscox, J.A.; et al. Characterization of antigenic domains and epitopes in the ORF3 protein of a Chinese isolate of avian hepatitis E virus. Vet. Microbiol. 2013, 167, 242–249. [Google Scholar] [CrossRef]
- Guo, H.L.; Zhou, E.M.; Sun, Z.F.; Meng, X.J. Immunodominant epitopes mapped by synthetic peptides on the capsid protein of avian hepatitis E virus are non-protective. Viral Immunol. 2008, 21, 61–77. [Google Scholar] [CrossRef]
- Zhao, Q.; Syed, S.F.; Zhou, E.M. Antigenic properties of avian hepatitis E virus capsid protein. Vet. Microbiol. 2015, 180, 10–24. [Google Scholar] [CrossRef]
- Meng, X.J. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet. Microbiol. 2010, 140, 256–265. [Google Scholar] [CrossRef]
- Yarbough, P.O.; Tam, A.W.; Fry, K.E.; Krawczynski, K.; McCaustland, K.A.; Bradley, D.W.; Reyes, G.R. Hepatitis e virus: Identification of type-common epitopes. J. Virol. 1991, 65, 5790–5797. [Google Scholar] [CrossRef]
- Ghabrah, T.M.; Tsarev, S.; Yarbough, P.O.; Emerson, S.U.; Strickland, G.T.; Purcell, R.H. Comparison of tests for antibody to hepatitis E virus. J. Med. Virol. 1998, 55, 134–137. [Google Scholar] [CrossRef]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis e virus (hev): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Li, H.; Jing, S.R. Research Progress in ORF3 and Its Protein of Hepatitis E Virus. J. Microbiol. 2006, 5, 55–58. [Google Scholar]
- Kenney, S.P.; Pudupakam, R.S.; Huang, Y.W.; Pierson, F.W.; Leroith, T.; Meng, X.J. The PSAP Motif within the ORF3 Protein of an Avian Strain of the Hepatitis E Virus Is Not Critical for Viral Infectivity In Vivo but Plays a Role in Virus Release. J. Virol. 2012, 86, 5637–5646. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Primer Sequences (5′–3′) |
| Va-ORF3-F | GGATCCatgCGCCTCGGCTGCCAGCAC (BamHI GGATCC) |
| Va-ORF3-R | CTCGAGctaCATCTGGTACCGTGCG (XhoI CTCGAG) |
| YT-ORF3-F | GGATCCatgTGTCTTAGTTGCCAGTT (BamHI GGATCC) |
| YT-ORF3-R | CTCGAGctaCGTCTGGTACCGTGCGA (XhoI CTCGAG) |
| Days After Challenge | VaHEV Protein Immunization Group | YT aHEV Protein Immunization Group | Mixed Immunization Group | Negative Control Group |
|---|---|---|---|---|
| 2 | 5/8 | 5/8 | 3/8 | 8/8 |
| 4 | 1/8 | 0/8 | 0/8 | 8/8 |
| 7 | 0/8 | 0/8 | 0/8 | 8/8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Chi, Z.; Zhao, H.; Zhang, Y.; Zhang, Y.; Wang, Y.; Chang, S.; Zhao, P. Application of ORF3 Subunit Vaccine for Avian Hepatitis E Virus. Vet. Sci. 2022, 9, 676. https://doi.org/10.3390/vetsci9120676
Yan H, Chi Z, Zhao H, Zhang Y, Zhang Y, Wang Y, Chang S, Zhao P. Application of ORF3 Subunit Vaccine for Avian Hepatitis E Virus. Veterinary Sciences. 2022; 9(12):676. https://doi.org/10.3390/vetsci9120676
Chicago/Turabian StyleYan, Hongjian, Zengna Chi, Hui Zhao, Yawen Zhang, Yuduo Zhang, Yixin Wang, Shuang Chang, and Peng Zhao. 2022. "Application of ORF3 Subunit Vaccine for Avian Hepatitis E Virus" Veterinary Sciences 9, no. 12: 676. https://doi.org/10.3390/vetsci9120676
APA StyleYan, H., Chi, Z., Zhao, H., Zhang, Y., Zhang, Y., Wang, Y., Chang, S., & Zhao, P. (2022). Application of ORF3 Subunit Vaccine for Avian Hepatitis E Virus. Veterinary Sciences, 9(12), 676. https://doi.org/10.3390/vetsci9120676





