Less Typical Courses of Rhodococcus equi Infections in Foals
Simple Summary
Abstract
1. Introduction
2. Clinical Cases: History and Examination Results
2.1. Clinical Approach
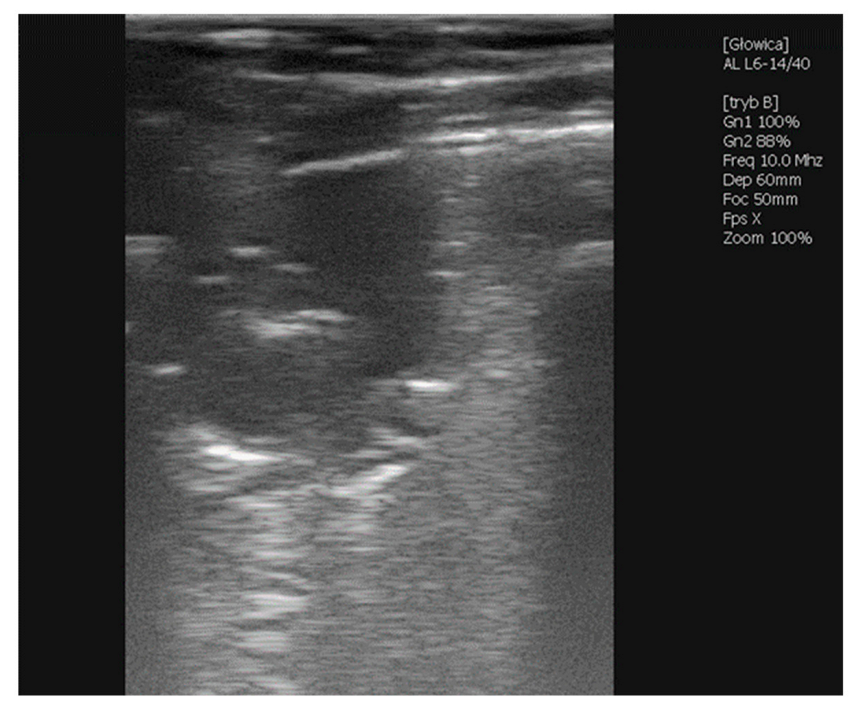
2.1.1. Case 1—Premature Foal
2.1.2. Case 2—Problematic Bloodline
2.1.3. Case 2.1
Dam 2 Gave Birth to a Healthy Colt at the End of January 2020
2.1.4. Case 2.2
Dam 1 Gave Birth to a Healthy Filly at the Beginning of June 2020
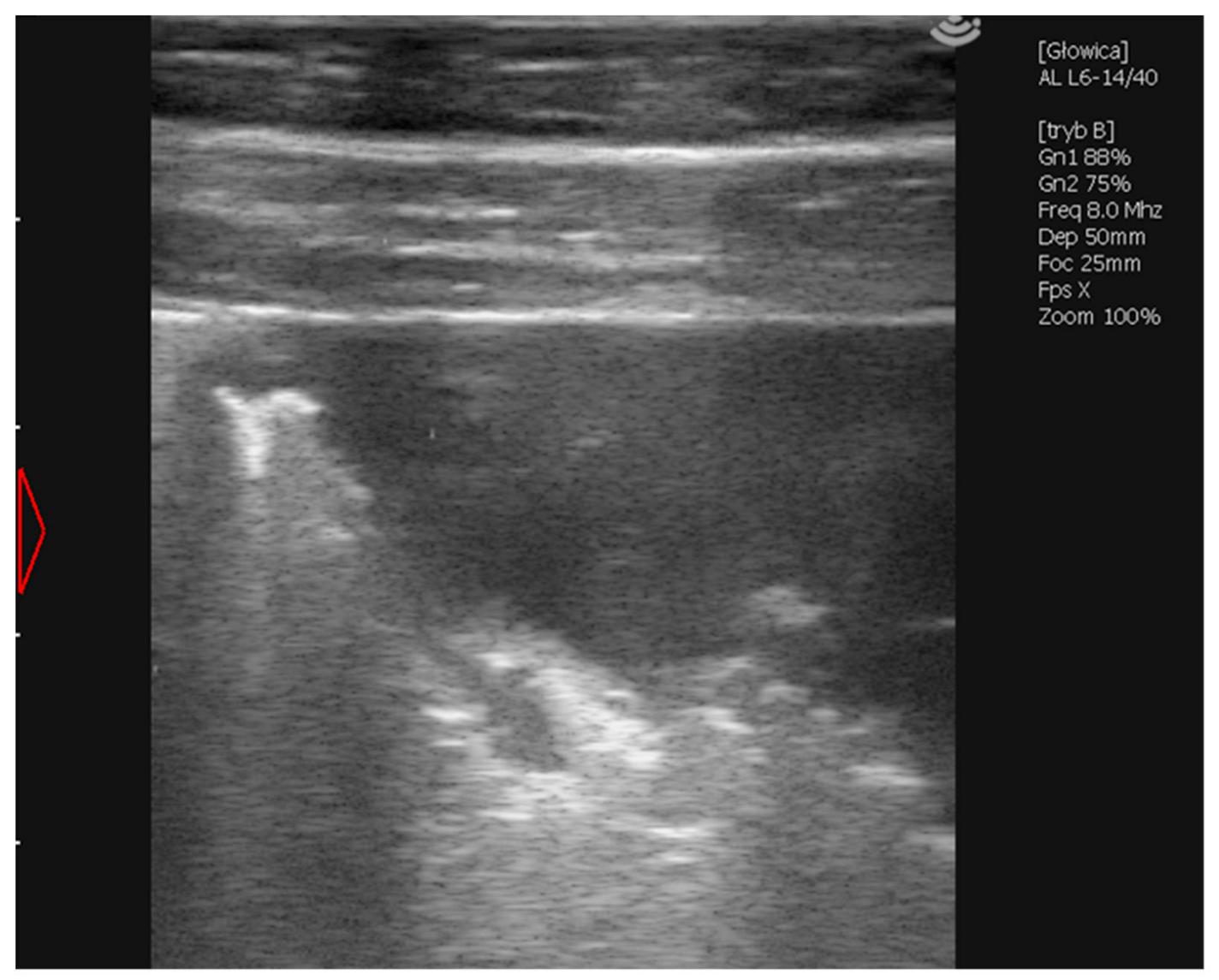
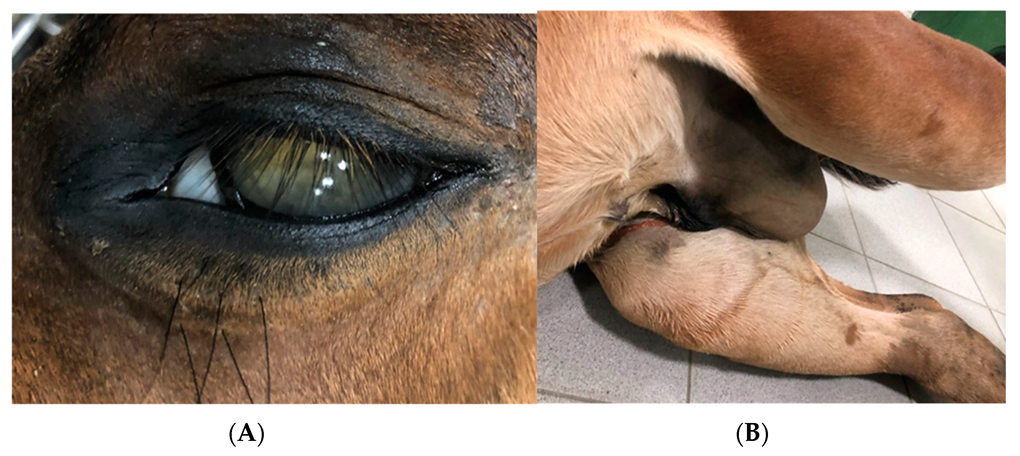
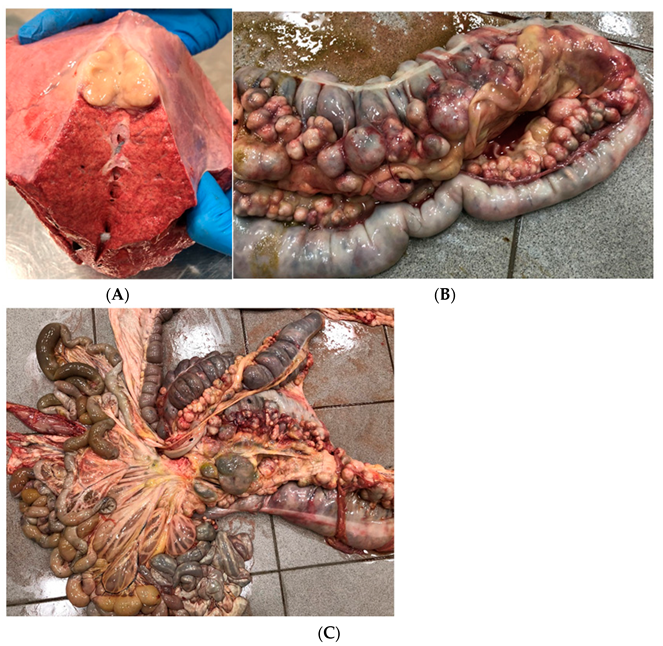
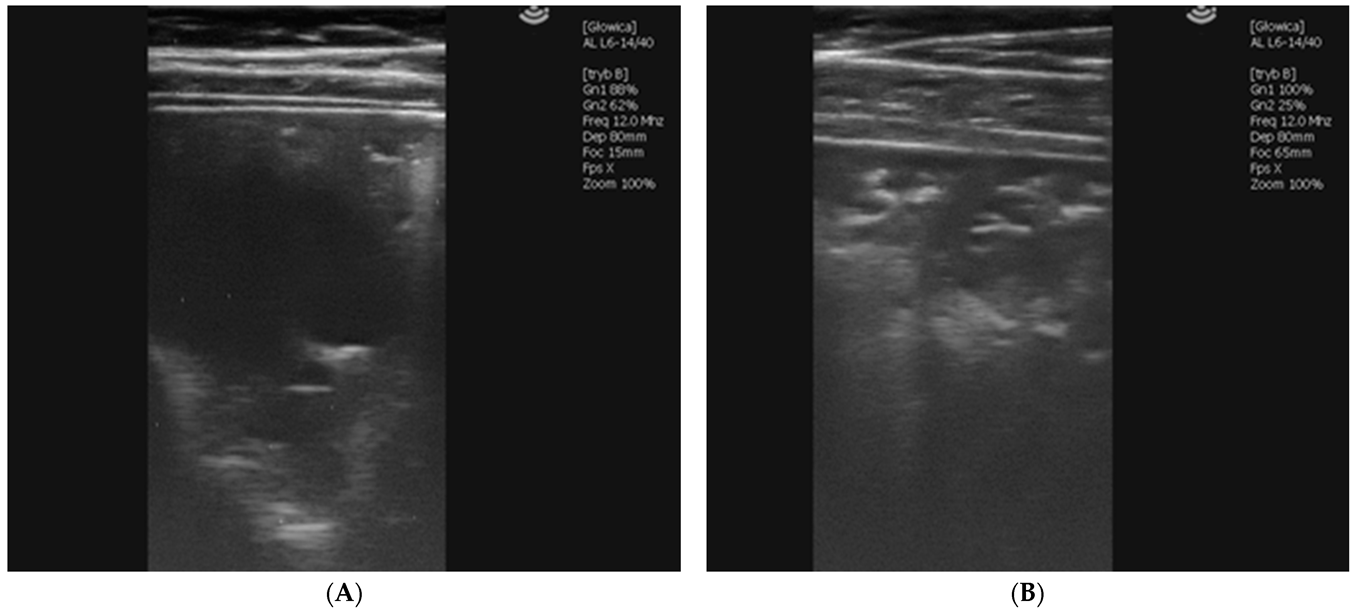
2.1.5. Case 3—Suspicion of Extrapulmonary Disorders
2.1.6. Case 4—Drug-Induced Hyperthermia
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giguère, S.; Cohen, N.D.; Keith Chaffin, M.; Slovis, N.M.; Hondalus, M.K.; Hines, S.A.; Prescott, J.F. Diagnosis, Treatment, Control, and Prevention of Infections Caused by Rhodococcus equi in Foals. J. Vet. Intern. Med. 2011, 25, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.C.; Jackson, K.A.; Watson, J.L. Identification of Immunologically Relevant Genes in Mare and Foal Dendritic Cells Responding to Infection by Rhodococcus equi. Vet. Immunol. Immunopathol. 2010, 136, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.R.M.Y.; Horohov, D.W.; Meijer, W.G.; Muscatello, G. Current Understanding of the Equine Immune Response to Rhodococcus equi. An Immunological Review of R. equi Pneumonia. Vet. Immunol. Immunopathol. 2010, 135, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muscatello, G.; Leadon, D.P.; Klay, M.; Ocampo-Sosa, A.; Lewis, D.A.; Fogarty, U.; Buckley, T.; Gilkerson, J.R.; Meijer, W.G.; Vazquez-Boland, J.A. Rhodococcus equi Infection in Foals: The Science of “Rattles”. Equine Vet. J. 2007, 39, 470–478. [Google Scholar] [CrossRef]
- Witkowski, L.; Kaba, J.; Rzewuska, M.; Nowicki, M.; Szaluś-Jordanow, O.; Kita, J. Development of ELISA Test for Determination of the Level of Antibodies against Rhodococcus equi in Equine Serum and Colostrum. Vet. Immunol. Immunopathol. 2012, 149, 280–285. [Google Scholar] [CrossRef]
- Tarancón, I.; Leiva, M.; Jose-Cunilleras, E.; Ríos, J.; Peña, T. Ophthalmologic Findings Associated with Rhodococcus equi Bronchopneumonia in Foals. Vet. Ophthalmol. 2019, 22, 660–665. [Google Scholar] [CrossRef]
- Reuss, S.M.; Chaffin, M.K.; Schmitz, D.G.; Norman, T.E. Sonographic Characteristics of Intraabdominal Abscessation and Lymphadenopathy Attributable to Rhodococcus equi Infections in Foals. Vet. Radiol. Ultrasound 2011, 52, 462–465. [Google Scholar] [CrossRef]
- Reuss, S.M. Rhodococcus equi, Extrapulmonary Disorders and Lack of Response to Therapy. Equine Vet. Educ. 2021, 34, 399–400. [Google Scholar] [CrossRef]
- Morresey, P.R.; Garrett, K.S.; Carter, D. Rhodococcus equi Occipital Bone Osteomyelitis, Septic Arthritis and Meningitis in a Neurological Foal. Equine Vet. Educ. 2011, 23, 398–402. [Google Scholar] [CrossRef]
- Lin, W.V.; Kruse, R.L.; Yang, K.; Musher, D.M. Diagnosis and Management of Pulmonary Infection Due to Rhodococcus equi. Clin. Microbiol. Infect. 2019, 25, 310–315. [Google Scholar] [CrossRef]
- Giguére, S.; Jacks, S.; Roberts, G.D.; Hernandez, J.; Long, M.T.; Ellis, C. Retrospective Comparison of Azithromycin, Clarithromycin, and Erythromycin for the Treatment of Foals with Rhodococcus equi Pneumonia. J. Vet. Intern. Med. 2004, 18, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wetzig, M.; Venner, M.; Giguère, S. Efficacy of the Combination of Doxycycline and Azithromycin for the Treatment of Foals with Mild to Moderate Bronchopneumonia. Equine Vet. J. 2019, 52, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Rutenberg, D.; Venner, M.; Giguère, S. Efficacy of Tulathromycin for the Treatment of Foals with Mild to Moderate Bronchopneumonia. J. Vet. Intern. Med. 2017, 31, 901–906. [Google Scholar] [CrossRef]
- Hildebrand, F.; Venner, M.; Giguère, S. Efficacy of Gamithromycin for the Treatment of Foals with Mild to Moderate Bronchopneumonia. J. Vet. Intern. Med. 2015, 29, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Leclere, M.; Magdesian, K.G.; Kass, P.H.; Pusterla, N.; Rhodes, D.M. Comparison of the Clinical, Microbiological, Radiological and Haematological Features of Foals with Pneumonia Caused by Rhodococcus equi and Other Bacteria. Vet. J. 2011, 187, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.V.; Mello, L.S.; Ribeiro, P.R.; Wentz, M.F.; Stolf, A.S.; Lopes, B.C.; de Andrade, C.P.; Snel, G.G.M.; Sonne, L.; Driemeier, D.; et al. Causes and Pathology of Equine Pneumonia and Pleuritis in Southern Brazil. J. Comp. Pathol. 2020, 179, 65–73. [Google Scholar] [CrossRef]
- McCracken, J.L.; Slovis, N.M. Use of Thoracic Ultrasound for the Prevention of Rhodococcus equi Pneumonia on Endemic Farms. Proc. Am. Assoc. Equine Pract. 2009, 55, 38–44. [Google Scholar]
- Huber, L.; Gressler, L.T.; Sanz, M.G.; Garbade, P.; Vargas, Á.; Silveira, B.P. Monitoring Foals by Thoracic Ultrasonography, Bacterial Culture, and PCR: Diagnostic of Rhodococcus equi Subclinical Pneumonia in South of Brazil. J. Equine Vet. Sci. 2018, 60, 104–108.e1. [Google Scholar] [CrossRef]
- Giguère, S.; Roberts, G.D. Association between Radiographic Pattern and Outcome in Foals with Pneumonia Caused by Rhodococcus equi. Vet. Radiol. Ultrasound 2012, 53, 601–604. [Google Scholar] [CrossRef]
- Venner, M.; Walther, S.M.; Münzer, B.; Stadler, P. Diagnostic of Pulmonary Abscesses in Foals-Comparison of Sonographic and Radiographic Examination. Pferdeheilkunde 2014, 30, 561–566. [Google Scholar] [CrossRef]
- Łobaczewski, A.; Czopowicz, M.; Moroz, A.; Mickiewicz, M.; Stabińska, M.; Petelicka, H.; Frymus, T.; Szaluś-Jordanow, O. Lung Ultrasound for Imaging of B-Lines in Dogs and Cats—A Prospective Study Investigating Agreement between Three Types of Transducers and the Accuracy in Diagnosing Cardiogenic Pulmonary Edema, Pneumonia and Lung Neoplasia. Animals 2021, 11, 3279. [Google Scholar] [CrossRef] [PubMed]
- Arnold-Lehna, D.; Venner, M.; Berghaus, L.J.; Berghaus, R.; Giguère, S. Changing Policy to Treat Foals with Rhodococcus equi Pneumonia in the Later Course of Disease Decreases Antimicrobial Usage without Increasing Mortality Rate. Equine Vet. J. 2020, 52, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Cauchard, S.; Giguère, S.; Venner, M.; Muscatello, G.; Cauchard, J.; Cohen, N.D.; Haas, A.; Hines, S.A.; Hondalus, M.K.; Horohov, D.W.; et al. Rhodococcus equi Research 2008-2012: Report of the Fifth International Havemeyer Workshop. Equine Vet. J. 2013, 45, 523–526. [Google Scholar] [CrossRef]
- Slovis, N.M.; McAuliffe, S.B. Color Atlas of Diseases and Disorders of the Foal; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780702028106. [Google Scholar]
- Reuss, S.M.; Chaffin, M.K.; Cohen, N.D. Extrapulmonary Disorders Associated with Rhodococcus equi Infection in Foals: 150 Cases (1987–2007). J. Am. Vet. Med. Assoc. 2009, 235, 855–863. [Google Scholar] [CrossRef]
- Wilkes, E.J.A.; Hughes, K.J.; Kessell, A.E.; Raidal, S.L. Successful Management of Multiple Extrapulmonary Complications Associated with Rhodococcus equi Pneumonia in a Foal. Equine Vet. Educ. 2016, 28, 186–192. [Google Scholar] [CrossRef]
- le Corre, S.; Janes, J.; Slovis, N.M. Multiple Extra-Pulmonary Disorders Associated with Rhodococcus equi Infection in a 2-Month-Old Foal. Equine Vet. Educ. 2021, 33, 396. [Google Scholar] [CrossRef]
- Kalinowski, M.; Jarosz, Ł.; Grądzki, Z. Assessment of Antimicrobial Susceptibility of Virulent Strains of Rhodococcus equi Isolated From Foals and Soil of Horse Breeding Farms With and Without Endemic Infections. J. Equine Vet. Sci. 2020, 91, 103114. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Giguère, S.; Slovis, N.M.; Carter, C.N.; Barr, B.S.; Cohen, N.D.; Elam, J.; Erol, E.; Locke, S.J.; Phillips, E.D.; et al. Emergence of Resistance to Macrolides and Rifampin in Clinical Isolates of Rhodococcus equi from Foals in Central Kentucky, 1995 to 2017. Antimicrob. Agents Chemother. 2018, 63. [Google Scholar] [CrossRef]
- Huber, L.; Giguère, S.; Cohen, N.D.; Slovis, N.M.; Hanafi, A.; Schuckert, A.; Berghaus, L.; Greiter, M.; Hart, K.A. Prevalence and Risk Factors Associated with Emergence of Rhodococcus equi Resistance to Macrolides and Rifampicin in Horse-Breeding Farms in Kentucky, USA. Vet. Microbiol. 2019, 235, 243–247. [Google Scholar] [CrossRef]
- Burton, A.J.; Giguère, S.; Sturgill, T.L.; Berghaus, L.J.; Slovis, N.M.; Whitman, J.L.; Levering, C.; Kuskie, K.R.; Cohen, N.D. Macrolide- and Rifampin-Resistant Rhodococcus equi on a Horse Breeding Farm, Kentucky, USA. Emerg. Infect. Dis. 2013, 19, 282–285. [Google Scholar] [CrossRef]
- Noah Cohen, B.D. Rhodococcus equi Pneumonia in Foals: An Update on Epidemiology, Diagnosis, Treatment, and Prevention. AAEP 2021. Available online: https://aaep.org/horsehealth/rhodococcus-equi-pneumonia-foals-update-epidemiology-diagnosis-treatment-and-prevention (accessed on 10 September 2022).
- Stieler, A.L.; Sanchez, L.C.; Mallicote, M.F.; Martabano, B.B.; Burrow, J.A.; MacKay, R.J. Macrolide-Induced Hyperthermia in Foals: Role of Impaired Sweat Responses. Equine Vet. J. 2016, 48, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Patterson Rosa, L.; Mallicote, M.F.; MacKay, R.J.; Brooks, S.A. Ion Channel and Ubiquitin Differential Expression during Erythromycin-Induced Anhidrosis in Foals. Animals 2021, 11, 3379. [Google Scholar] [CrossRef] [PubMed]
- Mcqueen, C.M.; Dindot, S.V.; Foster, M.J.; Cohen, N.D. Genetic Susceptibility to Rhodococcus equi. J. Vet. Intern. Med. 2015, 29, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Giguère, S.; Berghaus, L.J.; Hanafi, A.; Ryan, C. Fecal Shedding of Rhodococcus equi in Mares and Foals after Experimental Infection of Foals and Effect of Composting on Concentrations of R. equi in Contaminated Bedding. Vet. Microbiol. 2018, 223, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.D.; Chaffin, M.K.; Kuskie, K.R.; Syndergaard, M.K.; Blodgett, G.P.; Takai, S. Association of Perinatal Exposure to Airborne Rhodococcus equi with Risk of Pneumonia Caused by R Equi in Foals. Am. J. Vet. Res. 2013, 74, 102–109. [Google Scholar] [CrossRef]
- Madrigal, R.G.; Shaw, S.D.; Witkowski, L.A.; Sisson, B.E.; Blodgett, G.P.; Chaffin, M.K.; Cohen, N.D. Use of Serial Quantitative PCR of the VapA Gene of Rhodococcus equi in Feces for Early Detection of R. equi Pneumonia in Foals. J. Vet. Intern. Med. 2016, 30, 664–670. [Google Scholar] [CrossRef]
- Nordengrahn, A.; Rusvai, M.; Merza, M.; Ekstrijm, J.; Morein, B.; Bela, S. Equine Herpesvirus Type 2 (EHV-2) as a Predisposing Factor for Rhodococcus equi Pneumonia in Foals: Prevention of the Bifactorial Disease with EHV-2 Immunostimulating Complexes. Vet. Microbiol. 1996, 51, 55–68. [Google Scholar] [CrossRef]
- Perez-Ecija, A.; Mendoza, F.J.; Estepa, J.C.; Bautista, M.J.; Pérez, J. Equid Herpesvirus 1 and Rhodococcus equi Coinfection in a Foal with Bronchointerstitial Pneumonia. J. Vet. Med. Sci. 2016, 78, 1511–1513. [Google Scholar] [CrossRef][Green Version]
- Stewart, A.; Sowden, D.; Caffery, M.; Bint, M.; Broom, J. Rhodococcus equi Infection: A Diverse Spectrum of Disease. IDCases 2019, 15, e00487. [Google Scholar] [CrossRef]
- Zychska, M.; Witkowski, L.; Klementowska, A.; Rzewuska, M.; Kwiecien, E.; Stefanska, I.; Czopowicz, M.; Szalus-Jordanow, O.; Mickiewicz, M.; Moroz, A.; et al. Rhodococcus equi—Occurrence in Goats and Clinical Case Report. Pathogens 2021, 10, 1141. [Google Scholar] [CrossRef]
- Bordin, A.I.; Gressler, L.T.; Alexander, E.R.C.; Sule, P.; Cirillo, J.D.; Edwards, J.F.; Cohen, N.D. Guinea Pig Infection with the Intracellular Pathogen Rhodococcus equi. Vet. Microbiol. 2018, 215, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kubota, H.; Madarame, H.; Takase, F.; Takahashi, K.; Sasaki, Y.; Kakuda, T.; Takai, S. Pathogenicity and Genomic Features of VapN-Harboring Rhodococcus equi Isolated from Human Patients. Int. J. Med. Microbiol. 2021, 311, 151519. [Google Scholar] [CrossRef] [PubMed]
- Salmuna, Z.N.; Azim, W.A.W.A.; Harun, A. Rhodococcus equi Pulmonary Infection in an Immunocompromised Patient: Case Report and Literature Review. Clin. Microbiol. Newsl. 2018, 40, 128–130. [Google Scholar] [CrossRef]
- Boy, M.G.; Zhang, C.; Antczak, D.F.; Hamir, A.N.; Whitlock, R.H.; Baker, J.A. Unusual Selective Immunoglobulin Deficiency in an Arabian-Foal. J. Vet. Intern. Med. 1992, 6, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Weldon, A.D.; Zhang, C.; Antczak, D.F.; Rebhun, W.C. Selective IgM Deficiency and Abnormal B-Cell Response in a Foal. J. Am. Vet. Med. Assoc. 1992, 201, 1396–1398. [Google Scholar] [PubMed]
- Taintor, J.; Schleis, S. Equine Lymphoma. Equine Vet. Educ. 2011, 23, 205–213. [Google Scholar] [CrossRef]
- Perkins, G.A.; Nydam, D.V.; Flaminio, M.J.B.F.; Ainsworth, D.M. Serum IgM Concentrations in Normal, Fit Horses and Horses with Lymphoma or Other Medical Conditions. J. Vet. Intern. Med. 2003, 17, 337–342. [Google Scholar] [CrossRef]
| Horse | Results from the Beginning of May 2020 | Results from the End of August 2020 | |||
|---|---|---|---|---|---|
| IgG (Ref. Value 500–2000) | IgM (Ref. Value 90–150) | IgG (Ref. Value 500–2000) | IgM (Ref. Value 90–150) | IgA (Ref. Value 60–130) | |
| Dam 1 | 1480 mg/dL | 340.1 mg/dL | 2190 mg/dL | 272.3 mg/dL | 55.4 mg/dL |
| Filly from Dam 1 | 630 mg/dL | 4.5 mg/dL | 101.9 mg/dL | ||
| Dam 2 * | 1310 mg/dL | 34.8 mg/dL | |||
| Colt from Dam 2 | 1390 mg/dL | 14.2 mg/dL | |||
| RBC | MCV | HCT | PLT | MPV | WBC | HGB | MCH | MCHC | LYMF | GRAN | MID | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. value | 6.5–9.99 | 38–53 | 33–48 | 115–450 | - | 5.0–12.6 | 11–16 | 12–16 | 31–37 | 1.4–2.3 | 2.75–8.19 | - |
| Case 4 | 9.96 | 32 | 31.9 | 360 | 7.3 | 29.7 | 20.7 | 20.8 | 64.8 | 4.6 | 23.5 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakowska, A.; Marciniak-Karcz, A.; Bereznowski, A.; Cywińska, A.; Żychska, M.; Witkowski, L. Less Typical Courses of Rhodococcus equi Infections in Foals. Vet. Sci. 2022, 9, 605. https://doi.org/10.3390/vetsci9110605
Rakowska A, Marciniak-Karcz A, Bereznowski A, Cywińska A, Żychska M, Witkowski L. Less Typical Courses of Rhodococcus equi Infections in Foals. Veterinary Sciences. 2022; 9(11):605. https://doi.org/10.3390/vetsci9110605
Chicago/Turabian StyleRakowska, Alicja, Agnieszka Marciniak-Karcz, Andrzej Bereznowski, Anna Cywińska, Monika Żychska, and Lucjan Witkowski. 2022. "Less Typical Courses of Rhodococcus equi Infections in Foals" Veterinary Sciences 9, no. 11: 605. https://doi.org/10.3390/vetsci9110605
APA StyleRakowska, A., Marciniak-Karcz, A., Bereznowski, A., Cywińska, A., Żychska, M., & Witkowski, L. (2022). Less Typical Courses of Rhodococcus equi Infections in Foals. Veterinary Sciences, 9(11), 605. https://doi.org/10.3390/vetsci9110605





