Intratesticular Versus Intrafunicular Lidocaine to Reduce Perioperative Nociception and Immunological Response in Ponies Undergoing Field Castration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Anaesthesia Premedication, Induction, and Surgery
2.3. Cytokine Plasma Concentrations
2.4. Statistical Analysis
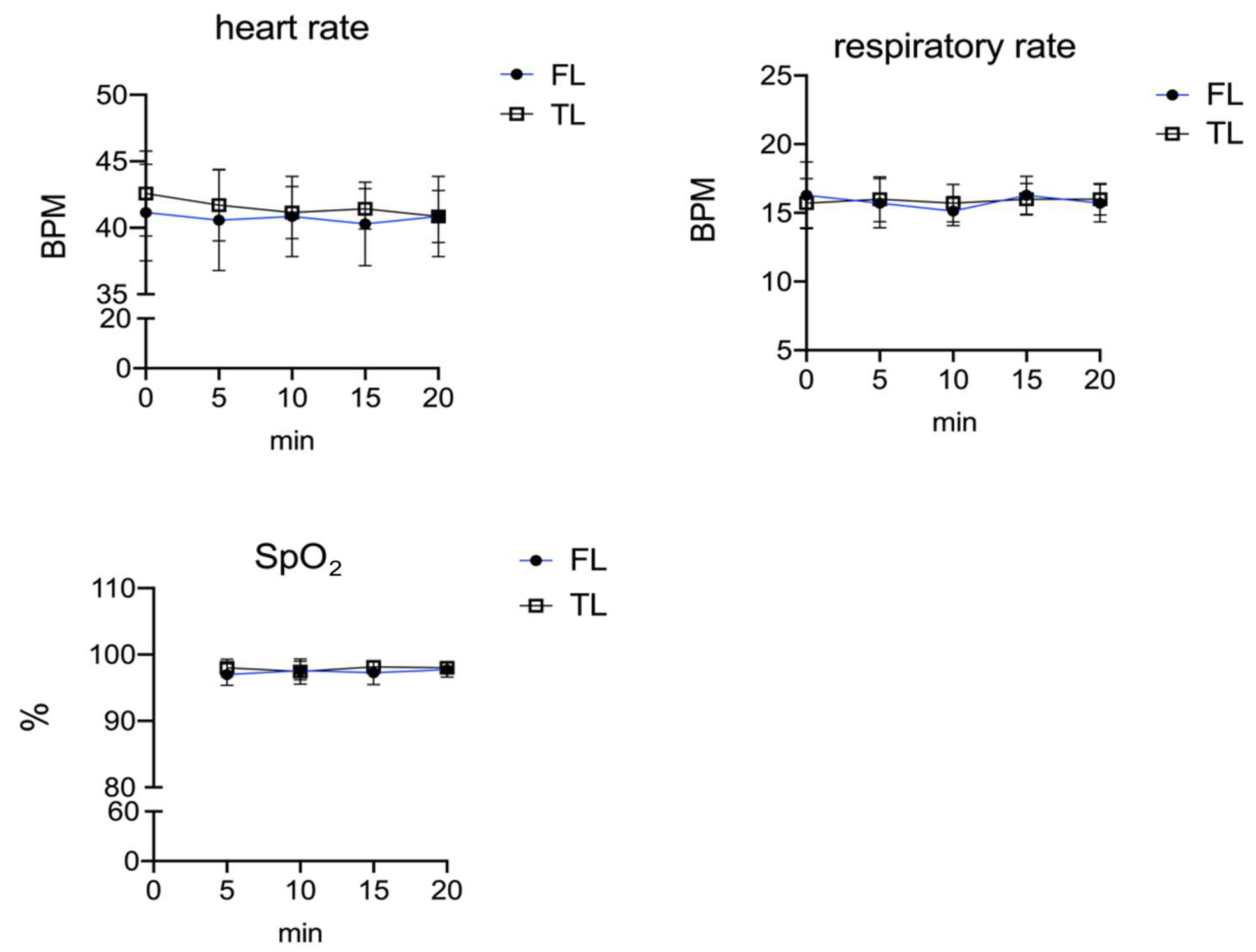
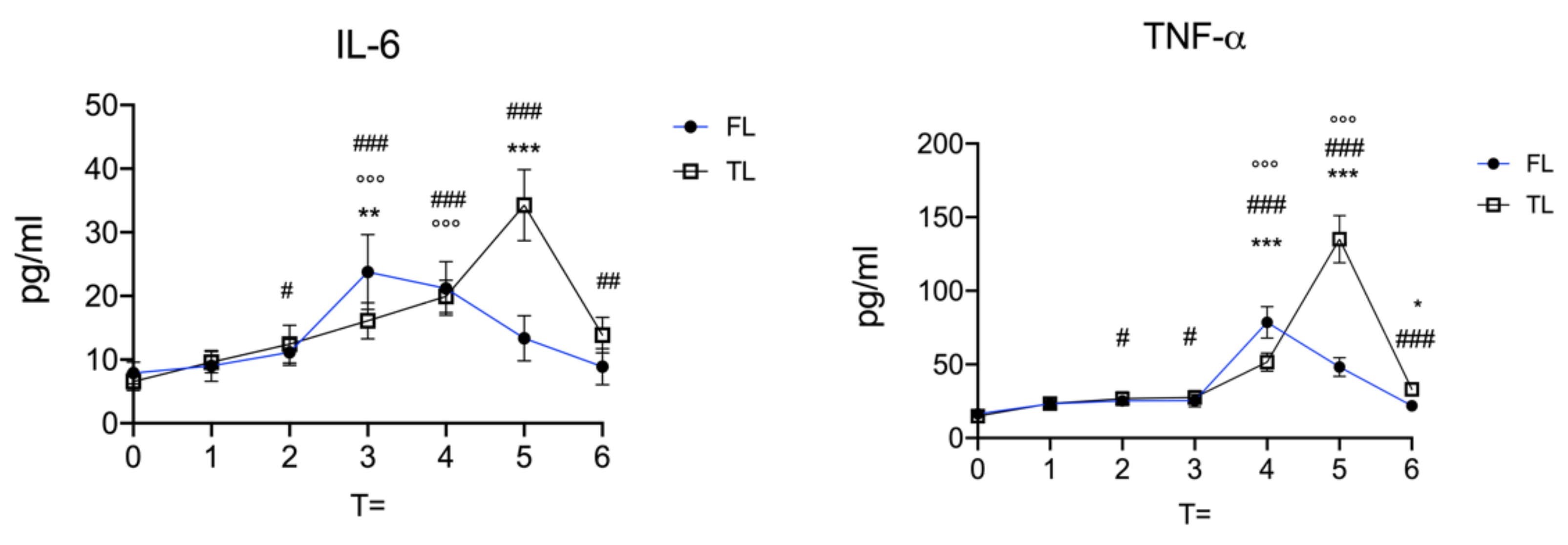
3. Results
4. Discussion
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.C.; Xi, C.H.; An, Y.F.; Dong, W.H.; Zhou, M. Perioperative inflammatory response and protein S-100beta concentrations—relationship with post-operative cognitive dysfunction in elderly patients. Acta Anaesthesiol. Scand. 2012, 56, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalak, B.B.; You, J.; Kurz, A.; Kot, M.; Bralliar, T.; Remzi, F.H.; Sessler, D.I. The effects of dexamethasone, light anesthesia, and tight glucose control on postoperative fatigue and quality of life after major noncardiac surgery: A randomized trial. J. Clin. Anesth. 2019, 55, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.; Bailey, J.; Janzen, E.; Wilson, D. Routine castration in 568 draught colts: Incidence of evisceration and omental herniation. Equine Vet. J. 2004, 36, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, L.C.; Thorin, C.; Meurice, A.; Dubus, A.; Touzot-Jourde, G.; Courouce, A.; Leroux, A.A. Comparison of Flunixin Meglumine, Meloxicam and Ketoprofen on Mild Visceral Post-Operative Pain in Horses. Animals 2022, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J. Complications of castration. Equine Vet. Educ. 1996, 8, 254–259. [Google Scholar] [CrossRef]
- Kilcoyne, I.; Spier, S.J. Castration Complications: A Review of Castration Techniques and How to Manage Complications. Vet. Clin. N. Am. Equine Pract. 2021, 37, 259–273. [Google Scholar] [CrossRef]
- Searle, D.; Dart, A.J.; Dart, C.M.; Hodgson, D.R. Equine castration: Review of anatomy, approaches, techniques and complications in normal, cryptorchid and monorchid horses. Aust. Vet. J. 1999, 77, 428–434. [Google Scholar] [CrossRef]
- Kilcoyne, I.; Watson, J.L.; Kass, P.H.; Spier, S.J. Incidence, management, and outcome of complications of castration in equids: 324 cases (1998–2008). J. Am. Vet. Med. Assoc. 2013, 242, 820–825. [Google Scholar] [CrossRef]
- Abass, M.; Picek, S.; Garzon, J.F.G.; Kuhnle, C.; Zaghlou, A.; Bettschart-Wolfensberger, R. Local mepivacaine before castration of horses under medetomidine isoflurane balanced anaesthesia is effective to reduce perioperative nociception and cytokine release. Equine Vet. J. 2018, 50, 733–738. [Google Scholar] [CrossRef]
- Portier, K.G.; Jaillardon, L.; Leece, E.A.; Walsh, C.M. Castration of horses under total intravenous anaesthesia: Analgesic effects of lidocaine. Vet. Anaesth. Analg. 2009, 36, 173–179. [Google Scholar] [CrossRef]
- Haga, H.A.; Lykkjen, S.; Revold, T.; Ranheim, B. Effect of intratesticular injection of lidocaine on cardiovascular responses to castration in isoflurane-anesthetized stallions. Am. J. Vet. Res. 2006, 67, 403–408. [Google Scholar] [CrossRef]
- Trindade, P.H.E.; Taffarel, M.O.; Luna, S.P.L. Spontaneous Behaviors of Post-Orchiectomy Pain in Horses Regardless of the Effects of Time of Day, Anesthesia, and Analgesia. Animals 2021, 11, 1629. [Google Scholar] [CrossRef]
- Jordan, S.C.; Choi, J.; Kim, I.; Wu, G.; Toyoda, M.; Shin, B.; Vo, A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Yang, H.W.; Yang, T.S.; Xie, W.; Hu, Z.H. TNF-alpha-mediated peripheral and central inflammation are associated with increased incidence of PND in acute postoperative pain. BMC Anesthesiol. 2021, 21, 79. [Google Scholar] [CrossRef]
- Esme, H.; Kesli, R.; Apiliogullari, B.; Duran, F.M.; Yoldas, B. Effects of flurbiprofen on CRP, TNF-alpha, IL-6, and postoperative pain of thoracotomy. Int. J. Med. Sci. 2011, 8, 216–221. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Muller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Interleukin-6 in surgery, trauma, and critical care part II: Clinical implications. J. Intensive Care Med. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Li, Q.Y.; Xu, H.Y.; Yang, H.J. [Effect of proinflammatory factors TNF-alpha, IL-1beta, IL-6 on neuropathic pain]. Zhongguo Zhong Yao Za Zhi 2017, 42, 3709–3712. [Google Scholar] [CrossRef]
- Zhang, H.; Tai, H.; Ma, Y.; Li, Y.; Dang, Z.; Wang, J.; Zhao, L. Postoperative Serum Levels of Interleukin-1beta (IL-1beta), Interleukin-17 (IL-17), and Tumor Necrosis Factor-alpha (TNF-alpha) in Patients Following Hip Replacement Surgery for Traumatic Fractured Femoral Neck: A Retrospective Study. Med. Sci. Monit. 2019, 25, 6120–6127. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Carroll, C.L.; Huntington, P.J. Body condition scoring and weight estimation of horses. Equine Vet. J. 1988, 20, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Vullo, C.; Carluccio, A.; Robbe, D.; Meligrana, M.; Petrucci, L.; Catone, G. Guaiphenesin-ketamine-xylazine infusion to maintain anesthesia in mules undergoing field castration. Acta Vet. Scand. 2017, 59, 67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maimone, S.; Saffioti, F.; Filomia, R.; Caccamo, G.; Saitta, C.; Pallio, S.; Consolo, P.; Sabatini, S.; Sitajolo, K.; Franze, M.S.; et al. Elective endoscopic variceal ligation is not a risk factor for bacterial infection in patients with liver cirrhosis. Dig Liver Dis 2018, 50, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Pistocchini, E.; Cito, G.; Ritondo, B.L.; Verri, S.; Rogliani, P. Inflammatory and contractile profile in LPS-challenged equine isolated bronchi: Evidence for IL-6 as a potential target against AHR in equine asthma. Pulm. Pharmacol. Ther. 2022, 73-74, 102125. [Google Scholar] [CrossRef] [PubMed]
- White, F.A.; Bhangoo, S.K.; Miller, R.J. Chemokines: Integrators of pain and inflammation. Nat. Rev. Drug Discov. 2005, 4, 834–844. [Google Scholar] [CrossRef]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef]
- Hess, A.; Axmann, R.; Rech, J.; Finzel, S.; Heindl, C.; Kreitz, S.; Sergeeva, M.; Saake, M.; Garcia, M.; Kollias, G.; et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 3731–3736. [Google Scholar] [CrossRef]
- Vazquez, E.; Kahlenbach, J.; von Banchet, G.S.; Konig, C.; Schaible, H.G.; Ebersberger, A. Spinal interleukin-6 is an amplifier of arthritic pain in the rat. Arthritis Rheum. 2012, 64, 2233–2242. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Mancuso, G.; Midiri, A.; Di Paola, R.; Cuzzocrea, S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1beta and IL-18. Biochem. Pharmacol. 2018, 155, 150–161. [Google Scholar] [CrossRef]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef]
- Cunha, F.; Poole, S.; Lorenzetti, B.; Ferreira, S. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992, 107, 660. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221. [Google Scholar] [CrossRef]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef]
| FL | TL | |
|---|---|---|
| TEMP °C | 37.56 ± 0.395 | 37.6 ± 0.793 |
| Anaesthesia time min. | 44.29 ± 6.499 | 45.71 ± 6.395 |
| Surgery time min. | 25.86 ± 4.562 | 26.86 ± 4.980 |
| Recovery time min. | 38.49 ± 5.442 | 39.29 ± 3.638 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vullo, C.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; Gugliandolo, E.; Biondi, V.; Catone, G. Intratesticular Versus Intrafunicular Lidocaine to Reduce Perioperative Nociception and Immunological Response in Ponies Undergoing Field Castration. Vet. Sci. 2022, 9, 664. https://doi.org/10.3390/vetsci9120664
Vullo C, Crupi R, Di Paola R, Cuzzocrea S, Gugliandolo E, Biondi V, Catone G. Intratesticular Versus Intrafunicular Lidocaine to Reduce Perioperative Nociception and Immunological Response in Ponies Undergoing Field Castration. Veterinary Sciences. 2022; 9(12):664. https://doi.org/10.3390/vetsci9120664
Chicago/Turabian StyleVullo, Cecilia, Rosalia Crupi, Rosanna Di Paola, Salvatore Cuzzocrea, Enrico Gugliandolo, Vito Biondi, and Giuseppe Catone. 2022. "Intratesticular Versus Intrafunicular Lidocaine to Reduce Perioperative Nociception and Immunological Response in Ponies Undergoing Field Castration" Veterinary Sciences 9, no. 12: 664. https://doi.org/10.3390/vetsci9120664
APA StyleVullo, C., Crupi, R., Di Paola, R., Cuzzocrea, S., Gugliandolo, E., Biondi, V., & Catone, G. (2022). Intratesticular Versus Intrafunicular Lidocaine to Reduce Perioperative Nociception and Immunological Response in Ponies Undergoing Field Castration. Veterinary Sciences, 9(12), 664. https://doi.org/10.3390/vetsci9120664









