HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Caseload
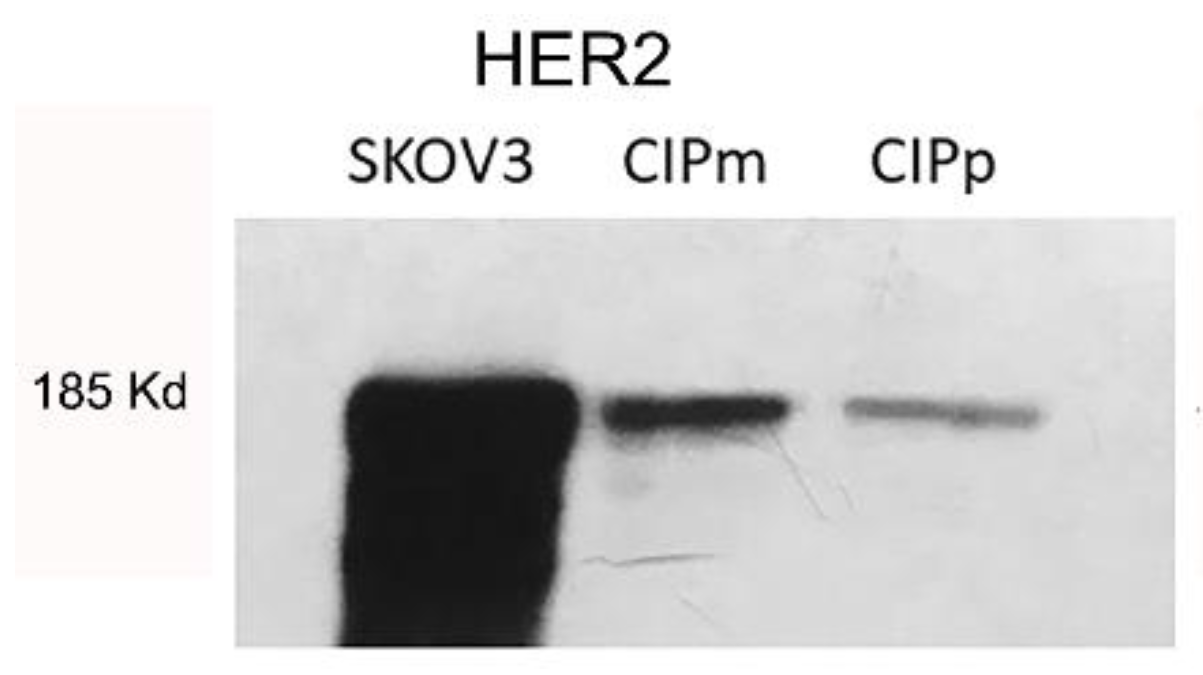
2.2. Western Blot for Antibody Validation
2.3. Immunohistochemistry
2.4. Tissue Microarray
2.5. Fluorescent in Situ Hybridization Experiment Design and Blast Analysis
2.6. Fluorescence in Situ Hybridization Method
2.7. Follow-Up Data
2.8. Statistical Analysis
3. Results
3.1. Caseload, Histological Diagnosis, and Grade
3.2. HER2 Expression in Canine Carcinoma Mammary Cell Lines
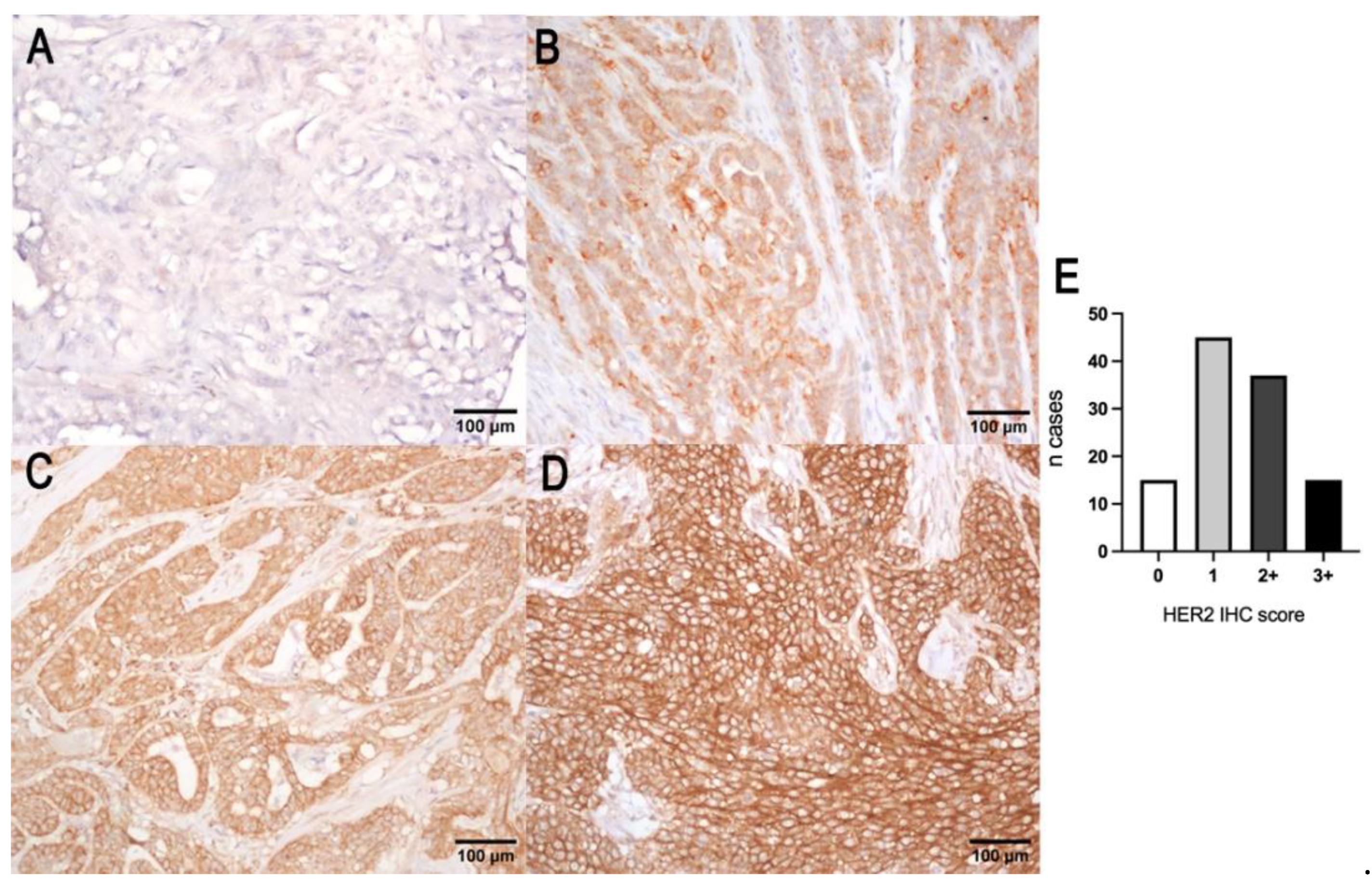
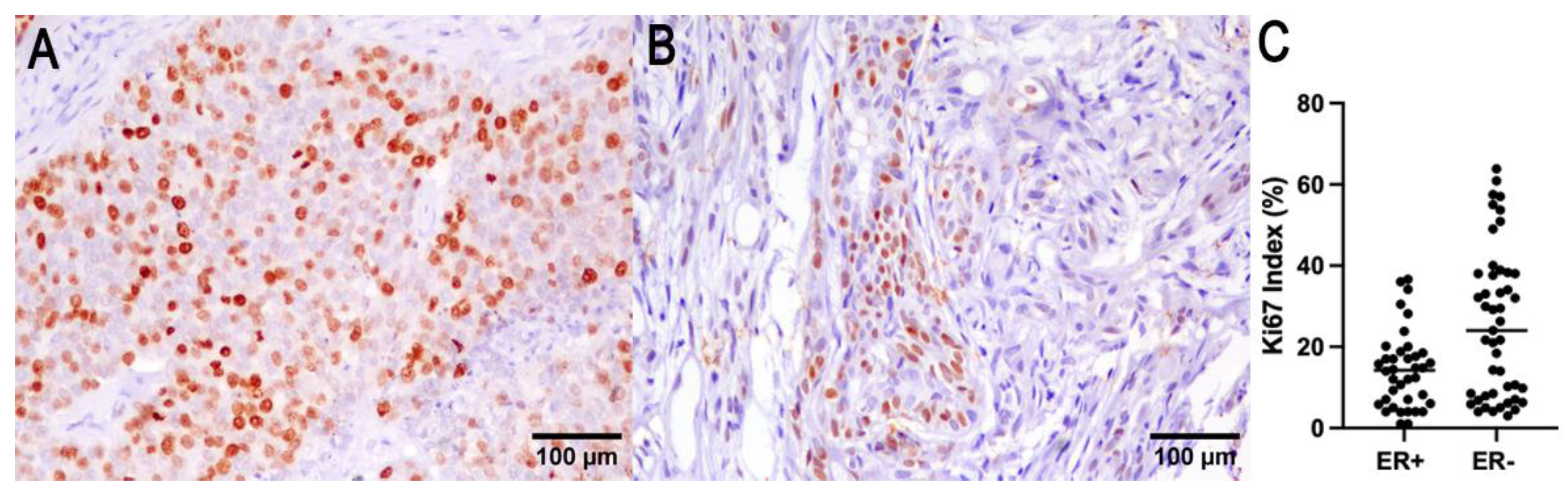
3.3. HER2 Protein Expression, Ki67 Proliferation Index, and Estrogen Receptors Expression
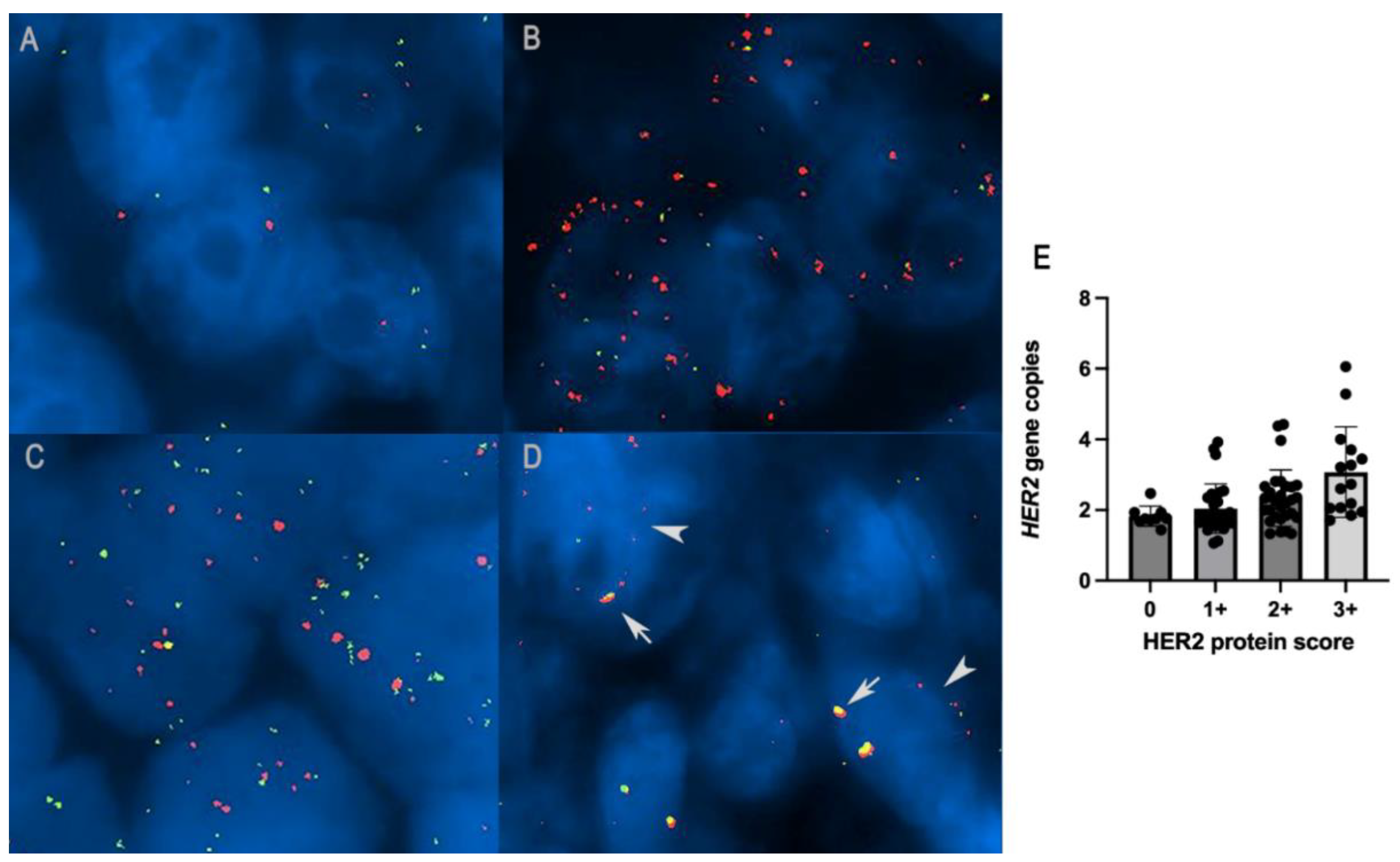
3.4. HER2 and CRYBA1 Copy Number Variations
- (1)
- Cases with mean HER2 copy numbers per nucleus > 6 (4/8 cases).
- (2)
- Cases with mean HER2 copy numbers ≥4 < 6 and with positive immunohistochemical expression (HER2 protein score 3+) (1/8 cases).
- (3)
- Cases with mean HER2 copy numbers ≥4 < 6 and with equivocal immunohistochemical expression (HER2 protein score 2+) that underwent dual-probe evaluation and showed a HER2/CRYBA1 copy number ratio > 2 (3/8 cases).
- (1)
- Cases with HER2/CRYBA1 > 2, with a mean of HER2 copy numbers per nucleus > 4, regardless of the immunohistochemical expression (7/8).
- (2)
- Cases with HER2/CRYBA1 < 2, with a mean of HER2 copy numbers per nucleus > 4, and with positive immunohistochemical expression (HER2 protein score 3+) (1/8).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marotta, M.; Onodera, T.; Johnson, J.; Budd, G.T.; Watanabe, T.; Cui, X.; Giuliano, A.E.; Niida, A.; Tanaka, H. Palindromic Amplification of the ERBB2 Oncogene in Primary HER2-Positive Breast Tumors. Sci. Rep. 2017, 7, 41921. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Allison, K.H.; Ellis, I.O.; Horii, R.; Masuda, S.; Penault-Llorca, F.; Tsuda, H.; Vincent-Salomon, A. Invasive breast carcinoma: General overview. In Breast Tumours; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 82–101. [Google Scholar]
- Lester, S. The Breast. In Robbins and Cotran Pathological Basis of Desease; Kumar, V., Abbas, A., Aster, J., Eds.; Elsevier Inc.: New York, NY, USA, 2021; pp. 1037–1064. [Google Scholar]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef] [PubMed]
- Miligy, I.M.; Toss, M.S.; Gorringe, K.L.; Lee, A.H.S.; Ellis, I.O.; Green, A.R.; Rakha, E.A. The Clinical and Biological Significance of HER2 Over-Expression in Breast Ductal Carcinoma in Situ: A Large Study from a Single Institution. Br. J. Cancer 2019, 120, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Bang, Y.-J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef]
- Campos, L.C.; Lavalle, G.E.; Estrela-Lima, A.; Melgaço de Faria, J.C.; Guimarães, J.E.; Dutra, Á.P.; Ferreira, E.; de Sousa, L.P.; Rabelo, É.M.L.; Vieira da Costa, A.F.D.; et al. CA15.3, CEA and LDH in Dogs with Malignant Mammary Tumors. J. Vet. Intern. Med. 2012, 26, 1383–1388. [Google Scholar] [CrossRef]
- Soares, N.P.; Medeiros, A.A.; Castro, I.D.P.; Andrade, M.B. Fatores de Prognóstico Em Carcinomas Mamários Caninos e Sua Relação Com Expressão de HER2. Acta Sci. Vet. 2017, 45, 1443. [Google Scholar]
- Ressel, L.; Puleio, R.; Loria, G.R.; Vannozzi, I.; Millanta, F.; Caracappa, S.; Poli, A. HER-2 Expression in Canine Morphologically Normal, Hyperplastic and Neoplastic Mammary Tissues and Its Correlation with the Clinical Outcome. Res. Vet. Sci. 2013, 94, 299–305. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Huang, H.-M.; Liao, J.-W.; Wong, M.-L.; Chang, S.-C. Increased Survival in Dogs with Malignant Mammary Tumours Overexpressing HER-2 Protein and Detection of a Silent Single Nucleotide Polymorphism in the Canine HER-2 Gene. Vet. J. 2009, 180, 116–123. [Google Scholar] [CrossRef]
- Dutra, A.P.; Granja, N.V.M.; Schmitt, F.C.; Cassali, G.D. C-ErbB-2 Expression and Nuclear Pleomorphism in Canine Mammary Tumors. Braz. J. Med. Biol. Res. 2004, 37, 1673–1681. [Google Scholar] [CrossRef]
- Muhammadnejad, A.; Keyhani, E.; Mortazavi, P.; Behjati, F.; Haghdoost, I.S. Overexpression of Her-2/Neu in Malignant Mammary Tumors; Translation of Clinicopathological Features from Dog to Human. Asian Pac. J. Cancer Prev. 2012, 13, 6415–6421. [Google Scholar] [CrossRef]
- Beha, G.; Brunetti, B.; Asproni, P.; Muscatello, L.V.; Millanta, F.; Poli, A.; Sarli, G.; Benazzi, C. Molecular Portrait-Based Correlation between Primary Canine Mammary Tumor and Its Lymph Node Metastasis: Possible Prognostic-Predictive Models and/or Stronghold for Specific Treatments? BMC Vet. Res. 2012, 8, 219. [Google Scholar] [CrossRef]
- Millanta, F.; Impellizeri, J.; McSherry, L.; Rocchigiani, G.; Aurisicchio, L.; Lubas, G. Overexpression of HER-2 via Immunohistochemistry in Canine Urinary Bladder Transitional Cell Carcinoma—A Marker of Malignancy and Possible Therapeutic Target. Vet. Comp. Oncol. 2018, 16, 297–300. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Kato, D.; Kamoto, S.; Yamamoto, K.; Tsuboi, M.; Shinada, M.; Ikeda, N.; Tanaka, Y.; Yoshitake, R.; Eto, S.; et al. Immunohistochemical Evaluation of HER2 Expression in Canine Thyroid Carcinoma. Heliyon 2019, 5, e02004. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Kato, D.; Kamoto, S.; Yamamoto, K.; Tsuboi, M.; Shinada, M.; Ikeda, N.; Tanaka, Y.; Yoshitake, R.; Eto, S.; et al. Detection of Human Epidermal Growth Factor Receptor 2 Overexpression in Canine Anal Sac Gland Carcinoma. J. Vet. Med. Sci. 2019, 81, 1034–1039. [Google Scholar] [CrossRef]
- Tsuboi, M.; Sakai, K.; Maeda, S.; Chambers, J.K.; Yonezawa, T.; Matsuki, N.; Uchida, K.; Nakayama, H. Assessment of HER2 Expression in Canine Urothelial Carcinoma of the Urinary Bladder. Vet. Pathol. 2019, 56, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Maeda, S.; Saeki, K.; Yoshitake, R.; Goto-Koshino, Y.; Nakagawa, T.; Nishimura, R.; Yonezawa, T.; Matsuki, N. ErbB2 Copy Number Aberration in Canine Urothelial Carcinoma Detected by a Digital Polymerase Chain Reaction Assay. Vet. Pathol. 2020, 57, 56–65. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Kato, D.; Kamoto, S.; Yamamoto, K.; Tsuboi, M.; Shinada, M.; Ikeda, N.; Tanaka, Y.; Yoshitake, R.; Eto, S.; et al. Overexpression of Human Epidermal Growth Factor Receptor 2 in Canine Primary Lung Cancer. J. Vet. Med. Sci. 2020, 82, 804–808. [Google Scholar] [CrossRef]
- Brunetti, B.; Bacci, B.; Sarli, G.; Pancioni, E.; Muscatello, L.V. Immunohistochemical Screening of HER2 in Canine Carcinomas: A Preliminary Study. Animals 2021, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Martin de las Mulas, J.; Ordás, J.; Millán, Y.; Fernández-Soria, V.; Ramón y Cajal, S. Oncogene HER-2 in Canine Mammary Gland Carcinomas: An Immunohistochemical and Chromogenic in Situ Hybridization Study. Breast Cancer Res. Treat. 2003, 80, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V. Mammary Tumors; Davis-Thompson DVM Foundation: Chicago, IL, USA, 2019; ISBN 173374911X. [Google Scholar]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic Value of Histological Grading in Noninflammatory Canine Mammary Carcinomas in a Prospective Study with Two-Year Follow-up: Relationship with Clinical and Histological Characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.; Nakagawa, T.; Endo, Y.; Kamida, A.; Yoshida, K.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Establishment of a Pair of Novel Cloned Tumour Cell Lines with or without Metastatic Potential from Canine Mammary Adenocarcinoma. Res. Vet. Sci. 2012, 93, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, B.; Bacci, B.; Angeli, C.; Benazzi, C.; Muscatello, L.V. P53, ER, and Ki67 Expression in Canine Mammary Carcinomas and Correlation With Pathological Variables and Prognosis. Vet. Pathol. 2021, 58, 325–331. [Google Scholar] [CrossRef]
- Muscatello, L.V.; Di Oto, E.; Sarli, G.; Monti, V.; Foschini, M.P.; Benazzi, C.; Brunetti, B. HER2 Amplification Status in Feline Mammary Carcinoma: A Tissue Microarray-Fluorescence In Situ Hydridization-Based Study. Vet. Pathol. 2019, 56, 230–238. [Google Scholar] [CrossRef]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine Mammary Tumors: A Review and Consensus of Standard Guidelines on Epithelial and Myoepithelial Phenotype Markers, HER2, and Hormone Receptor Assessment Using Immunohistochemistry. Vet. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef]
- Muscatello, L.V.; Di Oto, E.; Dignazzi, M.; Murphy, W.J.; Porcellato, I.; De Maria, R.; Raudsepp, T.; Foschini, M.P.; Sforna, M.; Benazzi, C.; et al. HER2 Overexpression and Amplification in Feline Pulmonary Carcinoma. Vet. Pathol. 2021, 58, 527–530. [Google Scholar] [CrossRef]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic Significance of Canine Mammary Tumor Histologic Subtypes: An Observational Cohort Study of 229 Cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine Invasive Mammary Carcinomas as Models of Human Breast Cancer. Part 1: Natural History and Prognostic Factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro Jr, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Escrivá-de-Romaní, S.; Arumí, M.; Bellet, M.; Saura, C. HER2-Positive Breast Cancer: Current and New Therapeutic Strategies. Breast 2018, 39, 80–88. [Google Scholar] [CrossRef]
- Park, Y.; Kitahara, T.; Takagi, R.; Kato, R. Current Status of Therapy for Breast Cancer Worldwide and in Japan. World J. Clin. Oncol. 2011, 2, 125–134. [Google Scholar] [CrossRef]
- Sakai, H.; Tsurutani, J.; Iwasa, T.; Komoike, Y.; Sakai, K.; Nishio, K.; Nakagawa, K. HER2 Genomic Amplification in Circulating Tumor DNA and Estrogen Receptor Positivity Predict Primary Resistance to Trastuzumab Emtansine (T-DM1) in Patients with HER2-Positive Metastatic Breast Cancer. Breast Cancer 2018, 25, 605–613. [Google Scholar] [CrossRef]
- Lüder Ripoli, F.; Mohr, A.; Conradine Hammer, S.; Willenbrock, S.; Hewicker-Trautwein, M.; Hennecke, S.; Murua Escobar, H.; Nolte, I. A Comparison of Fresh Frozen vs. Formalin-Fixed, Paraffin-Embedded Specimens of Canine Mammary Tumors via Branched-DNA Assay. Int. J. Mol. Sci. 2016, 17, 724. [Google Scholar] [CrossRef]
- Kabir, F.M.L.; DeInnocentes, P.; Agarwal, P.; Mill, C.P.; Riese Nd, D.J.; Bird, R.C. Estrogen Receptor-α, Progesterone Receptor, and c-ErbB/HER-Family Receptor MRNA Detection and Phenotype Analysis in Spontaneous Canine Models of Breast Cancer. J. Vet. Sci. 2017, 18, 149–158. [Google Scholar] [CrossRef]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine Invasive Mammary Carcinomas as Models of Human Breast Cancer. Part 2: Immunophenotypes and Prognostic Significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef]
- Kim, J.H.; Im, K.S.; Kim, N.H.; Yhee, J.Y.; Nho, W.G.; Sur, J.H. Expression of HER-2 and Nuclear Localization of HER-3 Protein in Canine Mammary Tumors: Histopathological and Immunohistochemical Study. Vet. J. 2011, 189, 318–322. [Google Scholar] [CrossRef]
- Levi, M.; Muscatello, L.V.; Brunetti, B.; Benazzi, C.; Parenti, F.; Gobbo, F.; Avallone, G.; Bacci, B.; Zambon, E.; Valenti, P.; et al. High Intrinsic Expression of P-Glycoprotein and Breast Cancer Resistance Protein in Canine Mammary Carcinomas Regardless of Immunophenotype and Outcome. Animals 2021, 11, 658. [Google Scholar] [CrossRef]
- Burrai, G.P.; Tanca, A.; De Miglio, M.R.; Abbondio, M.; Pisanu, S.; Polinas, M.; Pirino, S.; Mohammed, S.I.; Uzzau, S.; Addis, M.F.; et al. Investigation of HER2 Expression in Canine Mammary Tumors by Antibody-Based, Transcriptomic and Mass Spectrometry Analysis: Is the Dog a Suitable Animal Model for Human Breast Cancer? Tumour Biol. 2015, 36, 9083–9091. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Miller, M.A. When Tissue Antigens and Antibodies Get along: Revisiting the Technical Aspects of Immunohistochemistry--the Red, Brown, and Blue Technique. Vet. Pathol. 2014, 51, 42–87. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Webster, J.D.; DuSold, D.; Miller, M.A. Immunohistochemical Evaluation of the Effects of Paraffin Section Storage on Biomarker Stability. Vet. Pathol. 2014, 51, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Moatamed, N.A.; Nanjangud, G.; Pucci, R.; Lowe, A.; Shintaku, I.P.; Shapourifar-Tehrani, S.; Rao, N.; Lu, D.Y.; Apple, S.K. Effect of Ischemic Time, Fixation Time, and Fixative Type on HER2/Neu Immunohistochemical and Fluorescence in Situ Hybridization Results in Breast Cancer. Am. J. Clin. Pathol. 2011, 136, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.; Alves, A.; Schmitt, F. Identification of Molecular Phenotypes in Canine Mammary Carcinomas with Clinical Implications: Application of the Human Classification. Virchows Arch. 2008, 453, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, K.A.; Ferreira, E.; Estrela-Lima, A.; Gamba, C.d.O.; Miranda, F.F.; Alves, M.R.; Rocha, R.M.; de Barros, A.L.B.; Cassali, G.D. HER-2 and EGFR MRNA Expression and Its Relationship with Versican in Malignant Matrix-Producing Tumors of the Canine Mammary Gland. PLoS ONE. 2016, 11, e0160419. [Google Scholar] [CrossRef]
- Campos, L.C.; Silva, J.O.; Santos, F.S.; Araújo, M.R.; Lavalle, G.E.; Ferreira, E.; Cassali, G.D. Prognostic Significance of Tissue and Serum HER2 and MUC1 in Canine Mammary Cancer. J. Vet. Diagn. Investig. 2015, 27, 531–535. [Google Scholar] [CrossRef]
- Gibbons-Fideler, I.-S.; Nitta, H.; Murillo, A.; Tozbikian, G.; Banks, P.; Parwani, A.V.; Li, Z. Identification of HER2 Immunohistochemistry-Negative, FISH-Amplified Breast Cancers and Their Response to Anti-HER2 Neoadjuvant Chemotherapy. Am. J. Clin. Pathol. 2019, 151, 176–184. [Google Scholar] [CrossRef]
- Ogiwara, H.; Kohno, T.; Nakanishi, H.; Nagayama, K.; Sato, M.; Yokota, J. Unbalanced Translocation, a Major Chromosome Alteration Causing Loss of Heterozygosity in Human Lung Cancer. Oncogene 2008, 27, 4788–4797. [Google Scholar] [CrossRef]
- Mukherjee, K.; Storici, F. A Mechanism of Gene Amplification Driven by Small DNA Fragments. PLoS Genet. 2012, 8, e1003119. [Google Scholar] [CrossRef]
- Albertson, D.G.; Collins, C.; McCormick, F.; Gray, J.W. Chromosome Aberrations in Solid Tumors. Nat. Genet. 2003, 34, 369–376. [Google Scholar] [CrossRef]
- Iafrate, A.J.; Feuk, L.; Rivera, M.N.; Listewnik, M.L.; Donahoe, P.K.; Qi, Y.; Scherer, S.W.; Lee, C. Detection of Large-Scale Variation in the Human Genome. Nat. Genet. 2004, 36, 949–951. [Google Scholar] [CrossRef]
| Histological Grade | Tumor Size | Lymph Node Metastases | Systemic Metastases | OS | HER2 Protein Expression | Ki67 Index | ER exPression | HER2 Gene Copies | |
|---|---|---|---|---|---|---|---|---|---|
| Histological grade | - | p = 0.01 R = 0.37 | p < 0.0001 R = 0.64 | p = 0.004 R = 0.50 | p = 0.01 | ns | ns | p = 0.003 R = −0.31 | ns |
| Tumor size | p = 0.01 R = 0.372 | - | ns | ns | p = 0.01 R = −039 | ns | p = 0.006 R = 0.37 | p = 0.009 R−0.365 | ns |
| Lymph node metastases | p < 0.0001 R = 0.64 | ns | - | ns | ns | ns | p < 0.0001 R = 0.67 | ns | ns |
| Systemic metastases | p = 0.004 R = 0.50 | ns | ns | - | ns | ns | p = 0.006 R = 0.53 | ns | ns |
| OS | p = 0.01 | p = 0.01 R = −039 | ns | ns | - | ns | ns | p = 0.04 | ns |
| HER2 protein expression | ns | ns | ns | ns | ns | - | ns | ns | p = 0.01 R = 0.24 |
| Ki67 index | ns | p = 0.006 R = 0.37 | p < 0.0001 R = 0.67 | p = 0.006 R = 0.53 | ns | ns | - | p = 0.01 R = −0.28 | Ns |
| ER expression | p = 0.003 R = −0.31 | p = 0.009 R−0.36 | ns | ns | p = 0.04 | ns | p = 0.01 R = −0.28 | - | ns |
| HER2 gene copies | ns | ns | ns | ns | ns | p = 0.01 R = 0.24 | ns | ns | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscatello, L.V.; Gobbo, F.; Di Oto, E.; Sarli, G.; De Maria, R.; De Leo, A.; Tallini, G.; Brunetti, B. HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas. Vet. Sci. 2022, 9, 583. https://doi.org/10.3390/vetsci9110583
Muscatello LV, Gobbo F, Di Oto E, Sarli G, De Maria R, De Leo A, Tallini G, Brunetti B. HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas. Veterinary Sciences. 2022; 9(11):583. https://doi.org/10.3390/vetsci9110583
Chicago/Turabian StyleMuscatello, L. V., F. Gobbo, E. Di Oto, G. Sarli, R. De Maria, A. De Leo, G. Tallini, and B. Brunetti. 2022. "HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas" Veterinary Sciences 9, no. 11: 583. https://doi.org/10.3390/vetsci9110583
APA StyleMuscatello, L. V., Gobbo, F., Di Oto, E., Sarli, G., De Maria, R., De Leo, A., Tallini, G., & Brunetti, B. (2022). HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas. Veterinary Sciences, 9(11), 583. https://doi.org/10.3390/vetsci9110583







