Ovarian Response and Fertility after Short-Term Progestagen/eCG Treatments Are Compromised in Nulliparous Sheep during Non-Breeding Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Ethical Issues, and Experimental Design
2.2. Occurrence and Timing of Estrous Behavior
2.3. Occurrence and Timing of Ovulation
2.4. Ovulation Rate
2.5. Fertility and Prolificacy
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montossi, F.; Font-i-Furnols, M.; Del Campo, M.; San Julián, R.; Brito, G.; Sañudo, C. Sustainable sheep production and consumer preference trends: Compatibilities, contradictions, and unresolved dilemmas. Meat Sci. 2013, 95, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, A. Sustainable food production: The contribution of genome editing in livestock. Sustainability 2021, 13, 6788. [Google Scholar] [CrossRef]
- Redden, R.; Thorne, J.W. Reproductive management of sheep and goats. In Animal Agriculture; Sustainability, Challenges and Innovations; Fuller, W., Bazer, G., Lamb, C., Wu, G., Eds.; Department of Animal Science, Texas A&M University: College Station, TX, USA, 2020; pp. 211–230. [Google Scholar] [CrossRef]
- Rivero, M.J.; Lopez-Villalobos, N.; Evans, A.; Berndt, A.; Cartmill, A.; Neal, A.L.; McLaren, A.; Farruggia, A.; Mignolet, C.; Chadwick, D.; et al. Key traits for ruminant livestock across diverse production systems in the context of climate change: Perspectives from a global platform of research farms. Reprod. Fertil. Dev. 2021, 33, 1–19. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/dairy-production-products/production/dairy-animals/small-ruminants/en/ (accessed on 5 May 2022).
- Amiridis, G.S.; Cseh, S. Assisted reproductive technologies in the reproductive management of small ruminants. Anim. Reprod. Sci. 2012, 130, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.L.; Karsch, F.J.; Olster, D.H.; Ryan, K.D.; Yellon, S.M. Determinants of puberty in a seasonal breeder. In Proceedings of the 1985 Laurentian Hormone Conference; Academic Press: Cambridge, MA, USA, 1986; pp. 331–384. [Google Scholar] [CrossRef]
- Arroyo, J. Estacionalidad reproductiva de la oveja en México. Trop. Subtrop. Agroecosystems 2011, 14, 829–845. Available online: https://www.scielo.org.mx/pdf/tsa/v14n3/v14n3a1 (accessed on 15 February 2022).
- Rosa, H.J.; Bryant, M.J. Seasonality of reproduction in sheep. Small Rumin. Res. 2003, 48, 155–171. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef]
- Kareta, W.; Korman, K.; Cegła, M. Ovulation level and prolificacy in ewes depending on their age, birth type and percentage of prolific genotype. Reprod. Biol. 2006, 6, 73–78. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.640.867&rep=rep1&type= (accessed on 5 May 2022).
- Martemucci, G.; D’Alessandro, A.G. Synchronization of oestrus and ovulation by short time combined FGA, PGF2α, GnRH, eCG treatments for natural service or AI fixed-time. Anim. Reprod. Sci. 2011, 123, 32–39. [Google Scholar] [CrossRef]
- Abecia, J.A.; Forcada, F.; Gonzalez-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef]
- Wildeus, S. Current concepts in synchronization of estrus: Sheep and goats. J. Anim. Sci. 2000, 77, 47–53. [Google Scholar] [CrossRef]
- Menchaca, A.; Miller, V.; Salveraglio, V.; Rubianes, E. Endocrine, luteal and follicular responses after the use of the short-term protocol to synchronize ovulation in goats. J. Anim. Sci. 2007, 102, 76–87. [Google Scholar] [CrossRef]
- Gallegos-Sánchez, J.; Malpaux, B.; Thiéry, J.C. Control of pulsatile LH secretion during seasonal anoestrus in the ewe. Reprod. Nutr. Dev. 1998, 38, 3–15. [Google Scholar] [CrossRef][Green Version]
- Lozano, H.; Raes, M.; Vargas, J.J.; Ballieu, A.; Grajales, H.; Manrique, C.; Beckers, J.F.; Kirschvink, N. Onset of puberty and regularity of oestral cycles in ewe lambs of four breeds under high-altitude conditions in a non-seasonal country. Trop. Anim. Health Prod. 2020, 52, 3395–3402. [Google Scholar] [CrossRef]
- Santos-Jimenez, Z.; Guillen-Gargallo, S.; Encinas, T.; Berlinguer, F.; Veliz-Deras, F.G.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Use of propylene-glycol as a cosolvent for GnRH in synchronization of estrus and ovulation in sheep. Animals 2020, 10, 897. [Google Scholar] [CrossRef]
- Martinez-Ros, P.; Astiz, S.; Garcia-Rosello, E.; Rios-Abellan, A.; Gonzalez-Bulnes, A. Effects of short-term intravaginal progestagens on the onset and features of estrus, preovulatory LH surge and ovulation in sheep. Anim. Reprod. Sci. 2018, 197, 317–323. [Google Scholar] [CrossRef]
- Martinez-Ros, P.; Gonzalez-Bulnes, A.; Garcia-Rosello, E.; Rios-Abellan, A.; Astiz, S. Effects of short-term intravaginal progestagen treatment on fertility and prolificacy after natural breeding in sheep at different reproductive seasons. J. Appl Anim. Res. 2019, 47, 201–205. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Menchaca, A.; Martin, G.B.; Martinez-Ros, P. Seventy years progestagen treatments for management of the sheep oestrous cycle: Where we are and where we should go. Reprod. Fertil. Dev. 2020, 32, 441–452. [Google Scholar] [CrossRef]
- Leboeuf, B.; Manfredi, E.; Boue, P.; Piacere, A.; Brice, G.; Baril, G.; Terqui, M. Artificial insemination of dairy goats in France. Livest. Prod. Sci. 1998, 55, 193–203. [Google Scholar] [CrossRef]
- Joseph, I.B.J.K.; Currie, W.D.; Rawlings, N.C. Effects of time after ovariectomy, season and oestradiol on luteinizing hormone and follicle-stimulating hormone secretion in ovariectomized ewes. J. Reprod. Fertil. 1992, 94, 511–523. [Google Scholar] [CrossRef]
- Smith, J.T.; Clarke, I.J. Seasonal breeding as a neuroendocrine model for puberty in sheep. Mol. Cell. Endocrinol. 2010, 324, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J. Gonadotrophin-releasing hormone secretion (GnRH) in anoestrous ewes and the induction of GnRH surges by oestrogen. Endocrinology 1988, 117, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Nys, C.; Kendrick, K.M.; Scaramuzzi, R.J. The “ram effect”: New insights into neural modulation of the gonadotropic axis by male odors and socio-sexual interactions. Front. Neurosci. 2015, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Bedenbaugh, M.N.; Bowdridge, E.C.; Hileman, S.M. Role of neurokinin B in ovine puberty. Domest. Anim. Endocrinol. 2020, 73, 106442. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.A. Reproduction in the ewe and the goat. The estrous cycle. In Reproduction in Domestic Animals, 3rd ed.; Cole, H.H., Cupps, P.T., Eds.; Academic Press: Cambridge, MA, USA, 1977; pp. 410, 475–498. [Google Scholar]
- Skinner, D.C.; Caraty, A.; Allingham, R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: No colocalization with gonadotropin-releasing neurons. Endocrinology 2001, 142, 573–579. [Google Scholar] [CrossRef]
- Robinson, J.E. Gamma amino-butyric acid and the control of GnRH secretion in sheep. J. Reprod. Fert. 1995, 49, 221–230. Available online: https://www.biosciproceedings.org/bp/0003/bp0003rdr17.pdf (accessed on 10 November 2022). [CrossRef]
- Viñoles, C.; Forsberg, M.; Rubianes, E. Ovarian follicular dynamics during the estrous cycle in the ewe. In Proceedings of the 14th International Congress on Animal Reproduction, Stockholm, Sweden, 2–6 July 2000; Volume 1, p. 26. [Google Scholar]
- Fleisch, A.; Piechotta, M.; Bollwein, H.; Janett, F. Fruchtbarkeit nach 6-und 12-tägiger Behandlung mit Eazi-breed™ CIDR® G ausserhalb der Zuchtsaison beim Lacaune Milchschaf. Schweiz. Arch. Tierheilkd. 2013, 155, 391–398. [Google Scholar] [CrossRef][Green Version]
- NAM-National Academy of Medicine. Co-Produced by the National Academy of Medicine–Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International. In Guide for the Care and Use of Laboratory Animals, 1st ed.; Harlan: Mexico City, Mexico, 2010. [Google Scholar]
- FASS. Guide for the Care and Use of Agricultural Animals. In Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Pallares, P.; Vazquez, M.I. Ultrasonographic imaging in small ruminant reproduction. Reprod. Domest. Anim. 2010, 45, 9–20. [Google Scholar] [CrossRef]
- Santos-Jimenez, Z.; Meza-Herrera, C.A.; Calderon-Leyva, G.; Martinez-Ros, P.; Guillen-Muñoz, J.M.; Gonzalez-Bulnes, A. Efficiency of hCG for Inducing Resumption of Ovarian Cyclicity and Synchronized Ovulations during the Seasonal Anestrous in Sheep. Animals 2021, 11, 3159. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Encinas, T.; McNeilly, A.S.; Gonzalez-Bulnes, A. Timing of preovulatory LH surge and ovulation in superovulated sheep are affected by follicular status at start of the FSH treatment. Reprod. Domest. Anim. 2008, 43, 92–98. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Rubianes, E. Effectiveness of short-term progestogen primings for the induction of fertile oestrus with eCG in ewes during late seasonal anoestrus. Anim. Sci. J. 1999, 68, 349–353. [Google Scholar] [CrossRef]
- Swelum, A.A.A.; Saadeldin, I.M.; Moumen, A.F.; Ali, M.A.; Alowaimer, A.N. Efficacy of controlled internal drug release (CIDR) treatment durations on the reproductive performance, hormone profiles, and economic profit of Awassi ewes. Small Rumin. Res. 2018, 166, 47–52. [Google Scholar] [CrossRef]
- Barrett, D.M.W.; Bartlewski, P.M.; Batista-Arteaga, M.; Symington, A.; Rawlings, N.C. Ultrasound and endocrine evaluation of the ovarian response to a single dose of 500 IU of eCG following a 12-day treatment with progestogen-releasing intravaginal sponges in the breeding and nonbreeding seasons in ewes. Theriogenology 2004, 61, 311–327. [Google Scholar] [CrossRef]
- Chemineau, P.; Pellicer-Rubio, M.T.; Lassoued, N.; Khaldi, G.; Monniaux, D. Male-induced short oestrous and ovarian cycles in sheep and goats: A working hypothesis. Reprod. Nutr. Dev. 2006, 46, 417–429. [Google Scholar] [CrossRef]
- Iglesias, R.R.; Ciccioli, N.H.; Ferrería, J.; Pevsner, D.A.; Rosas, C.A.; Rodríguez, M.M.; Pedrueza, J.R. Short-lived corpora lutea syndrome in anoestrous ewes following 17β-oestradiol or MAP treatments applied before an allogenic sexual stimulation with rams and oestrous ewes. Anim. Reprod. Sci. 2013, 136, 268–279. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Beard, A.P.; Cook, S.J.; Rawlings, N.C. Ovarian follicular dynamics during anoestrus in ewes. Reproduction 1998, 113, 275–285. [Google Scholar] [CrossRef]
- Baird, D.T. Factors regulating the growth of the preovulatory follicle in sheep and human. J. Reprod. Infertil. 1983, 69, 343–352. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Beard, A.P.; Cook, S.J.; Chandolia, R.K.; Honaramooz, A.; Rawlings, N.C. Ovarian antral follicular dynamics and their relationships with endocrine variables throughout the oestrous cycle in breeds of sheep differing in prolificacy. Reproduction 1999, 115, 111–124. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Veiga-Lopez, A.; Garcia, P.; Garcia-Garcia, R.M.; Ariznavarreta, C.; Sanchez, M.A.; Tresguerres, J.A.F.; Cocero, M.J.; Flores, J.M. Effects of progestagens and prostaglandin analogues on ovarian function and embryo viability in sheep. Theriogenology 2005, 63, 2523–2534. [Google Scholar] [CrossRef]
- Mulvaney, F.J.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; West, D.M.; Vinoles, C.; Glover, K.M.M. Comparison between the reproductive performance of ewe hoggets and mature ewes following a progesterone-based oestrus synchronization protocol. New Zealand J. Agric. Res. 2013, 56, 288–296. [Google Scholar] [CrossRef][Green Version]
- Martinez, M.F.; McLeod, B.; Tattersfield, G.; Smaill, B.; Quirke, L.D.; Juengel, J.L. Successful induction of oestrus, ovulation and pregnancy in adult ewes and ewe lambs out of the breeding season using a GnRH+ progesterone oestrus synchronization protocol. Anim. Reprod. Sci. 2015, 155, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Viguié, C.; Skinner, D.C.; Thiéry, J.C.; Chemineau, P. Control of the circannual rhythm of reproduction by melatonin in the ewe. Brain Res. Bull. 1997, 44, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J. Applied Animal Endocrinology; Cabi Publishing: Wallingford, UK, 2010. [Google Scholar]
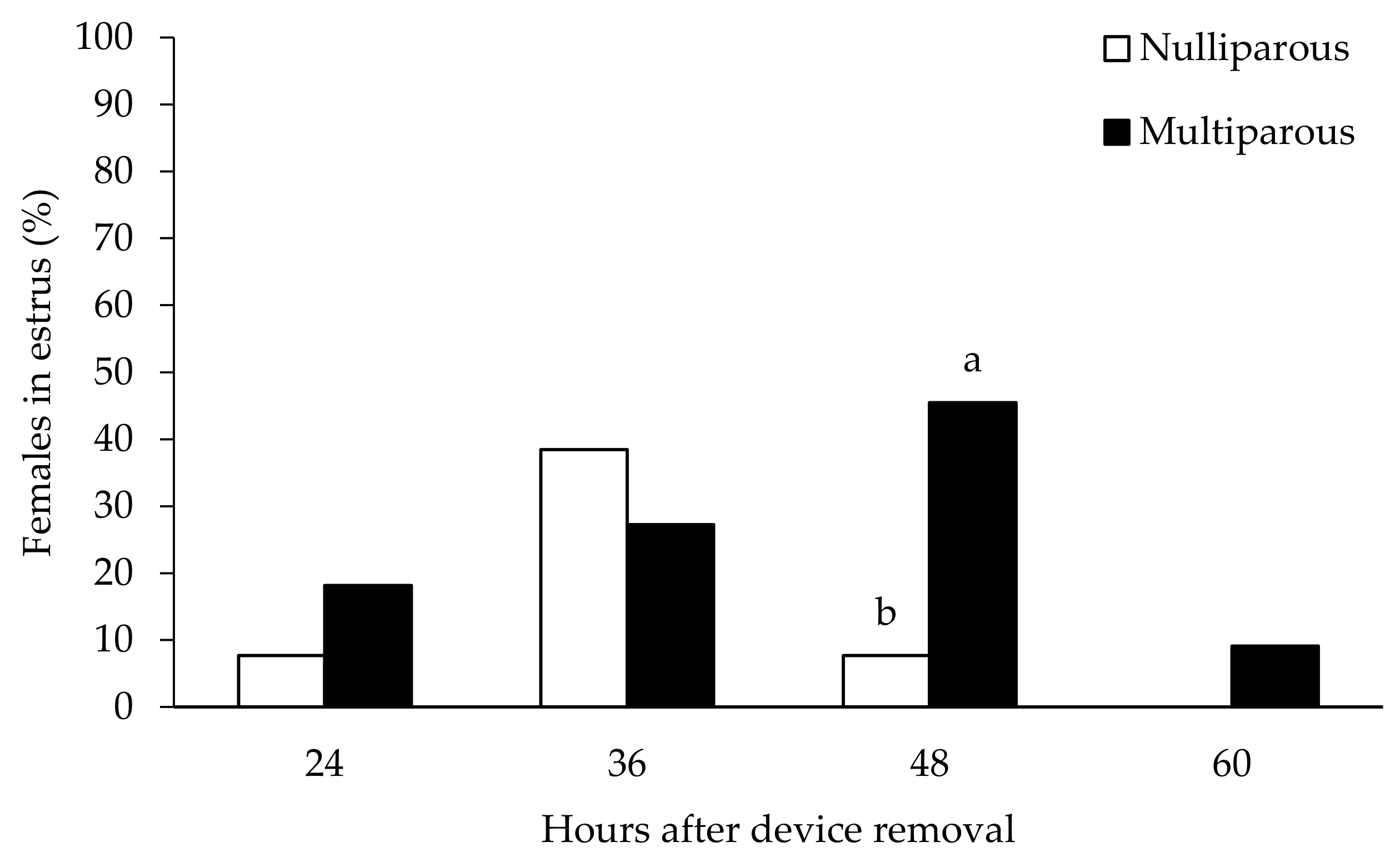
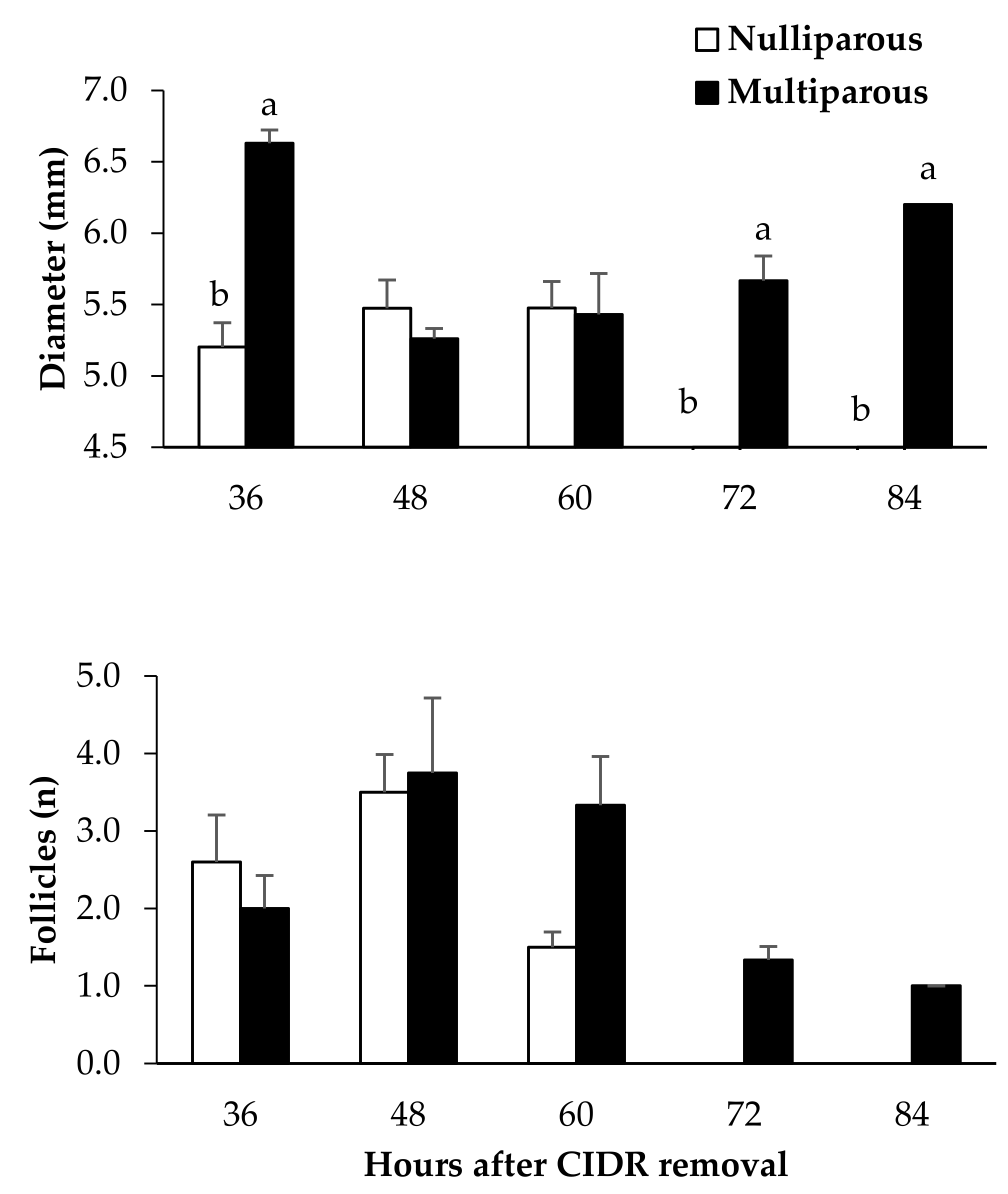
| Multiparous | Nulliparous | |
|---|---|---|
| Occurrence of estrous behavior (%) | 100 (11/11) a | 53.9 (7/13) b |
| Estrus duration (h) | 29.5 ± 4.08 | 24.0 ± 2.52 |
| Time of onset of estrus after CIDR removal (h) | 41.5 ± 3.4 | 36.0 ± 1.9 |
| Variables Evaluated | Multiparous | Nulliparous |
|---|---|---|
| Occurrence of ovulation (%) | 81.8 (9/11) | 100 (13/13) |
| Time of ovulation after CIDR removal (h) | 70.9 ± 3.0 | 63.4 ± 1.6 |
| Ovulation rate (number of corpora lutea) | 1.7 ± 0.3 | 2.2 ± 0.2 |
| Diameter CL (mm) | 12.31 ± 0.5 a | 11.96 ± 0.28 b |
| Fertility rate with regard to ewes treated (%) | 72.7 (8/11) a | 30.77 (4/13) b |
| Fertility rate with regard to ewes estrus (%) | 72.7 (8/11) | 57.1 (4/7) |
| Fertility rate with regard to ewes ovulating (%) | 88.9 (8/9) a | 30.77 (4/13) b |
| Numbers of embryos | 1.6 ± 0.2 | 1.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Jimenez, Z.; Martínez-Ros, P.; Encinas, T.; Morales-Cruz, J.L.; Guerrero-Gallegos, H.Z.; Gonzalez-Avalos, R.; Gonzalez-Bulnes, A.; Guillen-Muñoz, J.M. Ovarian Response and Fertility after Short-Term Progestagen/eCG Treatments Are Compromised in Nulliparous Sheep during Non-Breeding Season. Vet. Sci. 2022, 9, 663. https://doi.org/10.3390/vetsci9120663
Santos-Jimenez Z, Martínez-Ros P, Encinas T, Morales-Cruz JL, Guerrero-Gallegos HZ, Gonzalez-Avalos R, Gonzalez-Bulnes A, Guillen-Muñoz JM. Ovarian Response and Fertility after Short-Term Progestagen/eCG Treatments Are Compromised in Nulliparous Sheep during Non-Breeding Season. Veterinary Sciences. 2022; 9(12):663. https://doi.org/10.3390/vetsci9120663
Chicago/Turabian StyleSantos-Jimenez, Zurisaday, Paula Martínez-Ros, Teresa Encinas, Juan Luis Morales-Cruz, Hugo Zuriel Guerrero-Gallegos, Ramiro Gonzalez-Avalos, Antonio Gonzalez-Bulnes, and Juan Manuel Guillen-Muñoz. 2022. "Ovarian Response and Fertility after Short-Term Progestagen/eCG Treatments Are Compromised in Nulliparous Sheep during Non-Breeding Season" Veterinary Sciences 9, no. 12: 663. https://doi.org/10.3390/vetsci9120663
APA StyleSantos-Jimenez, Z., Martínez-Ros, P., Encinas, T., Morales-Cruz, J. L., Guerrero-Gallegos, H. Z., Gonzalez-Avalos, R., Gonzalez-Bulnes, A., & Guillen-Muñoz, J. M. (2022). Ovarian Response and Fertility after Short-Term Progestagen/eCG Treatments Are Compromised in Nulliparous Sheep during Non-Breeding Season. Veterinary Sciences, 9(12), 663. https://doi.org/10.3390/vetsci9120663







