Detection of Colistin Sulfate on Piglet Gastrointestinal Tract Microbiome Alterations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglets and Drugs
2.2. Study Design
2.3. DNA Isolation
2.4. Construction of a DNA Sequencing Library and Illumina MiSeq Sequencing
2.5. Processing and Analyzing Sequencing Data
2.6. Statistical Analysis
3. Results
3.1. Detection of Colistin on Piglet Gastrointestinal Tract Composition
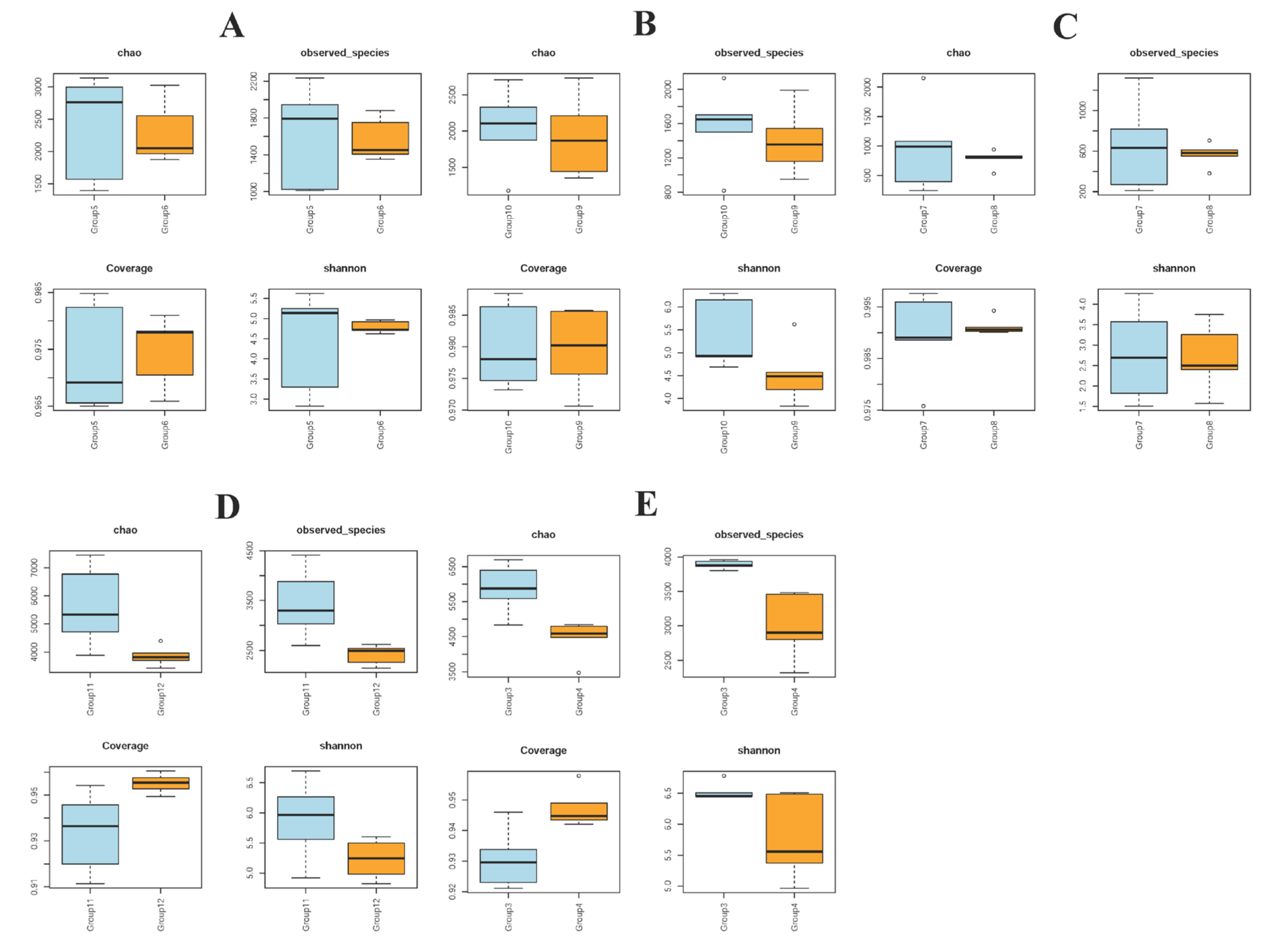
3.2. Alpha Diversity in Piglet Gut Microbiome Composition Is Influenced by Colistin
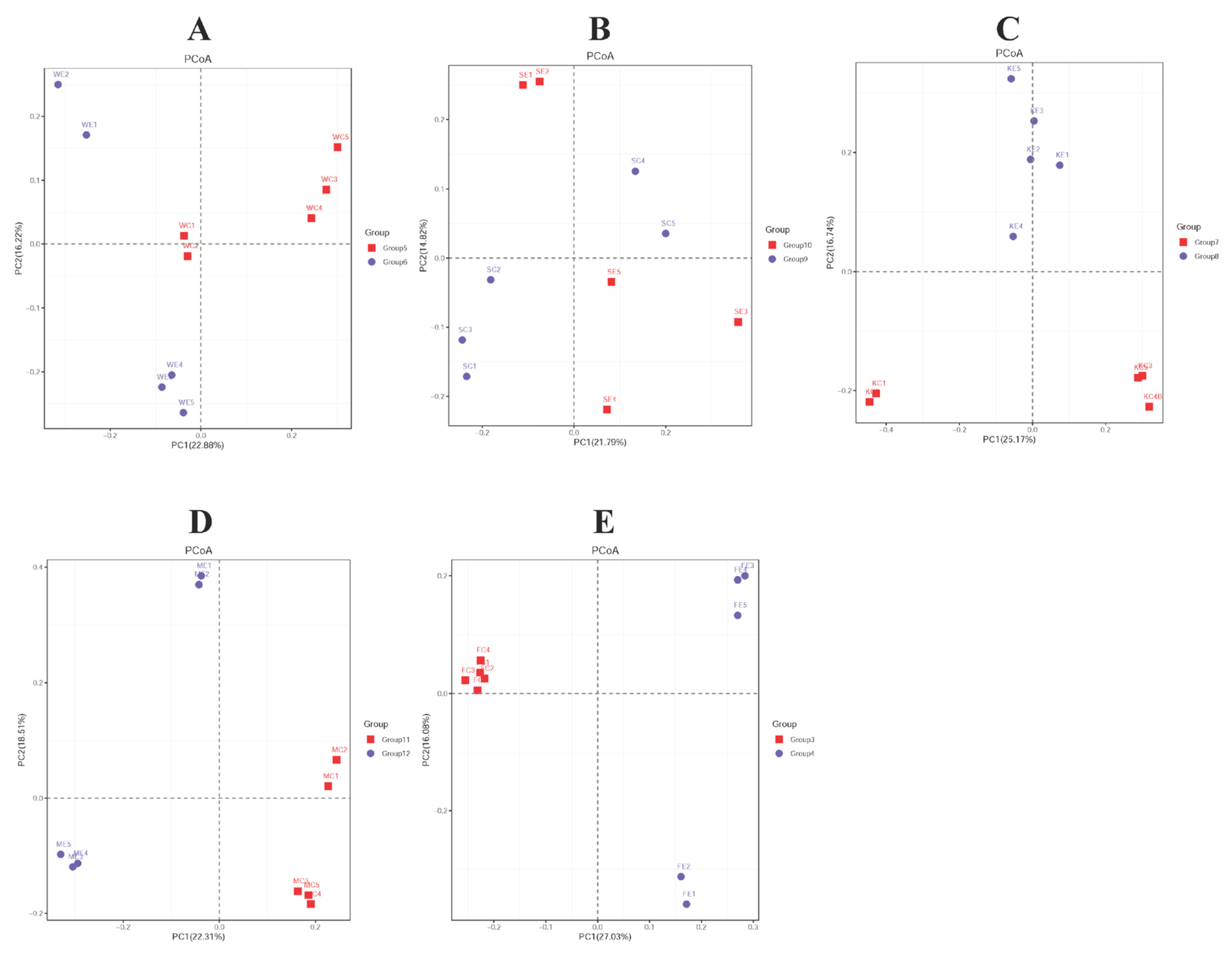
3.3. Beta Diversity in Piglet Gut Microbiome Composition Is Influenced by Colistin
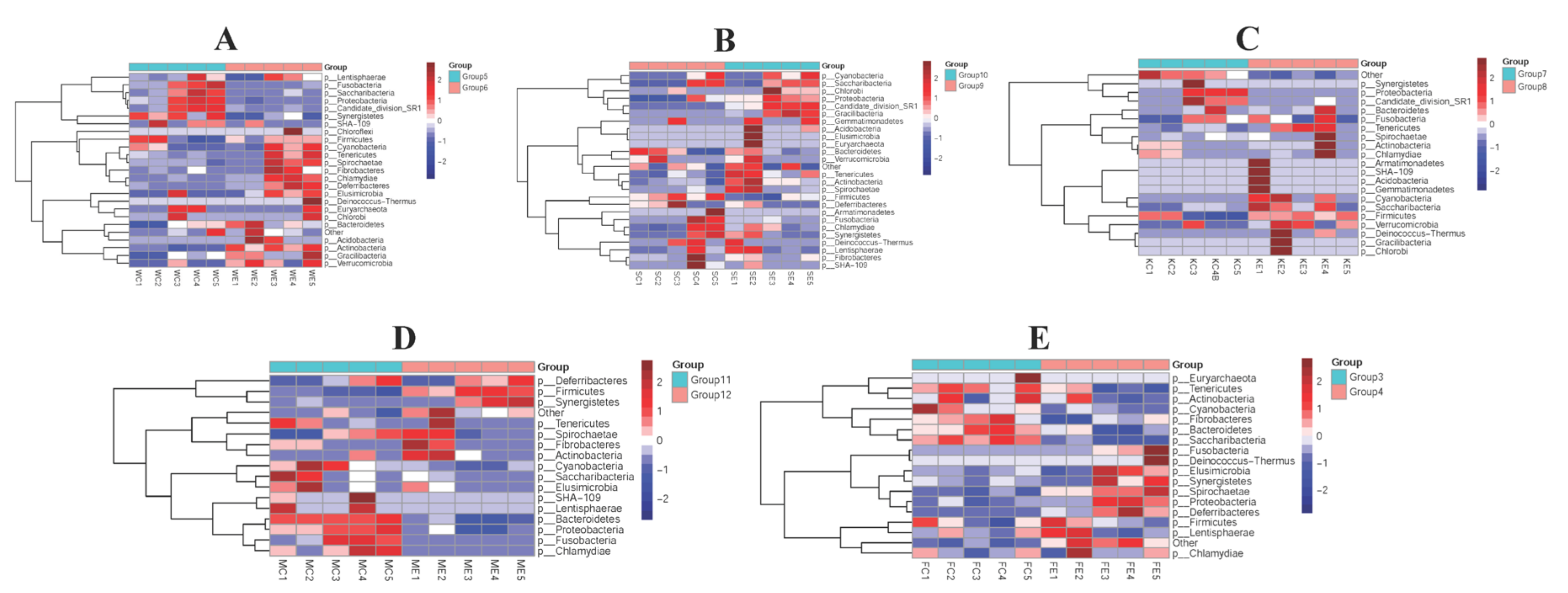
3.4. The Efficacy of Colistin on Piglet Microbiome Change at Phylum Levels
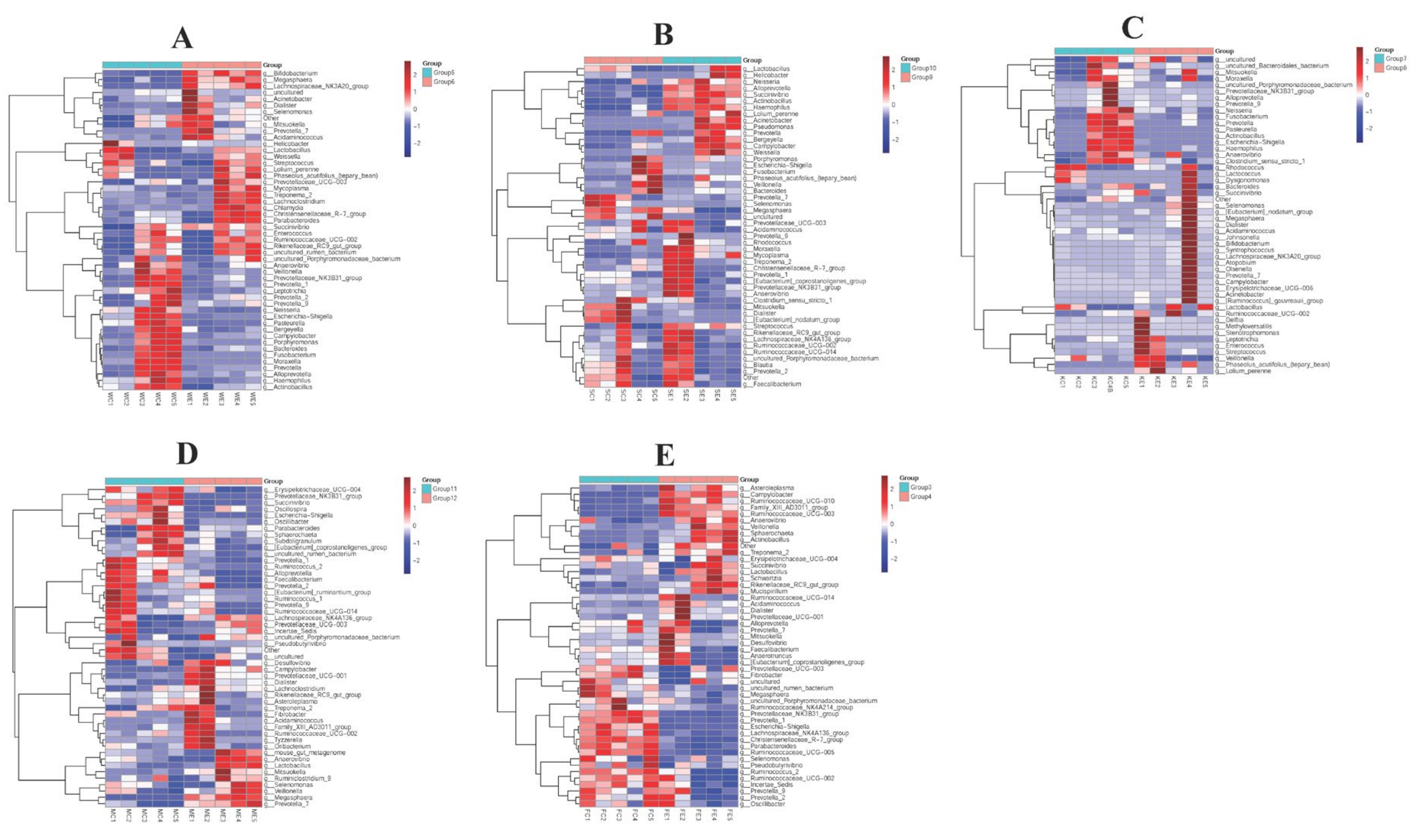
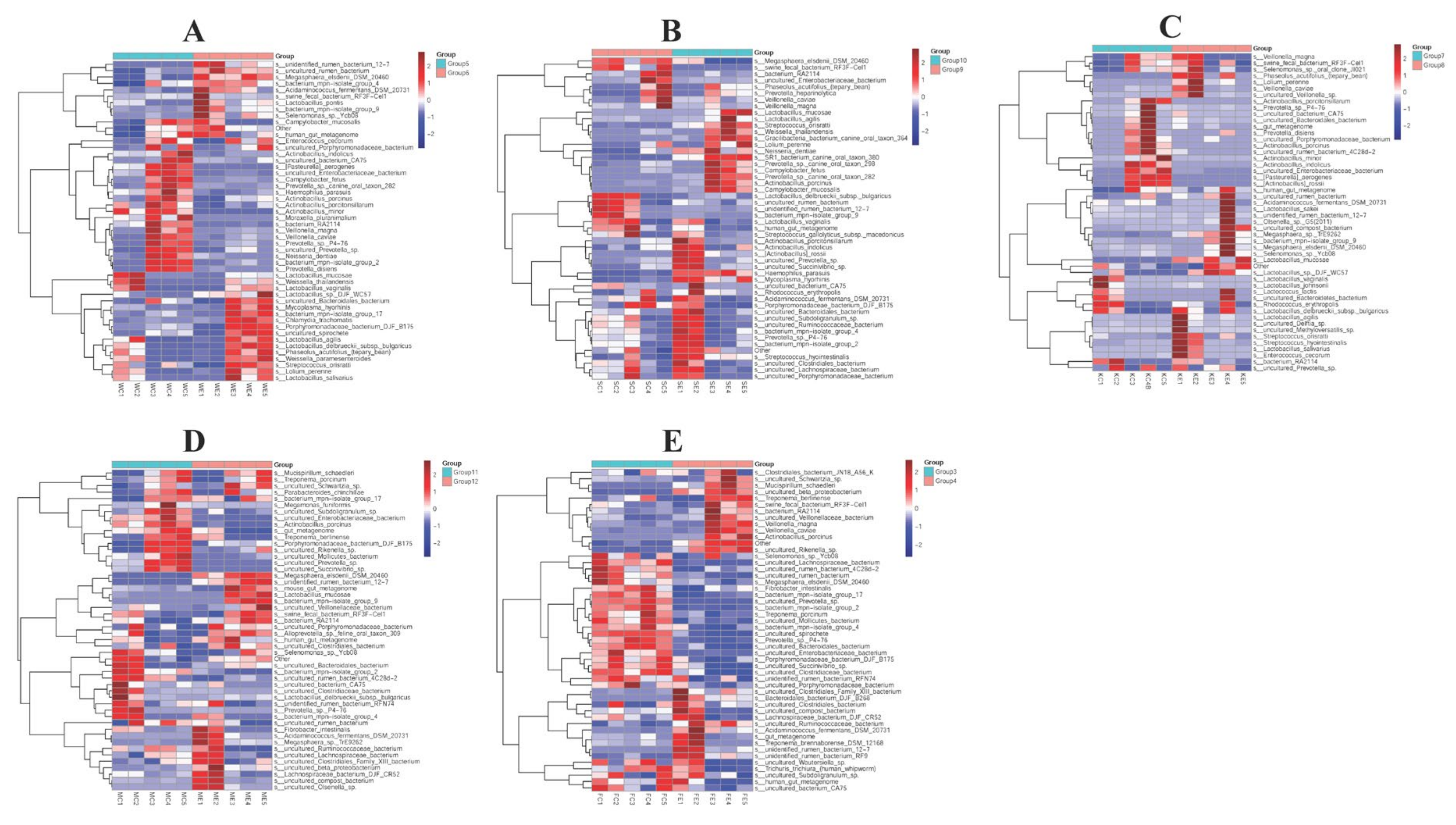
3.5. The Efficacy of Colistin on Piglet Microbiome Change at Genus Levels
3.6. The Efficacy of Colistin on Piglet Microbiome Change at Species Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Haq, Q.M.R. Current Update on Intrinsic and Acquired Colistin Resistance Mechanisms in Bacteria. Front. Med. 2021, 8, 677720. [Google Scholar] [CrossRef] [PubMed]
- Ilbeigi, K.; Askari Badouei, M.; Vaezi, H.; Zaheri, H.; Aghasharif, S.; Kafshdouzan, K. Molecular survey of mcr1 and mcr2 plasmid mediated colistin resistance genes in Escherichia coli isolates of animal origin in Iran. BMC Res. Notes 2021, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Urmi, U.L.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Iqbal, S.; Hossain, M.M.; Mosaddek, A.S.M.; Nahar, S. High abundance of the colistin resistance gene mcr-1 in chicken gut-bacteria in Bangladesh. Sci. Rep. 2020, 10, 17292. [Google Scholar] [CrossRef] [PubMed]
- Ara, B.; Urmi, U.L.; Haque, T.A.; Nahar, S.; Rumnaz, A.; Ali, T.; Alam, M.S.; Mosaddek, A.S.M.; Rahman, N.A.A.; Haque, M.; et al. Detection of mobile colistin-resistance gene variants (mcr-1 and mcr-2) in urinary tract pathogens in Bangladesh: The last resort of infectious disease management colistin efficacy is under threat. Expert Rev. Clin. Pharmacol. 2021, 14, 513–522. [Google Scholar] [CrossRef]
- Guo, R.; Ding, X.; Zhong, X.; Gao, S.; Sun, Y. Molecular and ultrastructural insights into the earthworm Eisenia fetida of the assessment of ecotoxicity during colistin exposure. Environ. Sci. Pollut. Res. Int. 2014, 21, 13405–13411. [Google Scholar] [CrossRef]
- Fu, R.; Liang, C.; Chen, D.; Yan, H.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of dietary Bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 898–907. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.M.; Ariza, J.J.; Martín-Platero, A.M.; Casabuena-Rincón, Ó.; Vázquez-Chas, P.; Guillamón, E.; Aguinaga-Casañas, M.A.; Maqueda, M.; et al. Allium Extract Implements Weaned Piglet’s Productive Parameters by Modulating Distal Gut Microbiota. Antibiotics 2021, 10, 269. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Z.; Azad, M.A.K.; Zhang, W.; Blachier, F.; Wang, Z.; Kong, X. Dietary supplementation with Bacillus mixture modifies the intestinal ecosystem of weaned piglets in an overall beneficial way. J. Appl. Microbiol. 2021, 130, 233–246. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Gao, Y.; Liu, L.; Duan, Y.; Mao, D.; Luo, Y. Colistin and amoxicillin combinatorial exposure alters the human intestinal microbiota and antibiotic resistome in the simulated human intestinal microbiota. Sci. Total Environ. 2021, 750, 141415. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, Q.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Ding, X.; Zeng, X.; Zhang, J. Effect of Antimicrobial Peptide Microcin J25 on Growth Performance, Immune Regulation, and Intestinal Microbiota in Broiler Chickens Challenged with Escherichia coli and Salmonella. Animals 2020, 10, 345. [Google Scholar] [CrossRef]
- Kim, S.E. Importance of nutritional therapy in the management of intestinal diseases: Beyond energy and nutrient supply. Intest. Res. 2019, 17, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lv, J.; Guo, F.; Li, J.; Jia, Y.; Jiang, D.; Wang, N.; Zhang, C.; Kong, L.; Liu, Y.; et al. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front. Microbiol. 2020, 11, 814. [Google Scholar] [CrossRef]
- Bullard, B.M.; VanderVeen, B.N.; McDonald, S.J.; Cardaci, T.D.; Murphy, E.A. Cross talk between the gut microbiome and host immune response in ulcerative colitis: Nonpharmacological strategies to improve homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fu, H.Y.; Qiu, S.N.; Teng, T.; Bai, G.D.; Ju, D.X.; Sun, Y.C.; Shi, B.M. Effects of early protein restriction on the growth performance and gut development of pigs fed diets with or without antibiotic. Animal 2020, 14, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Ab Majid, A.H. Metagenomic 16S rDNA amplicon data of microbial diversity of guts of fully fed tropical bed bugs, Cimex hemipterus (F.) (Hemiptera: Cimicidae). Data Brief. 2020, 30, 105575. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Lu, M.; Jiang, M.; Zhou, D.; Huang, H. Proteobacteria Acts as a Pathogenic Risk-Factor for Chronic Abdominal Pain and Diarrhea in Post-Cholecystectomy Syndrome Patients: A Gut Microbiome Metabolomics Study. Med. Sci. Monit. 2019, 25, 7312–7320. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Gui, Q.F.; Jin, H.L.; Zhu, F.; Lu, H.F.; Zhang, Q.; Xu, J.; Yang, Y.M.; Xiao, C. Gut microbiota signatures in Schistosoma japonicum infection-induced liver cirrhosis patients: A case-control study. Infect. Dis. Poverty 2021, 10, 43. [Google Scholar] [CrossRef]
- Karaiskos, I.; Souli, M.; Galani, I.; Giamarellou, H. Colistin: Still a lifesaver for the 21st century? Expert Opin. Drug Metab. Toxicol. 2017, 13, 59–71. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef]
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin. Pharm. 2017, 56, 1441–1460. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 2018, 6. [Google Scholar] [CrossRef]
- Jafari, F.; Elyasi, S. Prevention of colistin induced nephrotoxicity: A review of preclinical and clinical data. Expert Rev. Clin. Pharmacol. 2021, 14, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.H. Monitoring Colistin Resistance in Food Animals, An Urgent Threat. Expert Rev. Anti Infect. Ther. 2018, 16, 443–446. [Google Scholar] [CrossRef]
- Mu, C.; Pi, Y.; Zhang, C.; Zhu, W. Microbiomes in the Intestine of Developing Pigs: Implications for Nutrition and Health. Adv. Exp. Med. Biol. 2022, 1354, 161–176. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Cao, H.; Zong, C.; Dai, W.; Gao, Q.; Li, D.; Wu, X.; Li, D.; Tang, Y.W.; Wu, S. The Effects of Chinese Medicine QRD, Antibiotics, and Probiotics on Therapy and Gut Microbiota in Septic Rats. Front. Cell Infect. Microbiol. 2021, 11, 712028. [Google Scholar] [CrossRef]
- Ojima, M.N.; Gotoh, A.; Takada, H.; Odamaki, T.; Xiao, J.Z.; Katoh, T.; Katayama, T. Bifidobacterium bifidum Suppresses Gut Inflammation Caused by Repeated Antibiotic Disturbance Without Recovering Gut Microbiome Diversity in Mice. Front. Microbiol. 2020, 11, 1349. [Google Scholar] [CrossRef]
- Goldstein, E.J.; Tyrrell, K.L.; Citron, D.M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S98–S107. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, H.; Chen, J.; Luo, L.; Gu, Y.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; et al. Anti-Adhesion Effects of Lactobacillus Strains on Caco-2 Cells Against Escherichia Coli and Their Application in Ameliorating the Symptoms of Dextran Sulfate Sodium-Induced Colitis in Mice. Probiotics Antimicrob. Proteins 2021, 13, 1632–1643. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Li, L.; Wang, H.; Zhu, J.; Zhang, P.; Lee, Y.K.; Zhao, J.; Zhang, H.; Lu, W.; et al. Lactobacillus mucosae exerted different antiviral effects on respiratory syncytial virus infection in mice. Front. Microbiol. 2022, 13, 1001313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; San Andres, J.V.; Trenhaile-Grannemann, M.D.; van Sambeek, D.M.; Moore, K.C.; Winkel, S.M.; Fernando, S.C.; Burkey, T.E.; Miller, P.S. Effects of mannan oligosaccharides and Lactobacillus mucosae on growth performance, immune response, and gut health of weanling pigs challenged with Escherichia coli lipopolysaccharides. J. Anim. Sci. 2021, 99, skab286. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Pan, L.; Li, J.; Yu, Y.; Liu, B.; Zubair, M.; Wei, Y.; Pillay, B.; Olaniran, A.O.; et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) moonlights as an adhesin in Mycoplasma hyorhinis adhesion to epithelial cells as well as a plasminogen receptor mediating extracellular matrix degradation. Vet. Res. 2021, 52, 80. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ali, S.; Javed, S.O.; Shoukat, S.; Ahmad, S.; Ali, S.S.; Hussain, Z.; Waseem, M.; Rizwan, M.; Suleman, M.; et al. Proteome wide vaccine targets prioritization and designing of antigenic vaccine candidate to trigger the host immune response against the Mycoplasma genitalium infection. Microb. Pathog. 2021, 152, 104771. [Google Scholar] [CrossRef] [PubMed]
- Collingro, A.; Köstlbacher, S.; Horn, M. Chlamydiae in the Environment. Trends Microbiol. 2020, 28, 877–888. [Google Scholar] [CrossRef]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Meng, Q.; Zhang, D.; Zuo, S.; He, J.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Hu, C.A. Effect of Baicalin on Transcriptome Changes in Piglet Vascular Endothelial Cells Induced by a Combination of Glaesserella parasuis and Lipopolysaccharide. DNA Cell Biol. 2021, 40, 776–790. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Yuan, Y.; Tian, X.; Zhou, L.; Guo, L.; Zhang, D.; He, J.; Peng, C.; Qiu, Y.; Ye, C.; et al. Detection of Colistin Sulfate on Piglet Gastrointestinal Tract Microbiome Alterations. Vet. Sci. 2022, 9, 666. https://doi.org/10.3390/vetsci9120666
Fu S, Yuan Y, Tian X, Zhou L, Guo L, Zhang D, He J, Peng C, Qiu Y, Ye C, et al. Detection of Colistin Sulfate on Piglet Gastrointestinal Tract Microbiome Alterations. Veterinary Sciences. 2022; 9(12):666. https://doi.org/10.3390/vetsci9120666
Chicago/Turabian StyleFu, Shulin, Yuzhen Yuan, Xinyue Tian, Linglu Zhou, Ling Guo, Dan Zhang, Jing He, Chun Peng, Yinsheng Qiu, Chun Ye, and et al. 2022. "Detection of Colistin Sulfate on Piglet Gastrointestinal Tract Microbiome Alterations" Veterinary Sciences 9, no. 12: 666. https://doi.org/10.3390/vetsci9120666
APA StyleFu, S., Yuan, Y., Tian, X., Zhou, L., Guo, L., Zhang, D., He, J., Peng, C., Qiu, Y., Ye, C., Liu, Y., & Zong, B. (2022). Detection of Colistin Sulfate on Piglet Gastrointestinal Tract Microbiome Alterations. Veterinary Sciences, 9(12), 666. https://doi.org/10.3390/vetsci9120666






