Use of Transversus Abdominis Plane and Intercostal Blocks in Bitches Undergoing Laparoscopic Ovariectomy: A Randomized Controlled Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. TAP Group
2.2. FEN Group
2.3. Statistical Analysis
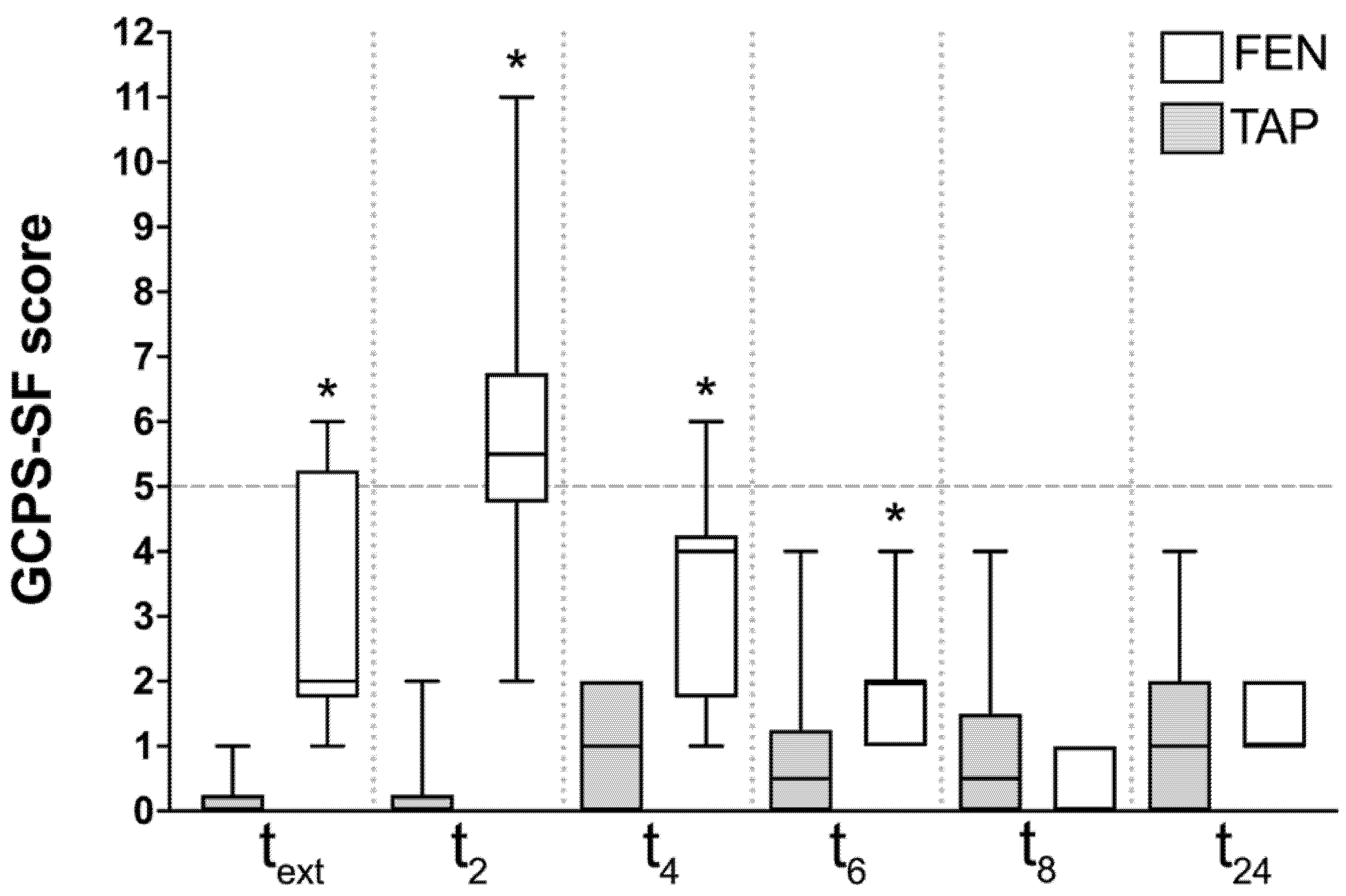
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lascelles, B.D.X.; Kirkby Shaw, K. An extended release local anaesthetic: Potential for future use in veterinary surgical patients? Vet. Med. Sci. 2016, 2, 229–238. [Google Scholar] [CrossRef]
- Grubb, T.; Lobprise, H. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef]
- Fozzard, H.; Lee, P.; Lipkind, G. Mechanism of Local Anesthetic Drug Action on Voltage-Gated Sodium Channels. CPD 2005, 11, 2671–2686. [Google Scholar] [CrossRef]
- Becker, D.E.; Reed, K.L. Local Anesthetics: Review of Pharmacological Considerations. Anesth. Prog. 2012, 59, 90–102. [Google Scholar] [CrossRef]
- Usunoff, K.G.; Popratiloff, A.; Schmitt, O.; Wree, A. Functional neuroanatomy of pain. Adv. Anat. Embryol. Cell Biol. 2006, 184, 1–115. [Google Scholar]
- Romano, M.; Portela, D.A.; Breghi, G.; Otero, P.E. Stress-related biomarkers in dogs administered regional anaesthesia or fentanyl for analgesia during stifle surgery. Vet. Anaesth. Analg. 2016, 43, 44–54. [Google Scholar] [CrossRef]
- El-Dawlatly, A.A.; Turkistani, A.; Kettner, S.C.; Machata, A.-M.; Delvi, M.B.; Thallaj, A.; Kapral, S.; Marhofer, P. Ultrasound-guided transversus abdominis plane block: Description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br. J. Anaesth. 2009, 102, 763–767. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Snyder, L.B.C.; Tearney, C.C.; Baker-Herman, T.L.; Schroeder, K.M. Ultrasound-guided transversus abdominis plane block in the dog: An anatomical evaluation. Vet. Anaesth. Analg. 2011, 38, 267–271. [Google Scholar] [CrossRef]
- Read, M.R.; Schroeder, C.A. The Trunk. In Small Animal Regional Anesthesia and Analgesia; Wiley: Hoboken, NJ, USA, 2013; pp. 167–198. ISBN 978-1-118-78338-2. [Google Scholar]
- Hermanson, J.W.; Evans, H.E.; Lahunta, A. de The Digestive Apparatus and Abdomen. In Miller and Evans’ Anatomy of the Dog—E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; Volume 7, p. 765. ISBN 978-0-323-54602-7. [Google Scholar]
- Castañeda-Herrera, F.E.; Buriticá-Gaviria, E.F.; Echeverry-Bonilla, D.F. Anatomical Evaluation of the Thoracolumbar Nerves Related to the Transversus Abdominis Plane Block Technique in the Dog. Anat. Histol. Embryol. 2017, 46, 373–377. [Google Scholar] [CrossRef]
- Romano, M.; Portela, D.A.; Thomson, A.; Otero, P.E. Comparison between two approaches for the transversus abdominis plane block in canine cadavers. Vet. Anaesth. Analg. 2021, 48, 101–106. [Google Scholar] [CrossRef]
- Drożdżyńska, M.; Monticelli, P.; Neilson, D.; Viscasillas, J. Ultrasound-guided subcostal oblique transversus abdominis plane block in canine cadavers. Vet. Anaesth. Analg. 2017, 44, 183–186. [Google Scholar] [CrossRef]
- Portela, D.A.; Romano, M.; Briganti, A. Retrospective clinical evaluation of ultrasound guided transverse abdominis plane block in dogs undergoing mastectomy. Vet. Anaesth. Analg. 2014, 41, 319–324. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, S.; Yuen, V.M.; Choi, S.-W.; Xu, X. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after caesarean delivery: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 7. [Google Scholar] [CrossRef]
- Odonnell, B. The Transversus Abdominis Plane (TAP) Block in Open Retropubic Prostatectomy. Reg. Anesth. Pain Med. 2006, 31, 91. [Google Scholar] [CrossRef]
- McDonnell, J.G.; Curley, G.; Carney, J.; Benton, A.; Costello, J.; Maharaj, C.H.; Laffey, J.G. The Analgesic Efficacy of Transversus Abdominis Plane Block After Cesarean Delivery: A Randomized Controlled Trial. Anesth. Analg. 2008, 106, 186–191. [Google Scholar] [CrossRef]
- Campoy, L.; Martin-Flores, M.; Boesch, J.M.; Moyal, M.N.; Gleed, R.D.; Radhakrishman, S.; Pavlinac, R.M.; Sieger, J.L.; Colon, C.S.; Magidenko, S.R. Transverse abdominis plane injection of bupivacaine with dexmedetomidine or a bupivacaine liposomal suspension yielded lower pain scores and requirement for rescue analgesia in a controlled, randomized trial in dogs undergoing elective ovariohysterectomy. Am. J. Vet. Res. 2022, 83, ajvr.22.03.0037. [Google Scholar] [CrossRef]
- Viscasillas, J.; Cañón, A.; Hernández, E.; Martínez, A.; Marti-Scharfhausen, R.; Lafuente, P.; Redondo, J.I. Clinical Assessment of Introducing Locoregional Anaesthesia Techniques as Part as the Intraoperative Analgesia Management for Canine Ovariohysterectomy in a Veterinary Teaching Hospital. Animals 2022, 12, 1939. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Schroeder, K.M.; Johnson, R.A. Transversus Abdominis Plane Block for Exploratory Laparotomy in a Canadian Lynx (Lynx canadensis). J. Zoo Wildl. Med. 2010, 41, 338–341. [Google Scholar] [CrossRef]
- Case, J.B.; Marvel, S.J.; Boscan, P.; Monnet, E.L. Surgical time and severity of postoperative pain in dogs undergoing laparoscopic ovariectomy with one, two, or three instrument cannulas. J. Am. Vet. Med. Assoc. 2011, 239, 203–208. [Google Scholar] [CrossRef]
- Della Rocca, G.; Colpo, R.; Reid, J.; Di Salvo, A.; Scott, M. Creation and validation of the Italian version of the Glasgow Composite Measure Pain Scale-Short Form (ICMPS-SF). Vet. Ital. 2018, 54, 251–260. [Google Scholar] [CrossRef]
- Skouropoulou, D.; Lacitignola, L.; Centonze, P.; Simone, A.; Crovace, A.M.; Staffieri, F. Perioperative analgesic effects of an ultrasound-guided transversus abdominis plane block with a mixture of bupivacaine and lidocaine in cats undergoing ovariectomy. Vet. Anaesth. Analg. 2018, 45, 374–383. [Google Scholar] [CrossRef]
- Portela, D.A.; Verdier, N.; Otero, P.E. Regional anesthetic techniques for the pelvic limb and abdominal wall in small animals: A review of the literature and technique description. Vet. J. 2018, 238, 27–40. [Google Scholar] [CrossRef]
- Jakobsson, J.G.; Wickerts, L.; Forsberg, S.; Ledin, G. Transversus abdominal plane (TAP) block for postoperative pain management: A review. F1000Research 2015, 4, 1359. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Yoshida, T.; Chuang, T.-Y.; Yang, S.-F.; Chang, C.-C.; Yao, H.-Y.; Tai, Y.-T.; Lin, J.-A.; Chen, K.-Y. Transversus Abdominis Plane Block: An Updated Review of Anatomy and Techniques. BioMed Res. Int. 2017, 2017, 8284363. [Google Scholar] [CrossRef]
- Umano, G.R.; Delehaye, G.; Noviello, C.; Papparella, A. The “Dark Side” of Pneumoperitoneum and Laparoscopy. Minim. Invasive Surg. 2021, 2021, 5564745. [Google Scholar] [CrossRef]
- Scott, J.E.; Singh, A.; Valverde, A.; Blois, S.L.; Foster, R.A.; Kilkenny, J.J.; Linden, A. zur Effect of pneumoperitoneum with warmed humidified or standard-temperature carbon dioxide during laparoscopy on core body temperature, cardiorespiratory and thromboelastography variables, systemic inflammation, peritoneal response, and signs of postoperative pain in healthy mature dogs. Am. J. Vet. Res. 2018, 79, 1321–1334. [Google Scholar] [CrossRef]
- Freeman, L.J.; Rahmani, E.Y.; Al-Haddad, M.; Sherman, S.; Chiorean, M.V.; Selzer, D.J.; Snyder, P.W.; Constable, P.D. Comparison of pain and postoperative stress in dogs undergoing natural orifice transluminal endoscopic surgery, laparoscopic, and open oophorectomy. Gastrointest. Endosc. 2010, 72, 373–380. [Google Scholar] [CrossRef]
- Corriveau, K.M.; Giuffrida, M.A.; Mayhew, P.D.; Runge, J.J. Outcome of laparoscopic ovariectomy and laparoscopic-assisted ovariohysterectomy in dogs: 278 cases (2003–2013). J. Am. Vet. Med. Assoc. 2017, 251, 443–450. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.W.; Paek, J.; Lee, S.H.; Yim, G.W.; Kim, J.H.; Kim, J.W.; Kim, Y.T.; Nam, E.J. Comparisons of Surgical Outcomes, Complications, and Costs Between Laparotomy and Laparoscopy in Early-Stage Ovarian Cancer. Int. J. Gynecol. Cancer 2011, 21, 251–256. [Google Scholar] [CrossRef]
- Bendinelli, C.; Properzi, R.; Boschi, P.; Bresciani, C.; Rocca, E.; Sabbioni, A.; Leonardi, F. Meloxicam vs robenacoxib for postoperative pain management in dogs undergoing combined laparoscopic ovariectomy and laparoscopic-assisted gastropexy. Vet. Surg. 2019, 48, 578–583. [Google Scholar] [CrossRef]
- Markham, A.; Faulds, D. Ropivacaine: A Review of its Pharmacology and Therapeutic Use in Regional Anaesthesia. Drugs 1996, 52, 429–449. [Google Scholar] [CrossRef]
- McKune, C.M.; Pascoe, P.J.; Lascelles, B.D.X.; Kass, P.H. The challenge of evaluating pain and a pre-incisional local anesthetic block. PeerJ 2014, 2, e341. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescott, A.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11 (Suppl. S2), S105-20. [Google Scholar]
- Simon, B.T.; Steagall, P.V. The present and future of opioid analgesics in small animal practice. J. Vet. Pharmacol. Therap. 2017, 40, 315–326. [Google Scholar] [CrossRef]
- Bruggink, S.M.; Schroeder, K.M.; Baker-Herman, T.L.; Schroeder, C.A. Weight-Based Volume of Injection Influences Cranial to Caudal Spread of Local Anesthetic Solution in Ultrasound-Guided Transversus Abdominis Plane Blocks in Canine Cadavers: Ultrasound-Guided Transversus Abdominis Plane Blocks in Canine Cadavers. Vet. Surg. 2012, 41, 455–457. [Google Scholar] [CrossRef]
- Faccenda, K.A.; Finucane, B.T. Complications of Regional Anaesthesia: Incidence and Prevention. Drug Saf. 2001, 24, 413–442. [Google Scholar] [CrossRef]
- Garbin, M.; Marangoni, S.; Finck, C.; Steagall, P.V. An Anatomical, Sonographic, and Computed Tomography Study of the Transversus Abdominis Plane Block in Cat Cadavers. Animals 2022, 12, 2674. [Google Scholar] [CrossRef]
- Freitag, F.A.V.; Bozak, V.L.; do Carmo, M.P.W.; Froes, T.R.; Duque, J.C.M. Continuous transversus abdominis plane block for analgesia in three dogs with abdominal pain. Vet. Anaesth. Analg. 2018, 45, 581–583. [Google Scholar] [CrossRef]
- Marhofer, D.; Marhofer, P.; Kettner, S.C.; Fleischmann, E.; Prayer, D.; Schernthaner, M.; Lackner, E.; Willschke, H.; Schwetz, P.; Zeitlinger, M. Magnetic Resonance Imaging Analysis of the Spread of Local Anesthetic Solution after Ultrasound-guided Lateral Thoracic Paravertebral Blockade. Anesthesiology 2013, 118, 1106–1112. [Google Scholar] [CrossRef]
- Grubb, T.; Lobprise, H. Local and regional anaesthesia in dogs and cats: Descriptions of specific local and regional techniques (Part 2). Vet. Med. Sci. 2020, 6, 218–234. [Google Scholar] [CrossRef]
| TAP | FEN | p Value | |
|---|---|---|---|
| Age (months) | 12 (7–24) | 24 (12–36) | 0.005 |
| Weight (kg) | 21.3 (15–36) | 30 (20–39) | 0.077 |
| Pain score at the time of premedication | 0 (0–3) | 0 (0–2) | 0.981 |
| Time spent for the block (min) | 25 (13–34) | 26 (15–36) | 0.867 |
| Peak intra-abdominal pressure (mmHg) | 8.5 (8–10) | 9 (8–12) | 0.633 |
| Baseline heart rate (bpm) | 66 (45–90) | 66.5 (44–70) | 0.381 |
| Baseline SAP (mmHg) | 111.5 (89–129) | 120 (108–130) | 0.027 |
| Baseline MAP (mmHg) | 75 (67–86) | 79.5 (69–96) | 0.085 |
| Median intra-operative EtISO (%) | 1.2 (1.1–1.3) | 1.2 (1.15–1.2) | 0.696 |
| Duration of surgery (min) | 82.5 (50–120) | 87 (75–195) | 0.446 |
| TAP | FEN | p Value | |
|---|---|---|---|
| Dogs requiring rescue fentanyl | 5/10 | 10/10 | 0.032 |
| Total fentanyl dose (mcg kg−1) | 1.5 (0–9) | 9 (3–15) | 0.013 |
| Dogs requiring rescue methadone | 0/10 | 8/10 | <0.001 |
| Total methadone dose (mg kg−1) | 0 | 0.2 (0–0.6) | <0.001 |
| Time to sternal recumbency (min) | 30 (19–80) | 45 (16–70) | 0.589 |
| Dogs showing dysphoria | 0/10 | 3/10 | 0.21 |
| Dogs accepting food at 6 h | 8/10 | 4/10 | 0.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolini, A.; Santoro, F.; Bianchi, A.; Collivignarelli, F.; Vignoli, M.; Scialanca, S.; Parrillo, S.; Falerno, I.; De Bonis, A.; Rosto, M.; et al. Use of Transversus Abdominis Plane and Intercostal Blocks in Bitches Undergoing Laparoscopic Ovariectomy: A Randomized Controlled Trial. Vet. Sci. 2022, 9, 604. https://doi.org/10.3390/vetsci9110604
Paolini A, Santoro F, Bianchi A, Collivignarelli F, Vignoli M, Scialanca S, Parrillo S, Falerno I, De Bonis A, Rosto M, et al. Use of Transversus Abdominis Plane and Intercostal Blocks in Bitches Undergoing Laparoscopic Ovariectomy: A Randomized Controlled Trial. Veterinary Sciences. 2022; 9(11):604. https://doi.org/10.3390/vetsci9110604
Chicago/Turabian StylePaolini, Andrea, Francesco Santoro, Amanda Bianchi, Francesco Collivignarelli, Massimo Vignoli, Silvia Scialanca, Salvatore Parrillo, Ilaria Falerno, Andrea De Bonis, Martina Rosto, and et al. 2022. "Use of Transversus Abdominis Plane and Intercostal Blocks in Bitches Undergoing Laparoscopic Ovariectomy: A Randomized Controlled Trial" Veterinary Sciences 9, no. 11: 604. https://doi.org/10.3390/vetsci9110604
APA StylePaolini, A., Santoro, F., Bianchi, A., Collivignarelli, F., Vignoli, M., Scialanca, S., Parrillo, S., Falerno, I., De Bonis, A., Rosto, M., & Tamburro, R. (2022). Use of Transversus Abdominis Plane and Intercostal Blocks in Bitches Undergoing Laparoscopic Ovariectomy: A Randomized Controlled Trial. Veterinary Sciences, 9(11), 604. https://doi.org/10.3390/vetsci9110604







