Digestibility, Blood Parameters, Rumen Fermentation, Hematology, and Nitrogen Balance of Goats after Receiving Supplemental Coffee Cherry Pulp as a Source of Phytochemical Nutrients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing of Dried Coffee Cheery Pulp
2.2. Animals, Treatments, and Experimental Design
2.3. Sampling Method and Data Collection
2.3.1. Fecal and Feed Sampling Methods
2.3.2. Urine Sampling Method
2.3.3. Rumen Fluid Sampling Method
2.3.4. Blood Metabolites
2.4. Statistical Analysis
3. Results
3.1. Influence of CoCP on Feed Intake and Nutrient Digestibility
3.2. Influence of CoCP on Rumen Characteristics and Blood Metabolites
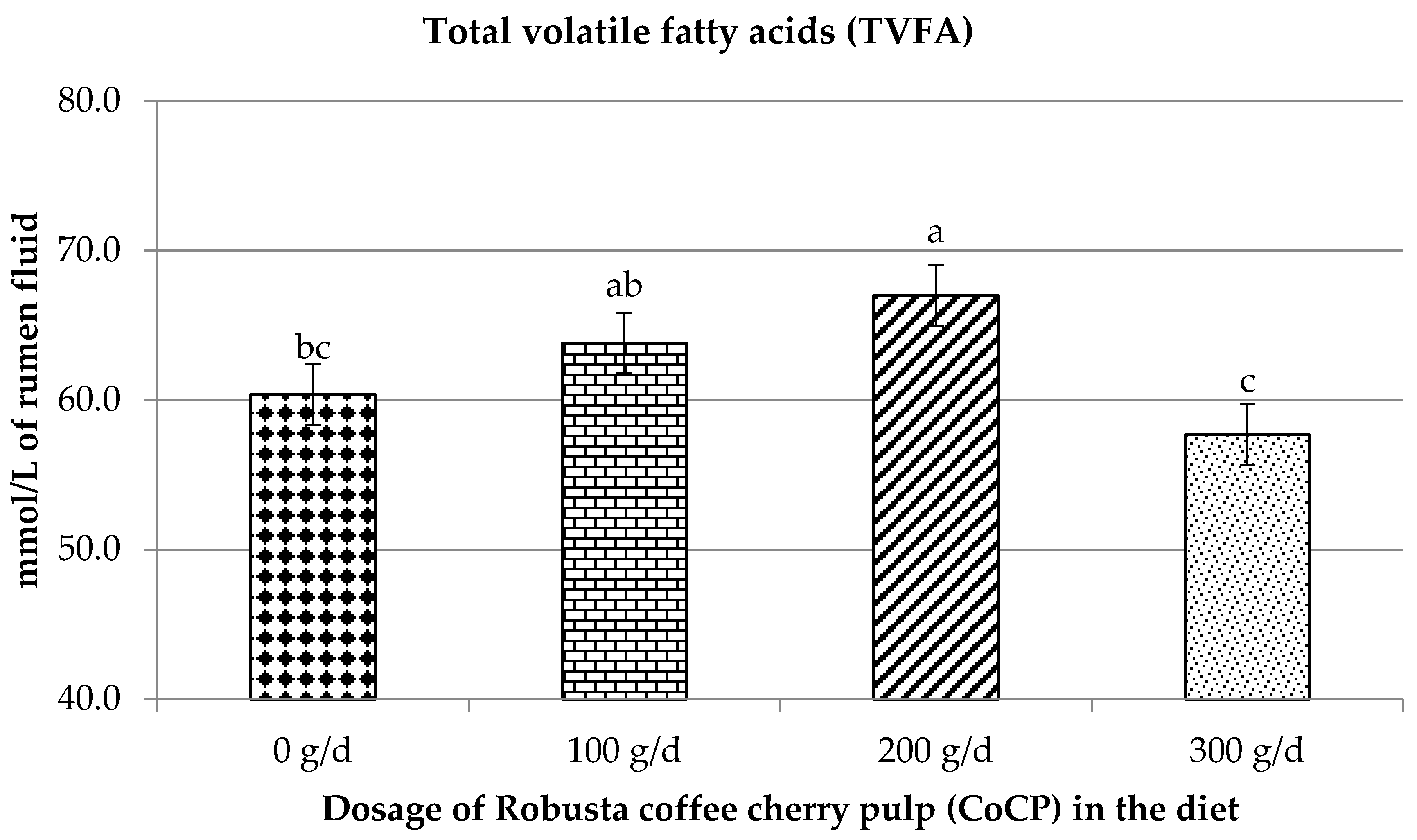
3.3. Influence of CoCP on Volatile Fatty Acid Profiles
3.4. Influence of CoCP on Microbial Population
3.5. Influence of CoCP on N Balance
4. Discussion
4.1. Nutrients and Phytochemical Composition of CoCP
4.2. Influence of CoCP on Digestibility and Intake
4.3. Influence of CoCP on Rumen Characteristics and Blood Metabolites
4.4. Influence of CoCP on Microbial Population
4.5. Influence of CoCP on Volatile Fatty Acid Profiles
4.6. Influence of CoCP on N Utilization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Sahoo, A.; Bhat, R.S.; Tripathi, M.K. Stall feeding in small ruminants: Emerging trends and future perspectives. Indian J. Anim. Nutr. 2015, 32, 353–372. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valenzuela, L.S.; Martínez, K.G.; Serna-Jimenez, J.A.; Hernández, M.C. Drying of coffee pulp: Process parameters, mathematical model and its effect over physicochemical properties. Inf. Tecnol. 2019, 30, 189–200. [Google Scholar] [CrossRef]
- Bakker, R.R.C. Availability of Lignocellulosic Feedstocks for Lactic Acid Production—Feedstock Availability, Lactic Acid Production Potential, and Selection Criteria; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2013. [Google Scholar]
- Gualtieri, A.M.J.; Villalta, R.C.; Díaz, T.L.E.; Medina, G.; Lapenna, E.; Rondón, M.E. Producción de biomasa de Saccharomyces cerevisiae y Candida utilis usando residuos de pulpa de Coffea arabica L. Rev. Inst. Nac. Hig. Rafael Rangel. 2007, 38, 31–37. [Google Scholar]
- Arellano, G.M.; Ramírez, C.A.; Mancera, T.T.; Pérez, M.G.; Saucedo, C.G. Antioxidant activity of fermented and non-fermented coffee (Coffea arabica) pulp extracts. Food Technol. Biotechnol. 2011, 49, 374–378. [Google Scholar]
- Salinas-Rios, T.; Sánchez-Torres, M.T.; Hernández-Bautista, J.; Díaz-Cruz, A.; Nava-Cuellar, C.; Zamora, V.; Cordero-Mora, J.L.; Vaquera-Huerta, H.; Velasco, J.L.F. Carcass characteristics, physicochemical changes and oxidative stress indicators of meat from sheep fed diets with coffee pulp. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1901–1908. [Google Scholar] [CrossRef][Green Version]
- Oliveira, L.S.; Franca, A.S. An overview of the potential uses for coffee husks. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 283–291. [Google Scholar]
- Fierro-Cabrales, N.; Contreras-Oliva, A.; González-Ríos, O.; Rosas-Mendoza, E.S.; Morales-Ramos, V. Chemical and nutritional characterization of coffee pulp (Coffea arabica L.). Agroproductividad 2018, 11, 9–13. [Google Scholar]
- de Souza, L.A.; Garcia, R.; Filho, S.C.V.; Rocha, F.C.; Campos, J.M.S.; Cabral, L.S.; Gobbi, K.F. Effects of feeding coffee hulls on intake, digestibility and milk yield and composition of lactating dairy cows. Rev. Bras. Zootec. 2005, 34, 2496–2504. [Google Scholar]
- Waghorn, G.C.; Tavendale, M.H.; Woodfield, D.R. Methanogenesis from forages fed to sheep. Proc. N. Z. Grassl. Assoc. 2002, 64, 167–171. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, A.F.; Paiva, P.C.A.; Pérez, J.R.O.; Santos, V.B.; Cardoso, R.M. Antinutritional factors of the hull and dehydrated pulp of coffee (Coffea arabica L.) stored in different periods. Rev. Bras. Zootec. 2001, 30, 1325–1331. [Google Scholar] [CrossRef]
- Cipriano, R.F.; García, R.; Freitas, A.W.; Lima de Souza, A.; Valadares, F.S.C.; Gomes, P.O.; Sampaio, R.J.P.; Gonçalves, T.R.; Cipriano, R.G. Intake and digestibility of lactating dairy cows fed diets containing coffee hulls. Rev. Bras. Zootec. 2006, 35, 2154–2162. [Google Scholar]
- Soares, O.A.; Souza, C.J.M.; Valadares, F.S.C.; Assis, A.J.; Monteiro, A.T.R.; Navajas, R.L.; Dos Santos, P.D.; Soares, O.G. Replacing corn with coffee hulls or soyhulls in diets of dairy cows: Chewing activity, ruminal metabolism, nitrogen utilization and microbial protein synthesis. Rev. Bras. Zootec. 2007, 36, 205–215. [Google Scholar]
- Soares, O.A.; Souza, C.J.M.; Valadares, F.S.C.; Assis, A.J.; Araújo, T.R.M.; Diniz, V.R.F.; Dos Santos, P.D.; Soares, O.G. Replacing corn with coffee hulls or soyhulls in dairy cows’ diets: Intake, nutrient digestibility, and milk production and composition. Rev. Bras. Zootec. 2007, 36, 1172–1182. [Google Scholar]
- Hagerman, A.E. Extraction of tannins from fresh and preserved leaves. J. Chem. Ecol. 1998, 14, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdian-rizi, M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. J. Appl. Hortic. 2008, 32, 17–20. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Robinson, P.; Givens, D.; Getachew, G. Evaluation of NRC, UC Davis and ADAS approaches to estimate the metabolizable energy values of feeds at maintenance energy intake from equations utilizing chemical assays and in vitro determinations. Anim. Feed Sci. Technol. 2004, 114, 75–90. [Google Scholar] [CrossRef]
- Mathew, S.; Sagathevan, S.; Thomas, J.; Mathen, G. An HPCL method for estimation of volatile fatty acids in ruminal fluid. Indian J. Anim. Sci. 1997, 67, 805–807. [Google Scholar]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Galyean, M. Laboratory Procedure in Animal Nutrition Research; Department of Animal and Range Sciences, New Mexico State University: Las Cruces, NM, USA, 1989. [Google Scholar]
- Statistical Analysis Systems. SAS/STAT User’s Guide: Version 8.1; SAS Inc.: Cary, NC, USA, 2000. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Patay, E.B.; Sali, N.; Koszegi, T.; Csepregi, R.; Balázs, V.L.; Németh, T.S.; Németh, T.; Papp, N. Antioxidant potential, tannin and polyphenol contents of seed and pericarp of three coffee species. Asian Pac. J. Trop. Med. 2016, 9, 366–371. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthong, A.; Seankamsorn, A.; Khonkhaeng, B.; Wanapat, M.; Uriyapongson, S.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. In vitro fermentation, digestibility and methane production as influenced by Delonix regia seed meal containing tannins and saponins. J. Anim. Feed Sci. 2017, 26, 123–130. [Google Scholar] [CrossRef]
- Patra, A.K.; Stiverson, J.; Yu, Z. Effects of quillaja and yucca saponins on communities and select populations of rumen bacteria and archaea, and fermentation in vitro. J. Appl. Microbiol. 2012, 113, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.; Wanapat, M.; Kang, S.; Cherdthong, A. effects of supplementation of eucalyptus (E. camaldulensis) leaf meal on feed intake and rumen fermentation efficiency in swamp buffaloes. Asian-Australas. J. Anim. Sci. 2015, 28, 951–957. [Google Scholar] [CrossRef]
- Wachirapakorn, C.; Pilachai, K.; Wanapat, M.; Pakdee, P.; Cherdthong, A. Effect of ground corn cobs as a fiber source in total mixed ration on feed intake, milk yield and milk composition in tropical lactating crossbred Holstein cows. Anim. Nutr. 2016, 2, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Gunun, P.; Wanapat, M.; Gunun, N.; Cherdthong, A.; Sirilaophaisan, S.; Kaewwongsa, W. Effects of condensed tannins in mao (Antidesma thwaitesianum Muell. Arg.) seed meal on rumen fermentation characteristics and nitrogen utilization in goats. Asian-Australas. J. Anim. Sci. 2016, 29, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Anantasook, N.; Wanapat, M.; Cherdthong, A.; Gunun, P. Effect of tannins and saponins in Samanea saman on rumen environment, milk yield and milk composition in lactating dairy cows. J. Anim. Physiol. Anim. Nutr. 2015, 99, 335–344. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Becker, K. Effects of Sesbania sesban and Carduus pycnocephalus leaves and Fenugreek (Trigonella foenum-graecum L.) seeds and their extracts on partitioning of nutrients from roughage-and concentrate based feeds to methane. Anim. Feed Sci. Technol. 2008, 147, 72–89. [Google Scholar] [CrossRef]
- Chanjula, P.; Suntara, C.; Cherdthong, A. The effects of oil palm fronds silage supplemented with urea-calcium hydroxide on rumen fermentation and nutrient digestibility of Thai native-Anglo Nubian goats. Fermentation 2021, 7, 218. [Google Scholar] [CrossRef]
- de Souza, A.L.; Garcia, R.; Bernardino, F.S.; Rocha, F.C.; Valadares-Filho, S.; Pereira, O.; Pires, A. Coffee hulls in the diet of sheep: Intake and apparent digestibility. Rev. Bras. Zootec. 2004, 33, 2170–2176. [Google Scholar]
- De Souza, A.L.; Garcia, R.; Cabral, L.S.; Pereira, M.L.A.; Valadares, R.F.D. Coffee hull in the diet of dairy heifers: Nitrogen balance and microbial protein synthesis. Rev. Bras. Zootec. 2010, 39, 1141–1145. [Google Scholar] [CrossRef]
- Hernández-Bautista, J.; Rodríguez-Magadán, H.M.; Villegas-Sánchez, J.A.; Salinas-Rios, T.; Ortiz-Muñoz, I.Y.; Aquino-Cleto, M.; Lozano-Trejo, S. Health status and productivity of sheep fed coffee pulp during fattening. Austral J. Vet. Sci. 2018, 50, 95–99. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Review effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Gunun, P.; Gunun, N.; Khejornsart, P.; Ouppamong, T.; Cherdthong, A.; Wanapat, M.; Sirilaophaisan, S.; Yuangklang, C.; Polyorach, S.; Kenchaiwong, W. Effects of Antidesma thwaitesianum Muell. Arg. pomace as a source of plant secondary compounds on digestibility, rumen environment, hematology, and milk production in dairy cows. Anim. Sci. J. 2019, 90, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Wanapat, M.; Cherdthong, A.; Pakdee, P.; Wanapat, S. Manipulation of rumen ecology by dietary lemongrass (Cymbopogon citratus Stapf.) powder supplementation. J. Anim. Sci. 2008, 86, 3497–3503. [Google Scholar] [CrossRef]
- Butler, S.T.; Pelton, S.H.; Butler, W.R. Energy balance, metabolic status, and the first postpartum ovarian follicle wave in cows administered propylene glycol. J. Dairy Sci. 2006, 89, 2938–2951. [Google Scholar] [CrossRef]
- Grummer, R.R.; Winkler, J.C.; Bertics, S.J.; Studer, V.A. Effect of propylene glycol dosage during feed restriction on metabolites in blood of prepartum Holstein heifers. J. Dairy. Sci. 1994, 77, 3618–3623. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Carta, S.; Tsiplakou, E.; Nicolussi, P.; Pulina, G.; Nudda, A. Effects of spent coffee grounds on production traits, haematological parameters, and antioxidant activity of blood and milk in dairy goats. Animal 2022, 16, 100501. [Google Scholar] [CrossRef]
- Cherdthong, A.; Prachumchai, R.; Wanapat, M.; Foiklang, S.; Chanjula, P. Effects of supplementation with royal poinciana seed meal (Delonix regia) on ruminal fermentation pattern, microbial protein synthesis, blood metabolites and mitigation of methane emissions in native Thai beef cattle. Animals 2019, 9, 625. [Google Scholar] [CrossRef]
- Junior, F.P.; Cassiano, E.C.O.; Martins, M.; Solorzano, L.A.R.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of tannins-rich extract from Acacia mearnsii or monensin as feed additives on ruminal fermentation efficiency in cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- Fagundes, G.M.; Benetel, G.; Carriero, M.M.; Sousa, R.L.; Muir, J.P.; Macedo, R.O.; Bueno, I.C.S. Tannin-rich forage as a methane mitigation strategy for cattle and the implications for rumen microbiota. Anim. Prod. Sci. 2020, 61, 26–37. [Google Scholar] [CrossRef]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef]
- Hu, W.; Wu, Y.; Liu, J.; Guo, Y.; Ye, J. Tea saponins affect in vitro fermentation and methanogenesis in faunated and defaunated rumen fluid. J. Zhejiang Univ. Sci. B. 2005, 6, 787–792. [Google Scholar] [CrossRef]
- Owens, F.N.; Zinn, R. Protein metabolism of ruminant animals. In The Ruminant Animal, Digestive Physiology and Nutrition; Church, D.C., Ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1988; pp. 227–249. [Google Scholar]

| Item | Basal Diet | CoCP 3 |
|---|---|---|
| Ingredients, %DM | ||
| Rice straw | 30.0 | |
| Ground corn | 45.0 | |
| Soybean meal | 7.30 | |
| Fish meal | 0.40 | |
| Leucaena leaves meal | 7.00 | |
| Palm kernel cake | 7.00 | |
| Molasses | 2.00 | |
| Dicalcium phosphate | 0.40 | |
| Salt | 0.20 | |
| Mineral and vitamin mix 1 | 0.70 | |
| Chemical composition, % | ||
| DM | 95.27 | 94.52 |
| OM | 94.60 | 99.03 |
| CP | 15.10 | 11.62 |
| EE | 1.90 | 3.52 |
| NDF | 47.33 | 43.58 |
| ADF | 24.34 | 10.41 |
| GE, Mcal/kg DM | 4.35 | 4.08 |
| ME, Mcal/kg DM 2 | 2.75 | 2.34 |
| Tannins, mg/100 g DM | - | 4.27 |
| Saponins, mg/100 g DM | - | 2.61 |
| Caffeine, mg/100 g DM | 0.92 | |
| Antioxidant activity content (mg Fe (II) equivalent/ 100 g DM) | - | 3.75 |
| Phenolic compound content (mg gallic acid equivalent/ 100 g DM) | - | 7.50 |
| Flavonoid content (mg catechin equivalent/ 100 g DM) | - | 4.37 |
| Item | Supplement Levels (g/d) of CoCP 1 | SEM 2 | Contrast p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | Linear | Quadratic | ||
| Dry matter intake | |||||||
| Total DMI, kg/d | 0.851 | 0.885 | 0.898 | 0.869 | 0.04 | 0.75 | 0.50 |
| DMI, %BW | 3.51 | 3.84 | 3.87 | 3.58 | 0.16 | 0.71 | 0.04 |
| DMI, g/kg W0.75 | 77.84 | 84.14 | 84.90 | 79.32 | 3.50 | 0.69 | 0.06 |
| Nutrient intake, kg/d | |||||||
| OMI | 0.786 | 0.819 | 0.824 | 0.786 | 0.03 | 0.97 | 0.41 |
| CPI | 0.126 | 0.133 | 0.137 | 0.132 | 0.01 | 0.74 | 0.46 |
| EEI | 0.016 b | 0.018 ab | 0.022 a | 0.022 a | 0.01 | <0.01 | 0.41 |
| NDFI, kg/d | 0.351 | 0.376 | 0.390 | 0.410 | 0.03 | 0.07 | 0.93 |
| ADFI, kg/d | 0.186 b | 0.201 ab | 0.225 a | 0.244 a | 0.01 | 0.04 | 0.91 |
| Apparent digestibility, % | |||||||
| DM | 69.38 a | 71.10 a | 70.21 a | 63.82 b | 1.15 | 0.03 | 0.03 |
| OM | 70.95 a | 72.28 a | 70.59 a | 64.73 b | 1.27 | 0.01 | 0.04 |
| CP | 71.35 ab | 74.42 a | 73.72 a | 67.16 b | 1.30 | 0.11 | 0.02 |
| EE | 70.10 b | 79.61 a | 83.40 a | 81.29 a | 2.56 | <0.01 | 0.03 |
| NDF | 56.93 a | 59.07 a | 57.56 a | 50.44 b | 1.20 | 0.09 | 0.05 |
| ADF | 34.65 a | 38.94 a | 37.41 a | 25.51 b | 2.50 | 0.06 | 0.04 |
| Estimated energy intake 4 | |||||||
| ME, Mcal/d | 2.12 | 2.25 | 2.21 | 1.94 | 0.12 | 0.30 | 0.12 |
| ME, Mcal/kg DM | 2.49 a | 2.54 a | 2.46 a | 2.23 b | 0.04 | <0.01 | 0.02 |
| Item | Supplement Levels (g/d) of CoCP 1 | SEM 2 | Contrast p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | Linear | Quadratic | ||
| Temperature, °C | 39.30 | 39.40 | 39.30 | 39.10 | 0.16 | 0.83 | 0.25 |
| Ruminal pH | 6.00 | 6.04 | 6.04 | 5.96 | 0.05 | 0.76 | 0.48 |
| NH3-N, mg/dL | 20.58 | 23.47 | 22.36 | 18.26 | 2.77 | 0.66 | 0.40 |
| BUN, mg/dL | 21.58 a | 17.39 b | 17.87 b | 17.37 b | 0.34 | <0.01 | <0.01 |
| GLU, mg/dL | 63.69 b | 66.25 ab | 66.75 a | 66.50 ab | 0.81 | 0.04 | 0.43 |
| PCV, % | 28.63 | 29.25 | 29.50 | 29.37 | 0.69 | 0.43 | 0.59 |
| NEFA, µmol/L | 225.63 a | 210.13 ab | 205.63 b | 198.88 b | 3.25 | <0.01 | 0.67 |
| BHBA, µmol/L | 0.48 a | 0.42 ab | 0.35 b | 0.38 ab | 0.02 | 0.05 | 0.24 |
| Cr, mg/dL | 1.19 | 1.29 | 1.14 | 1.17 | 0.06 | 0.70 | 0.78 |
| Item | Supplement Levels (g/d) of CoCP 1 | SEM 2 | Contrast p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | Linear | Quadratic | ||
| VFA profiles, mol/100 mol | |||||||
| Acetic acid | 62.28 a | 58.40 b | 56.99 b | 57.35 b | 0.74 | 0.03 | 0.18 |
| Propionic acid | 22.58 c | 26.38 b | 28.04 a | 26.35 b | 0.36 | 0.02 | 0.03 |
| Butyric acid | 12.76 | 12.91 | 12.24 | 13.39 | 1.33 | 0.79 | 0.63 |
| Acetic/propionic acid ratio | 2.78 a | 2.28 b | 2.09 c | 2.23 bc | 0.04 | 0.01 | 0.04 |
| Estimated methane, mol/100 mol 4 | 26.92 a | 24.18 b | 22.83 c | 23.91 b | 0.29 | 0.01 | 0.04 |
| Item | Supplement Levels (g/d) of CoCP 1 | SEM 2 | Contrast p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | Linear | Quadratic | ||
| Bacteria (×1010 cells/mL) | 7.27 | 7.39 | 7.63 | 7.77 | 0.36 | 0.33 | 0.98 |
| Fungal zoospores (×106 cells/ mL) | 1.10 | 1.05 | 1.28 | 1.26 | 0.12 | 0.23 | 0.91 |
| Total protozoa (×106 cells/mL) | 3.02 | 2.99 | 2.81 | 2.41 | 0.26 | 0.06 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxiselly, Y.; Chiarawipa, R.; Somnuk, K.; Hamchara, P.; Cherdthong, A.; Suntara, C.; Prachumchai, R.; Chanjula, P. Digestibility, Blood Parameters, Rumen Fermentation, Hematology, and Nitrogen Balance of Goats after Receiving Supplemental Coffee Cherry Pulp as a Source of Phytochemical Nutrients. Vet. Sci. 2022, 9, 532. https://doi.org/10.3390/vetsci9100532
Maxiselly Y, Chiarawipa R, Somnuk K, Hamchara P, Cherdthong A, Suntara C, Prachumchai R, Chanjula P. Digestibility, Blood Parameters, Rumen Fermentation, Hematology, and Nitrogen Balance of Goats after Receiving Supplemental Coffee Cherry Pulp as a Source of Phytochemical Nutrients. Veterinary Sciences. 2022; 9(10):532. https://doi.org/10.3390/vetsci9100532
Chicago/Turabian StyleMaxiselly, Yudithia, Rawee Chiarawipa, Krit Somnuk, Puwadon Hamchara, Anusorn Cherdthong, Chanon Suntara, Rittikeard Prachumchai, and Pin Chanjula. 2022. "Digestibility, Blood Parameters, Rumen Fermentation, Hematology, and Nitrogen Balance of Goats after Receiving Supplemental Coffee Cherry Pulp as a Source of Phytochemical Nutrients" Veterinary Sciences 9, no. 10: 532. https://doi.org/10.3390/vetsci9100532
APA StyleMaxiselly, Y., Chiarawipa, R., Somnuk, K., Hamchara, P., Cherdthong, A., Suntara, C., Prachumchai, R., & Chanjula, P. (2022). Digestibility, Blood Parameters, Rumen Fermentation, Hematology, and Nitrogen Balance of Goats after Receiving Supplemental Coffee Cherry Pulp as a Source of Phytochemical Nutrients. Veterinary Sciences, 9(10), 532. https://doi.org/10.3390/vetsci9100532









