Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection
2.3. Analysis of Samples
2.4. Histomorphology Analysis
2.5. Short Chain Fatty Acids (SCFAs) Analysis
2.6. Real-Time Quantitative PCR
2.7. Microbial Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Incidence
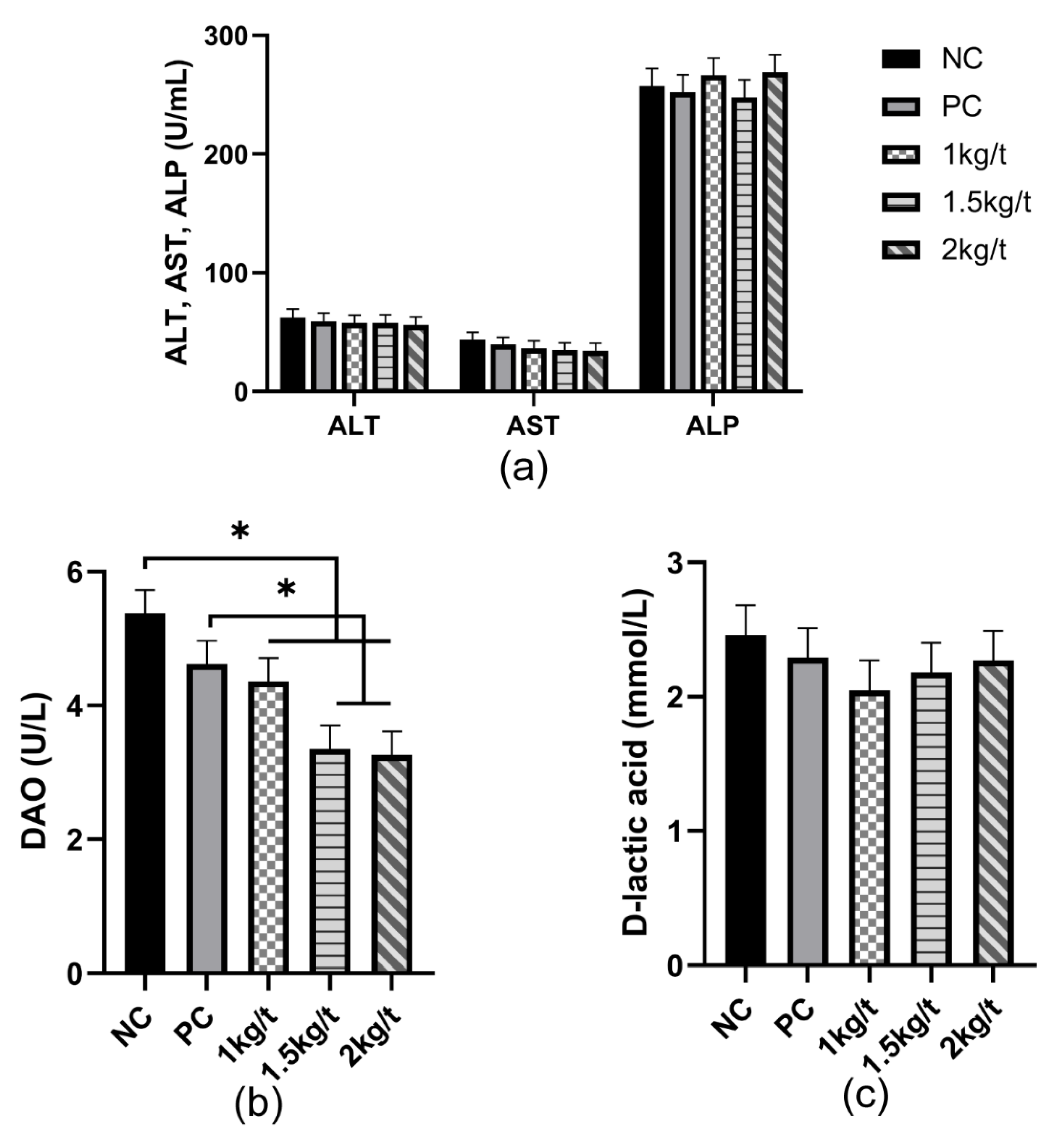
3.2. Liver Function and Intestinal Permeability
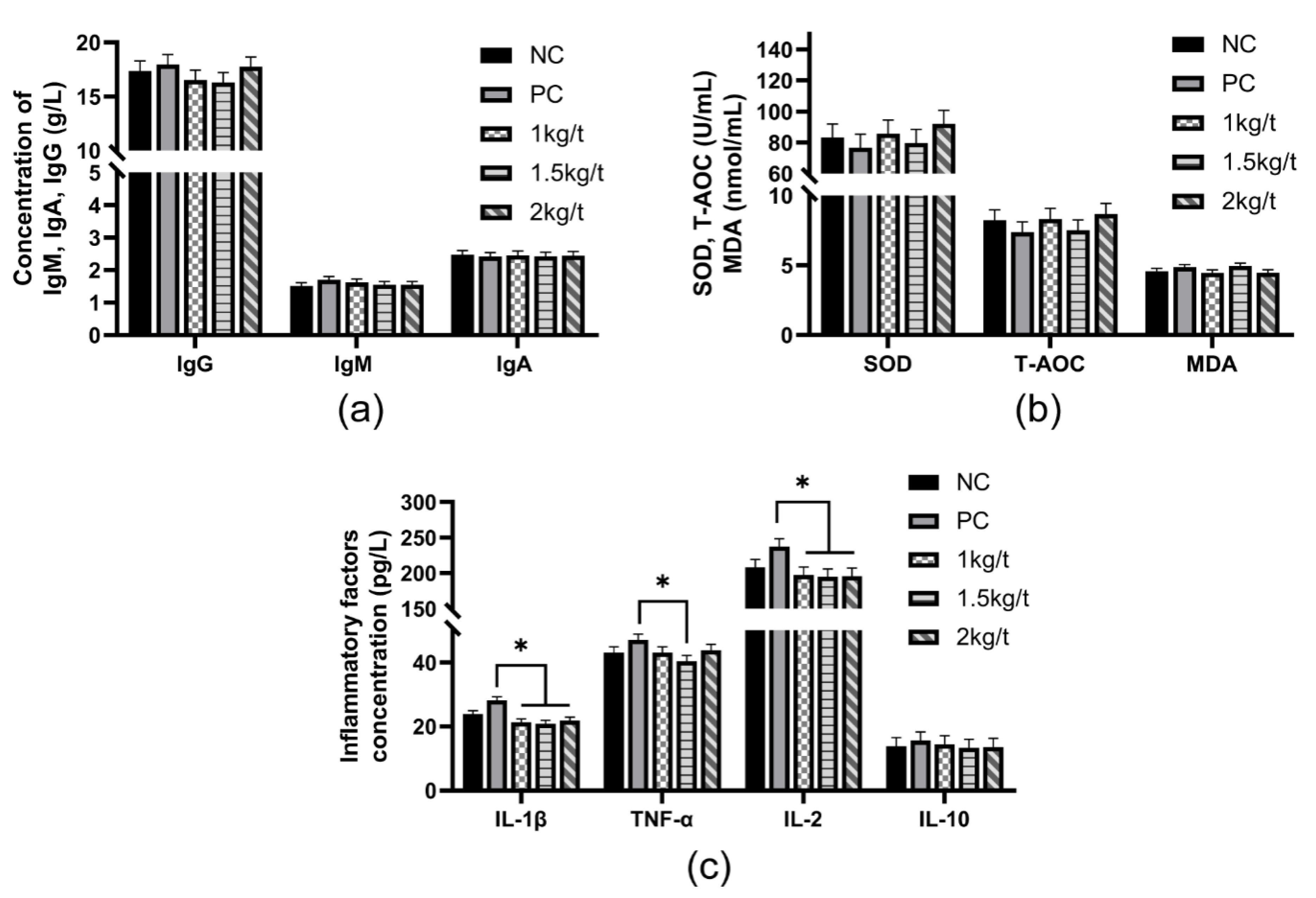
3.3. Serum Antioxidant Capacity and Immune Function
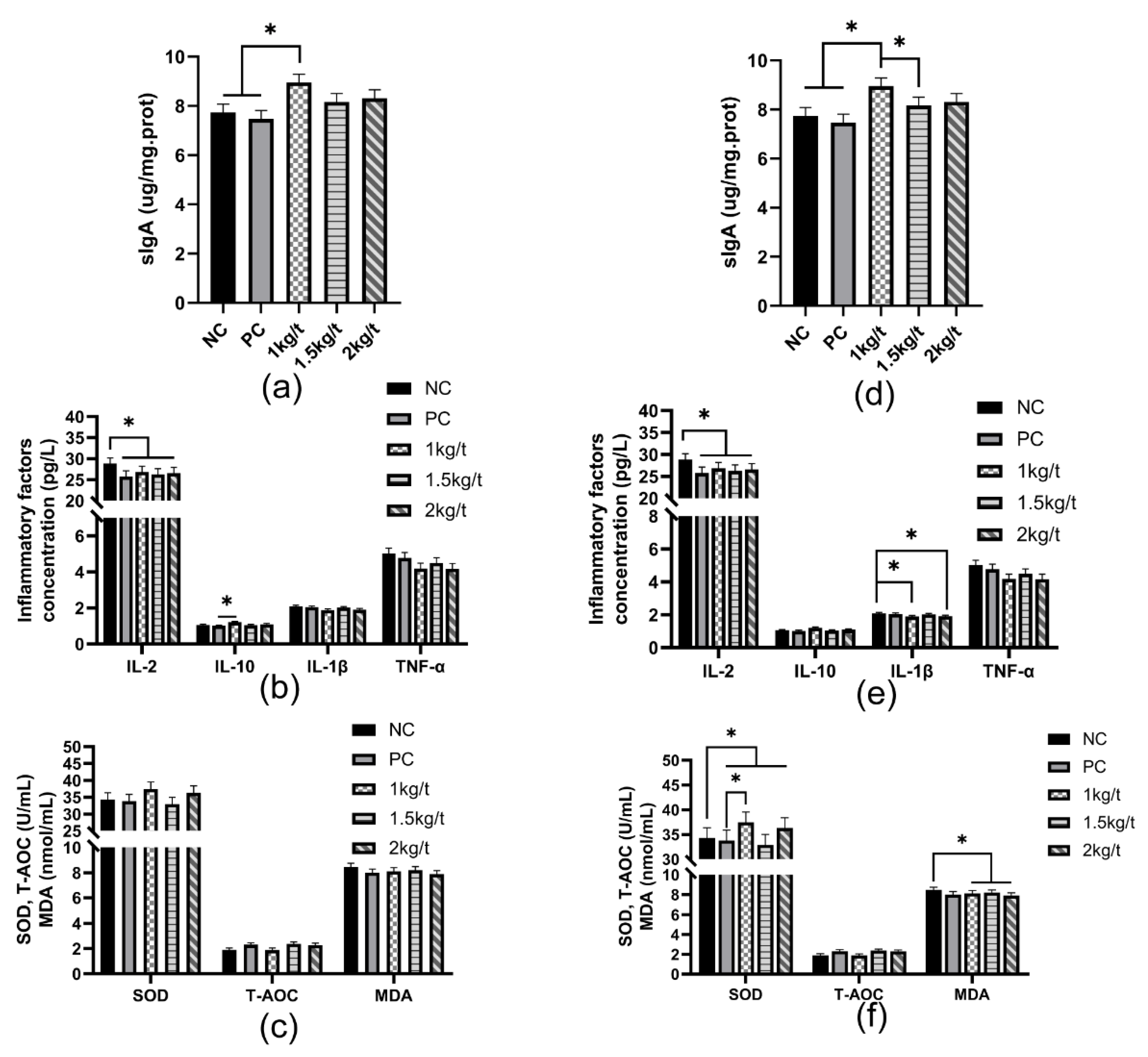
3.4. Intestinal Antioxidant Capacity and Immune Function
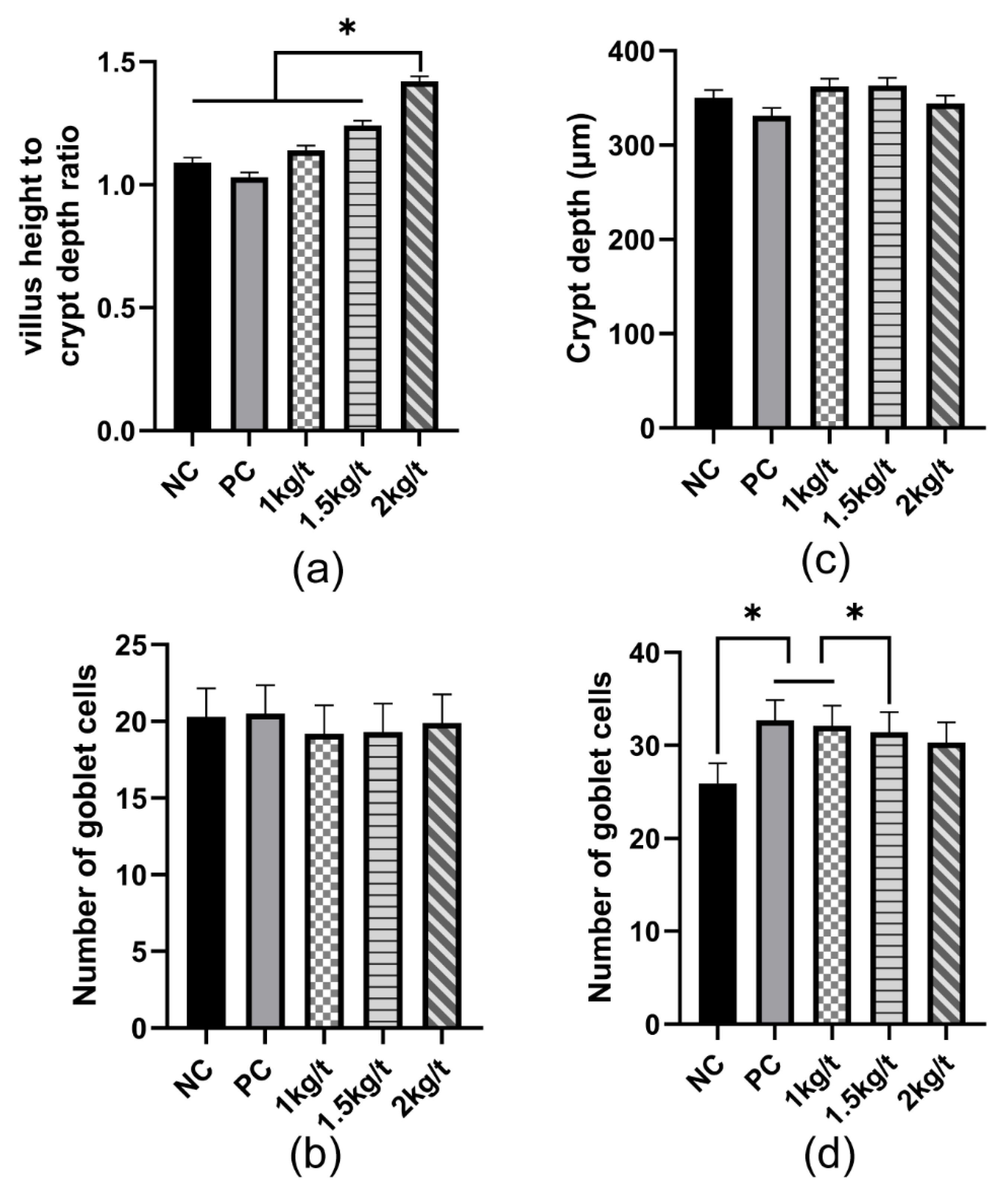
3.5. Intestinal Morphology and Number of Goblet Cells
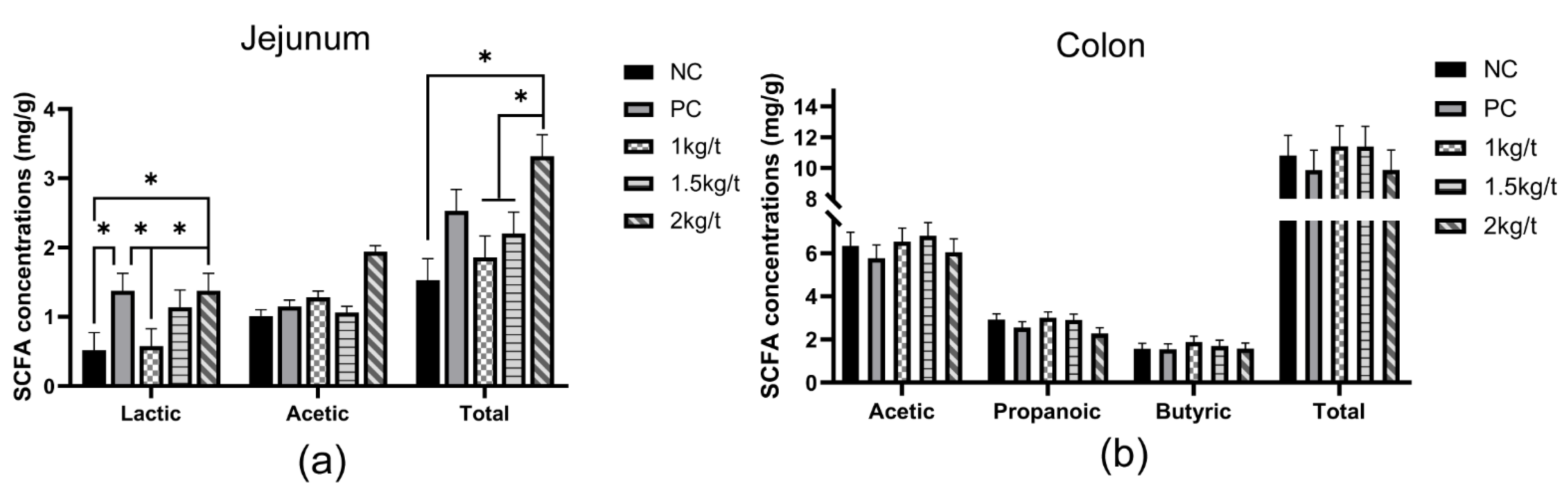
3.6. Lactic Acid and SCFA Concentrations
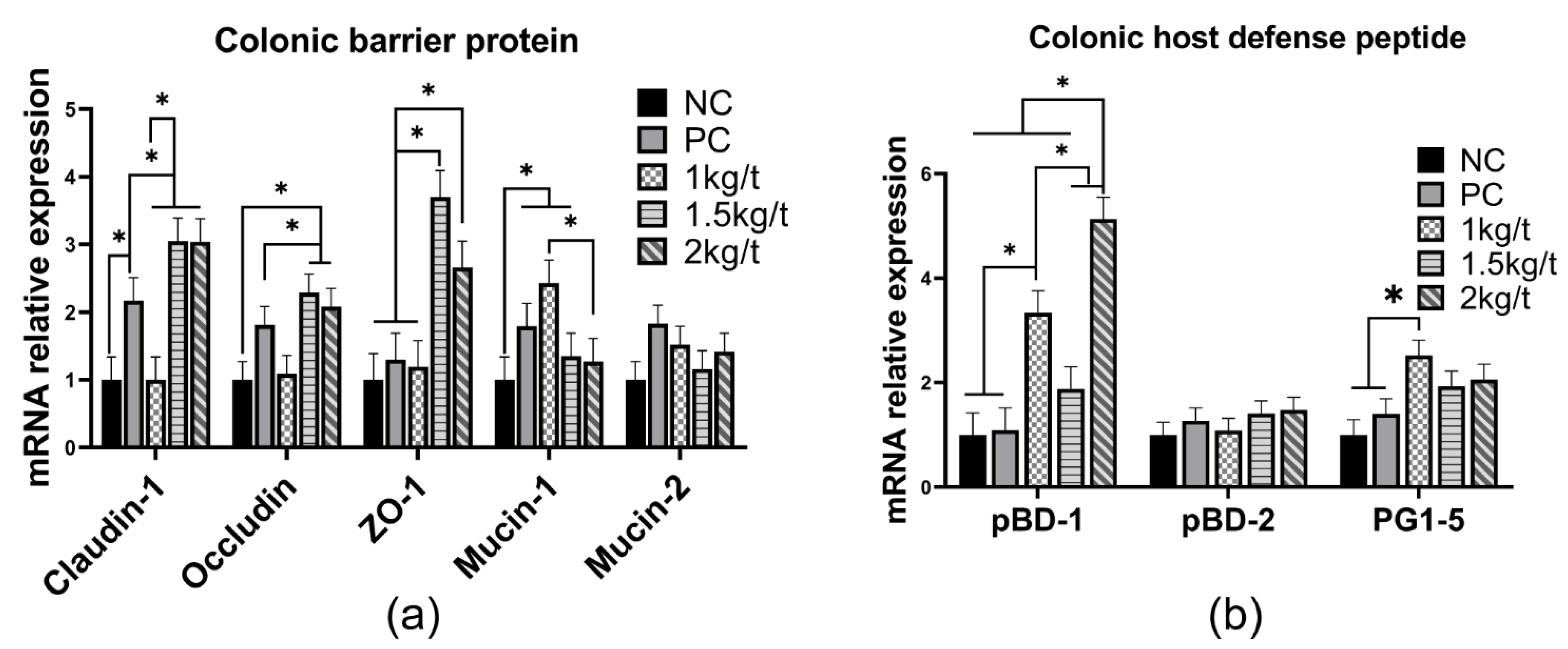
3.7. Colonic Expression of Tight Junction Functions and Host Defense Peptides
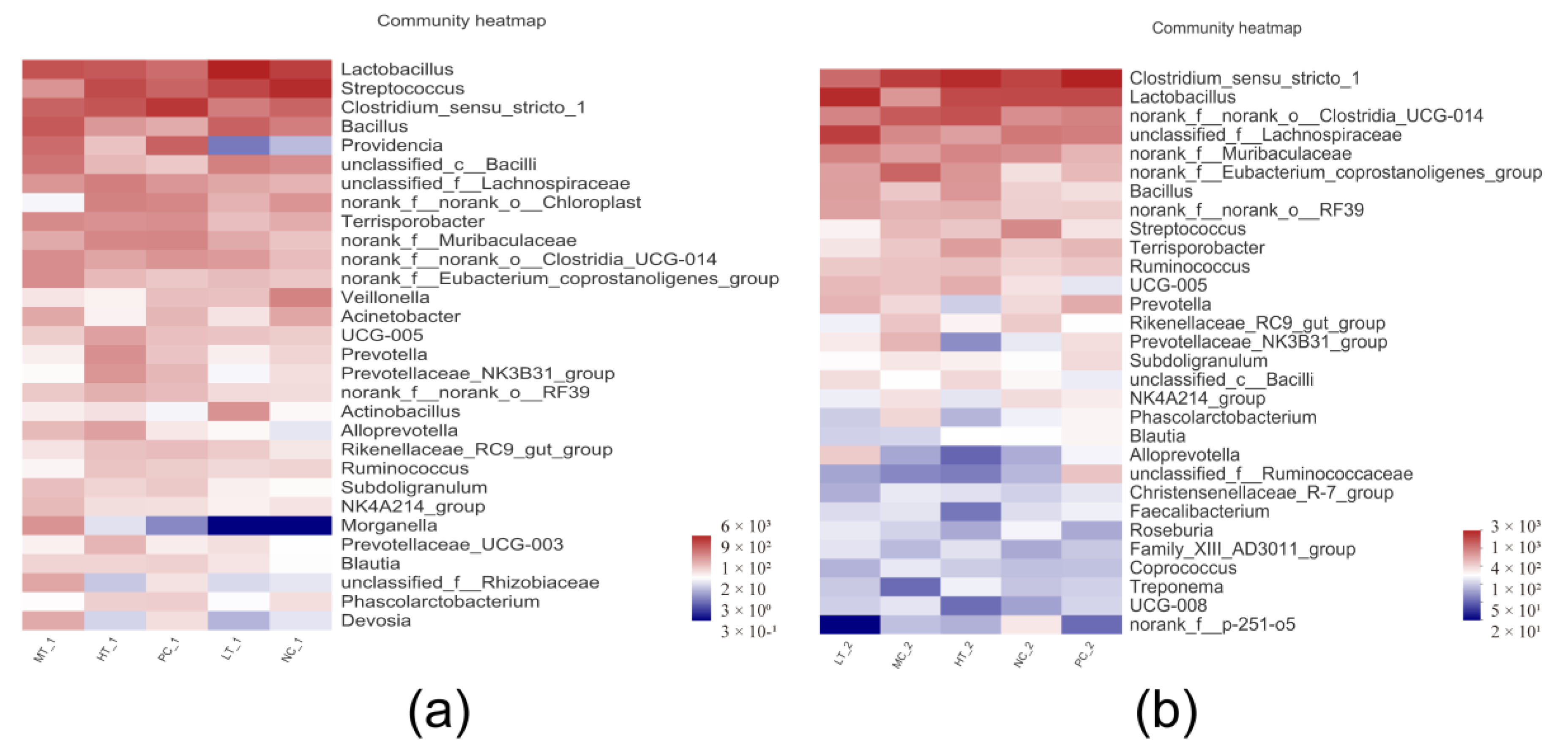
3.8. Microbial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef]
- Fang, H.; Han, L.; Zhang, H.; Long, Z.; Cai, L.; Yu, Y. Dissemination of antibiotic resistance genes and human pathogenic bacteria from a pig feedlot to the surrounding stream and agricultural soils. J. Hazard. Mater. 2018, 357, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Durand, H. Probiotics in animal nutrition and health. Benef. Microbes 2010, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Huang, K.; Zhang, M.; Wang, J.; Pan, X. Compound Lactobacillus sp. administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets. Appl. Microbiol. Biotechnol. 2020, 104, 6749–6765. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, S.; Liu, H.; Zhang, D.; Wang, Y.; Ji, H. Swine-derived probiotic lactobacillus plantarum mod-ulates porcine intestinal endogenous host defense peptide synthesis through tlr2/mapk/ap-1 signaling pathway. Front. Immunol. 2019, 10, 2691. [Google Scholar] [CrossRef]
- Rolfe, R.D. The Role of Probiotic Cultures in the Control of Gastrointestinal Health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef]
- Hu, Y.; Dun, Y.; Li, S.; Zhao, S.; Peng, N.; Liang, Y. Effects of bacillus subtilis kn-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 1131. [Google Scholar] [CrossRef]
- Hu, L.; Peng, X.; Chen, H.; Yan, C.; Liu, Y.; Xu, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of intrauterine growth retardation and bacillus subtilis pb6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur. J. Nutr. 2017, 56, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Ji, S.; Yu, G.; Jia, P.; Niu, Y.; Zhang, H.; Zhang, X.; Wang, T.; Zhang, L. Effects of Bacillus subtilis on jejunal integrity, redox status, and microbial composition of intrauterine growth restriction suckling piglets. J. Anim. Sci. 2021, 99, skab255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Wu, H.; Wu, S.-D.; Lu, N.; Wang, Y.-T.; Liu, H.-N.; Dong, L.; Liu, T.-T.; Shen, X.-Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Wang, W.; Li, T.; Zuo, B.; Li, Z.; Wang, J. Differences in the Gut Microbiota Establishment and Metabolome Characteristics Between Low- and Normal-Birth-Weight Piglets During Early-Life. Front. Microbiol. 2018, 9, 1798. [Google Scholar] [CrossRef] [PubMed]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Maison, T.; Koontanatechanon, A.; Prapasarakul, N. Use of Lactobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci. Rep. 2021, 11, 12028. [Google Scholar] [CrossRef]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J.; et al. Effects of Bacillus subtilis dsm32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, T.; Wei, X.; Zuo, B.; Davis, E.; Rehberger, T.; Hernandez, S.; Jochems, E.J.; Maxwell, C.V.; Zhao, J. Effect of lactylate and Bacillus subtilis on growth performance, peripheral blood cell profile, and gut microbiota of nursery pigs. Microorganisms 2021, 9, 803. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Weng, M.; Jiang, S.; Gao, J. Diagnostic and Clinical Significance of Serum Levels of D-Lactate and Diamine Oxidase in Patients with Crohn’s Disease. Gastroenterol. Res. Pract. 2019, 2019, 8536952. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 Promotes Intestinal Development Through Modulation of Gut Microbiota in Weaning Piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Montagne, L.; Pluske, J.; Hampson, D. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed. Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; He, T.; Li, D.; Su, J.-H.; Guo, L.; Wang, J.-F.; Zhu, Y.-H. Oral Administration of a Select Mixture of Lactobacillus and Bacillus Alleviates Inflammation and Maintains Mucosal Barrier Integrity in the Ileum of Pigs Challenged with Salmonella Infantis. Microorganisms 2019, 7, 135. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Ma, C.; Azad, A.K.; Tang, W.; Zhu, Q.; Wang, W.; Gao, Q.; Kong, X. Maternal probiotics supplementation improves immune and antioxidant function in suckling piglets via modifying gut microbiota. J. Appl. Microbiol. 2022, 64, 671–695. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Y.; Hu, S.; Zhu, C.; Wang, L.; Wen, X.; Yang, X.; Jiang, Z. Lactobacillus plantarum inhibited the inflammatory response induced by enterotoxigenic Escherichia coli K88 via modulating MAPK and NF-κB signalling in intestinal porcine epithelial cells. J. Appl. Microbiol. 2020, 130, 1684–1694. [Google Scholar] [CrossRef]
- Wang, K.; Kong, X.; Azad, M.; Kalam, A.; Zhu, Q.; Xiong, L.; Zheng, Y.; Hu, Z.; Yin, Y.; He, Q. Maternal probiotic or synbiotic supplementation modulates jejunal and colonic antioxidant capacity, mitochondrial function, and microbial abundance in bama mini-piglets. Oxidative Med. Cell. Longev. 2021, 2021, 6618874. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, K.; Zhang, J.; Xu, W.; Huang, Q.; Wang, T. Dietary effects of bacillus subtilis fmbj on the antioxidant ca-pacity of broilers at an early age. Poult. Sci. 2017, 96, 3564–3573. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, S.; Wang, Y.; Chu, X.; Ji, H. Lactobacillus plantarum Exhibits Antioxidant and Cytoprotective Activities in Porcine Intestinal Epithelial Cells Exposed to Hydrogen Peroxide. Oxidative Med. Cell. Longev. 2021, 2021, 8936907. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. An-timicrobial peptides: Features, action, and their resistance mechanisms in bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef]
- Blasig, I.E.; Bellmann, C.; Cording, J.; Del Vecchio, G.; Zwanziger, D.; Huber, O.; Haseloff, R.F. Occludin Protein Family: Oxidative Stress and Reducing Conditions. Antioxid. Redox Signal. 2011, 15, 1195–1219. [Google Scholar] [CrossRef]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T.; et al. A Microbiota-Derived Bacteriocin Targets the Host to Confer Diarrhea Resistance in Early-Weaned Piglets. Cell Host Microbe 2018, 24, 817–832.e8. [Google Scholar] [CrossRef]
- Siggers, R.H.; Siggers, J.; Boye, M.; Thymann, T.; Mølbak, L.; Leser, T.; Jensen, B.B.; Sangild, P.T. Early Administration of Probiotics Alters Bacterial Colonization and Limits Diet-Induced Gut Dysfunction and Severity of Necrotizing Enterocolitis in Preterm Pigs. J. Nutr. 2008, 138, 1437–1444. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.Y.; Kim, Y.; Lee, S.H.; Kim, S.; Kang, S.W.; Yang, J.Y.; Baek, I.J.; Sung, Y.H.; Park, Y.Y. Microbio-ta-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human me-tabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
| Ingredients, % | Control |
|---|---|
| Corn | 56.45 |
| Soybean meal | 13.50 |
| Whey powder | 10.00 |
| Fish meal | 3.00 |
| Soy protein concentrate | 5.00 |
| Extruded full-fat soybean | 5.00 |
| Sucrose | 2.00 |
| Soybean oil | 1.30 |
| Dicalcium phosphate | 1.20 |
| Limestone | 0.75 |
| NaCl | 0.20 |
| L-Lysine-HCl | 0.45 |
| DL-Methionine | 0.20 |
| L-Threonine | 0.15 |
| L-Tryptophan | 0.10 |
| L-Valine | 0.20 |
| Premix 1 | 0.50 |
| Nutritive levels, % | |
| Digestible energy, MJ/kg 2 | 14.82 |
| Crude protein | 18.95 |
| Calcium | 0.83 |
| Phosphorus | 0.66 |
| Target Genes | Primer | Primer Sequence (5′→3′) |
|---|---|---|
| β-Actin | Forward | CCAGGTCATCACCATCGGCAAC |
| Reverse | CAGCACCGTGTTGGCGTAGAG | |
| Mucin-1 | Forward | GTGCCGACGAAAGAACTG |
| Reverse | TGCCAGGTTCGAGTAAGAG | |
| Mucin-2 | Forward | CTGTGTGGGGCCTGACAA |
| Reverse | AGTGCTTGCAGTCGAACTCA | |
| ZO-1 | Forward | GCCATCCACTCCTGCCTAT |
| Reverse | CGGGACCTGCTCATAACTTC | |
| Occludin | Forward | CAGCAGCAGTGGTAACTTGG |
| Reverse | CAGCAGCAGTGGTAACTTGG | |
| Claudin-1 | Forward | AAGGACAAAACCGTGTGGGA |
| Reverse | CTCTCCCCACATTCGAGATGATT | |
| pBD-1 | Forward | TGCCACAGGTGCCGATCT |
| Reverse | CTGTTAGCTGCTTAAGGAATAAAGGC | |
| pBD-2 | Forward | CCAGAGGTCCGACCACTACA |
| Reverse | GGTCCCTTCAATCCTGTTGAA | |
| PG1-5 | Forward | GTAGGTTCTGCGTCTGTGTCG |
| Reverse | CAAATCCTTCACCGTCTACCA |
| Mixture of Probiotics | |||||||
|---|---|---|---|---|---|---|---|
| Items | NC | PC | LT | MT | HT | SEM | p-Value |
| Body weight | |||||||
| d 0 | 7.90 | 7.91 | 7.91 | 7.90 | 7.90 | 0.13 | 0.99 |
| d 14 | 11.88 | 12.12 | 12.17 | 11.98 | 11.99 | 0.32 | 0.91 |
| d 28 | 17.88 | 18.60 | 19.28 | 18.96 | 19.00 | 0.58 | 0.16 |
| d 42 | 27.50 | 28.70 | 29.82 | 29.28 | 29.34 | 0.92 | 0.11 |
| d 0–14 | |||||||
| ADFI | 418 | 427 | 440 | 411 | 434 | 15 | 0.75 |
| ADG | 285 | 303 | 307 | 293 | 294 | 9 | 0.45 |
| FCR | 1.47 | 1.41 | 1.45 | 1.41 | 1.48 | 0.01 | 0.41 |
| d 14–28 | |||||||
| ADFI | 720 c | 743 bc | 812 a | 762 ab | 777 ab | 17 | 0.01 |
| ADG | 430 c | 465 b | 511 a | 500 a | 502 a | 11 | 0.01 |
| FCR | 1.68 a | 1.6 ab | 1.59 ab | 1.53 b | 1.55 b | 0.01 | 0.01 |
| d 28–42 | |||||||
| ADFI | 1245 c | 1287 bc | 1412 a | 1322 b | 1352 ab | 18 | 0.01 |
| ADG | 692 c | 710 bc | 758 a | 741 ab | 743 ab | 12 | 0.01 |
| FCR | 1.81 | 1.82 | 1.87 | 1.79 | 1.82 | 0.02 | 0.23 |
| Diarrhea incidence, % | |||||||
| d 0–14 | 1.72 | 1.06 | 2.51 | 0.93 | 1.46 | 0.83 | 0.89 |
| d 14–28 | 3.57 | 1.98 | 2.65 | 1.72 | 3.44 | 1.04 | 0.74 |
| d 0–28 | 2.79 | 1.57 | 2.72 | 1.35 | 2.60 | 0.94 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gu, W.; Liu, X.; Zou, Y.; Wu, Y.; Xu, Y.; Han, D.; Wang, J.; Zhao, J. Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets. Vet. Sci. 2022, 9, 668. https://doi.org/10.3390/vetsci9120668
Liu Y, Gu W, Liu X, Zou Y, Wu Y, Xu Y, Han D, Wang J, Zhao J. Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets. Veterinary Sciences. 2022; 9(12):668. https://doi.org/10.3390/vetsci9120668
Chicago/Turabian StyleLiu, Yisi, Wei Gu, Xiaoyi Liu, Youwei Zou, Yujun Wu, Youhan Xu, Dandan Han, Junjun Wang, and Jinbiao Zhao. 2022. "Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets" Veterinary Sciences 9, no. 12: 668. https://doi.org/10.3390/vetsci9120668
APA StyleLiu, Y., Gu, W., Liu, X., Zou, Y., Wu, Y., Xu, Y., Han, D., Wang, J., & Zhao, J. (2022). Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets. Veterinary Sciences, 9(12), 668. https://doi.org/10.3390/vetsci9120668






