MyD88 Mediates Colitis- and RANKL-Induced Microfold Cell Differentiation
Abstract
:1. Introduction
2. Methods and Materials
2.1. Animals and Related Materials
2.2. Inflammatory Models
2.3. Histological Analysis
2.4. Western Blot Analysis
2.5. Murine Intestinal Organoids Culture
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
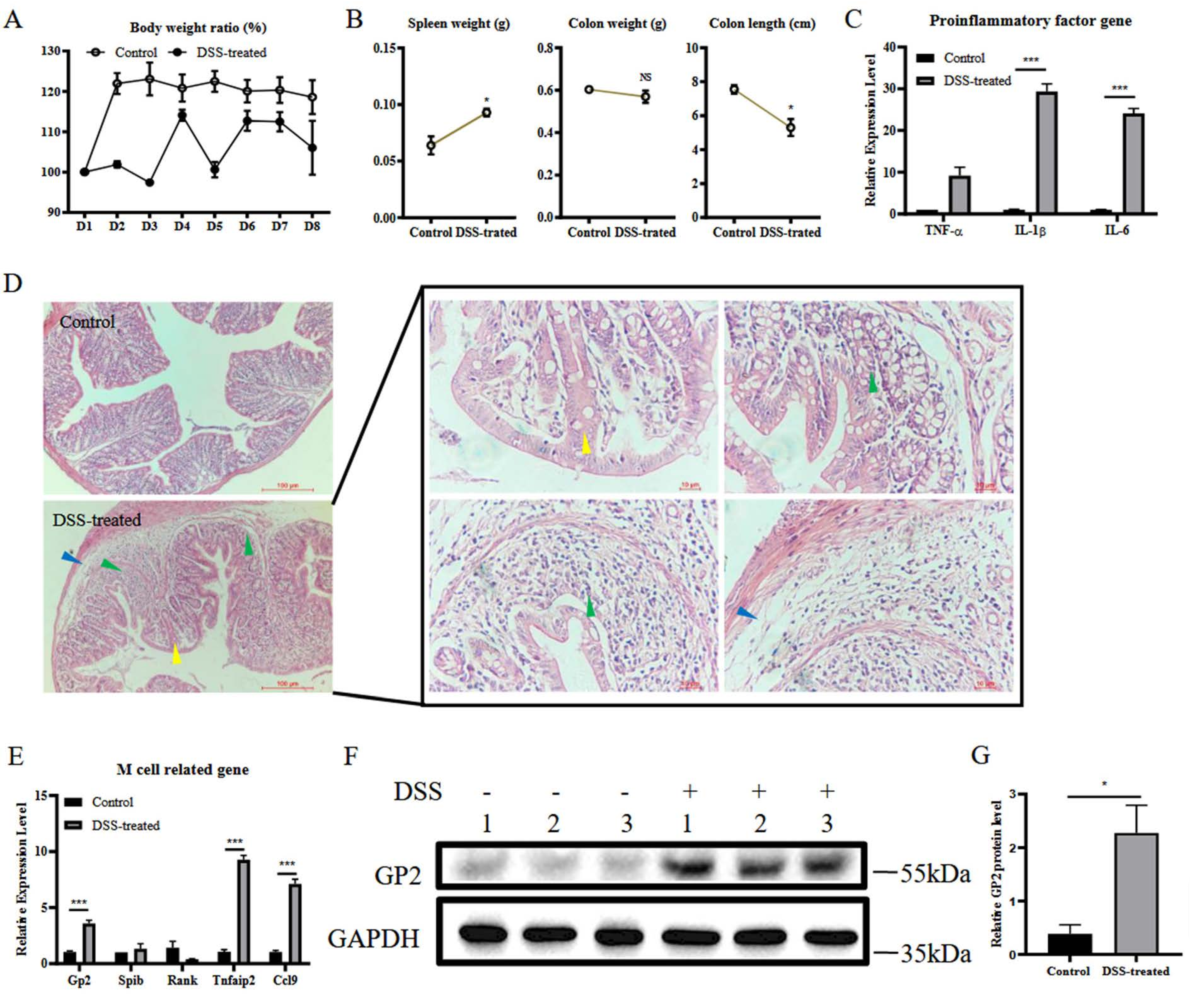
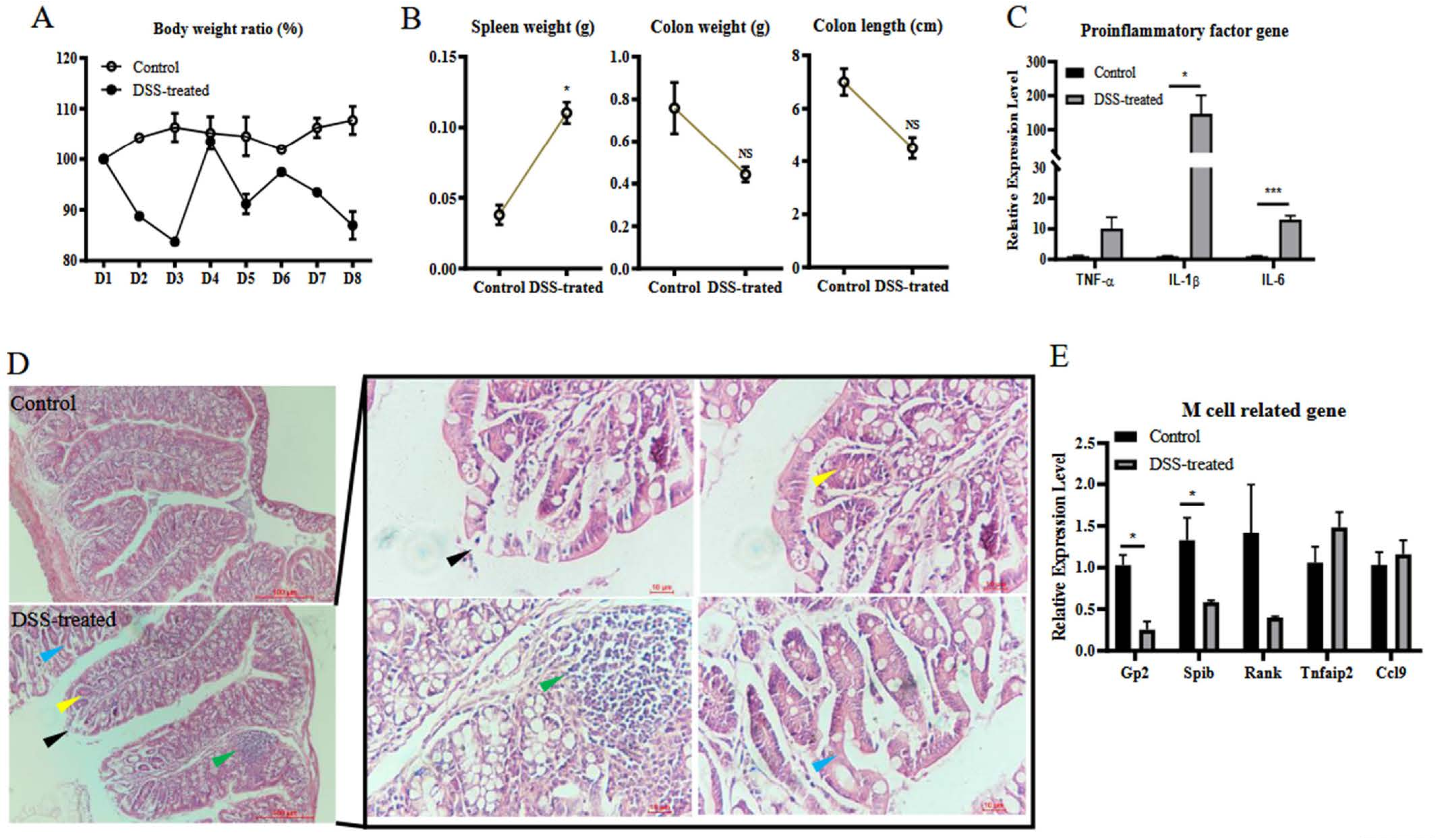
3.1. DSS Induces Colitis and M Cell Differentiation
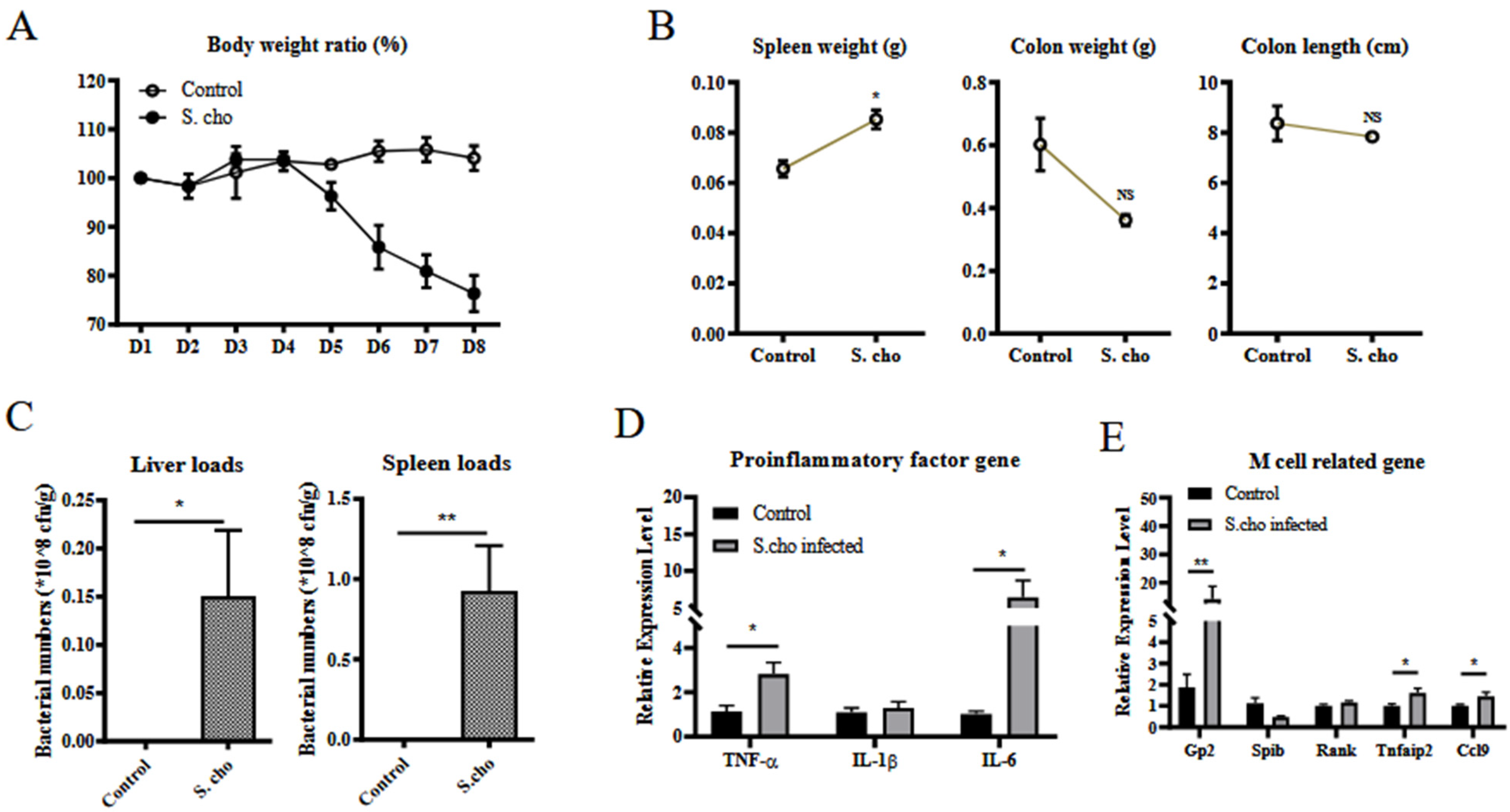
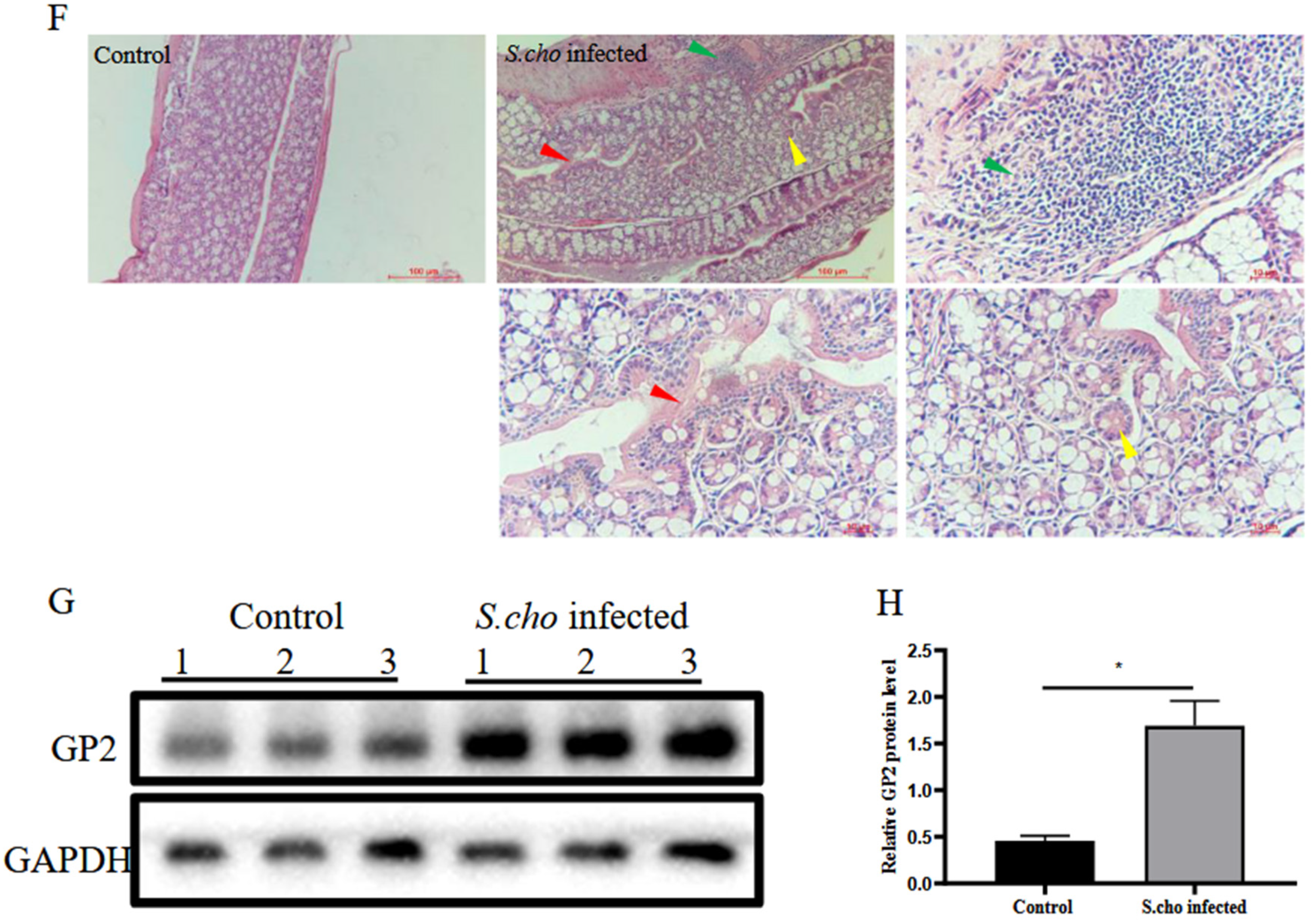
3.2. S. choleraesuis Infection Induces Colitis and M Cell Differentiation
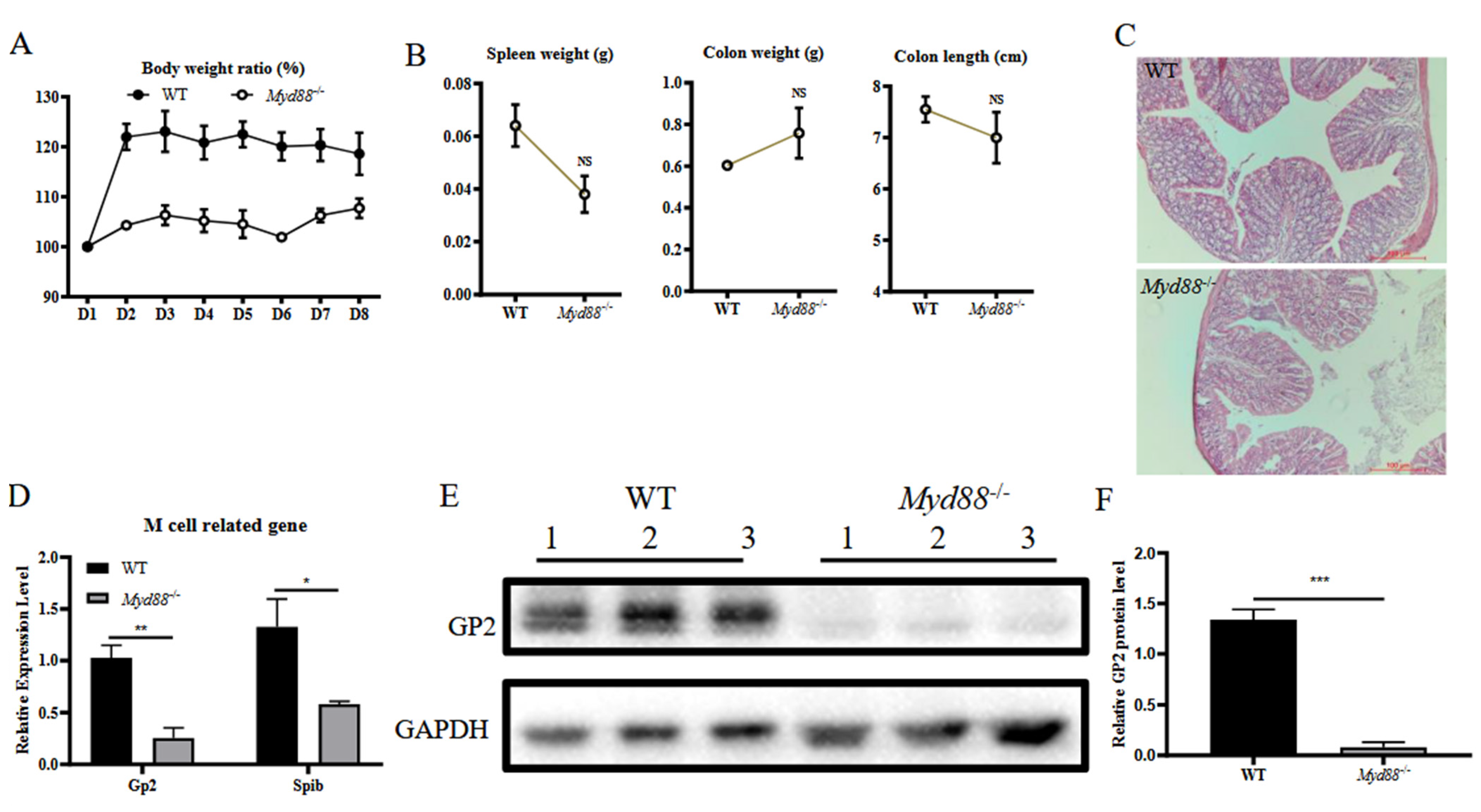
3.3. MyD88 Is a Critical Factor for Colonic M Cell Differentiation
3.4. MyD88 Is Involved in Colitis Induced M Cell Differentiation
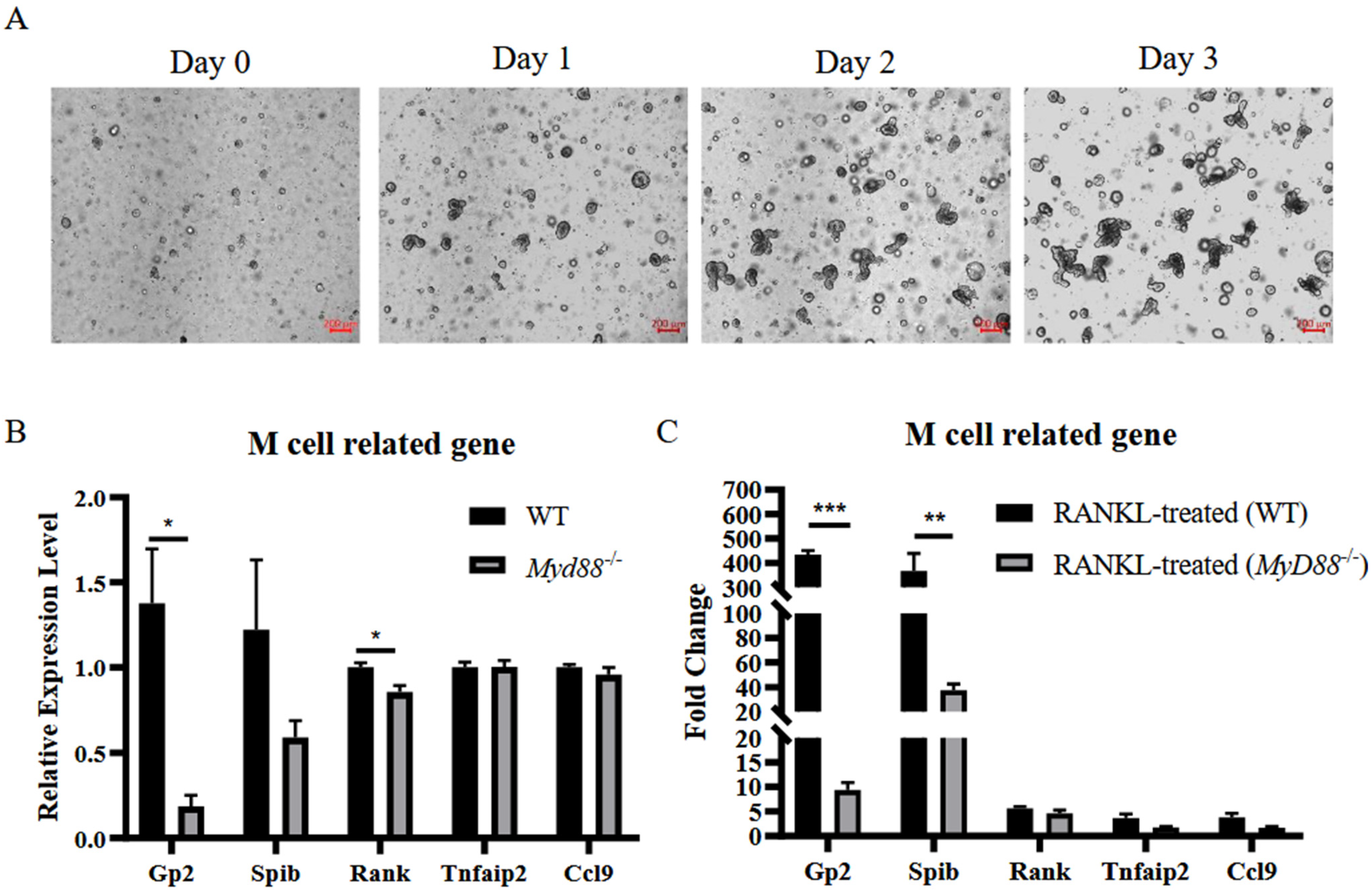
3.5. MyD88 Is Involved in RANKL-Induced M Cell Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schulz, O.; Pabst, O. Antigen Sampling in the Small Intestine. Trends Immunol. 2013, 34, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Valpotic, H.; Janjatovic, A.K.; Lackovic, G.; Bozic, F.; Dobranic, V.; Svoboda, D.; Valpotic, I.; Popovic, M. Increased Number of Intestinal Villous M Cells in Levamisole -Pretreated Weaned Pigs Experimentally Infected with F4ac(+) Enterotoxigenic Escherichia Coli Strain. Eur. J. Histochem. 2010, 54, e18. [Google Scholar] [CrossRef]
- Bennett, K.M.; Parnell, E.A.; Sanscartier, C.; Parks, S.; Chen, G.; Nair, M.G.; Lo, D.D. Induction of Colonic M Cells During Intestinal Inflammation. Am. J. Pathol. 2016, 186, 1166–1179. [Google Scholar] [CrossRef] [Green Version]
- Mutoh, M.; Kimura, S.; Takahashi-Iwanaga, H.; Hisamoto, M.; Iwanaga, T.; Iida, J. Rankl Regulates Differentiation of Microfold Cells in Mouse Nasopharynx-Associated Lymphoid Tissue (Nalt). Cell Tissue Res. 2016, 364, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) Cells: Important Immunosurveillance Posts in the Intestinal Epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Lo, D.D. Vigilance or Subversion? Constitutive and Inducible M Cells in Mucosal Tissues. Trends Immunol. 2017, 39, 185–195. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.B.; Liu, T.; Blanco, L.p.; Auble, H.; Payne, H.C.; Wobus, C.E. Murine Norovirus Transcytosis across an in Vitro Polarized Murine Intestinal Epithelial Monolayer Is Mediated by M-Like Cells. J. Virol. 2013, 87, 12685–12693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Hernandez, M.B.; Liu, T.; Payne, H.C.; Stencel-Baerenwald, J.E.; Ikizler, M.; Yagita, H.; Dermody, T.S.; Williams, I.R.; Wobus, C.E. Efficient Norovirus and Reovirus Replication in the Mouse Intestine Requires Microfold (M) Cells. J. Virol. 2014, 88, 6934–6943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.D.; Ghori, N.; Falkow, S. Salmonella Typhimurium Initiates Murine Infection by Penetrating and Destroying the Specialized Epithelial M Cells of the Peyer’s Patches. J. Exp. Med. 1994, 180, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Tang, J. Candida Albicans Infection and Intestinal Immunity. Microbiol. Res. 2017, 198, 27–35. [Google Scholar] [CrossRef]
- de Lau, W.; Kujala, P.; Schneeberger, K.; Middendorp, S.; Li, V.S.; Barker, N.; Martens, A.; Hofhuis, F.; DeKoter, R.P.; Peters, P.J.; et al. Peyer’s Patch M Cells Derived from Lgr5(+) Stem Cells Require Spib and Are Induced by Rankl in Cultured “Miniguts”. Mol. Cell. Biol. 2012, 32, 3639–3647. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Kaneto, S.; Shibata, N.; Takahashi, Y.; Okura, H.; Yuki, Y.; Kunisawa, J.; Kiyono, H. Transcription factor Spi-B–dependent and –independent pathways for the development of Peyer’s patch M cells. Mucosal Immunol. 2013, 6, 838–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.T.; Patel, S.R.; Lin, E.; Butler, B.R.; Lake, J.G.; Newberry, R.D.; Williams, I.R. Lymphotoxin-Independent Expression of Tnf-Related Activation-Induced Cytokine by Stromal Cells in Cryptopatches, Isolated Lymphoid Follicles, and Peyer’s Patches. J. Immunol. 2007, 178, 5659–5667. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.S.; Pollock, J.; Vohra, P.; Stevens, M.P.; Mabbott, N.A. Microbial Stimulation Reverses the Age-Related Decline in M Cells in Aged Mice. iScience 2020, 23, 101147. [Google Scholar] [CrossRef] [PubMed]
- Savidge, T.C.; Smith, M.W.; James, P.S.; Aldred, P. Salmonella-induced M-cell formation in germ-free mouse Peyer’s patch tissue. Am. J. Pathol. 1991, 139, 177–184. [Google Scholar] [PubMed]
- Moresco, E.M.; LaVine, D.; Beutler, B. Toll-Like Receptors. Curr. Biol. 2011, 21, R488–R493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-Like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, T.; Sakakibara, S.; Jinnohara, T.; Hachisuka, M.; Tachibana, N.; Hidano, S.; Kobayashi, T.; Kimura, S.; Iwanaga, T.; Nakagawa, T.; et al. Development of Intestinal M Cells and Follicle-Associated Epithelium Is Regulated by Traf6-Mediated Nf-Kappab Signaling. J. Exp. Med. 2018, 215, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Wang, B.; Yang, N.; Huang, X.; Chen, Q.; Geng, S.; Zhou, Y.; Shi, H.; Wang, L.; et al. Acute Porcine Epidemic Diarrhea Virus Infection Reshapes the Intestinal Microbiota. Virology 2020, 548, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, B.; Chen, Q.; Li, Y.; Li, B.; Yang, N.; Yang, S.; Geng, S.; Liu, G. Interleukin (Il)-21 Promotes the Differentiation of Iga-Producing Plasma Cells in Porcine Peyer’s Patches Via the Jak-Stat Signaling Pathway. Front. Immunol. 2020, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shi, H.; Yan, M.; Liu, G. Ifit5 Negatively Regulates the Type I Ifn Pathway by Disrupting Tbk1–Ikkε–Irf3 Signalosome and Degrading Irf3 and Ikkε. J. Immunol. 2021, 206, 2184–2197. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mimuro, H.; Kunisawa, J.; Furusawa, Y.; Takahashi, D.; Fujimura, Y.; Kaisho, T.; Kiyono, H.; Hase, K. Microfold Cell-Dependent Antigen Transport Alleviates Infectious Colitis by Inducing Antigen-Specific Cellular Immunity. Mucosal Immunol. 2020, 13, 679–690. [Google Scholar] [CrossRef]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through Glycoprotein 2 of Fimh+ Bacteria by M Cells Initiates Mucosal Immune Response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef]
- Kanaya, T.; Hase, K.; Takahashi, D.; Fukuda, S.; Hoshino, K.; Sasaki, I.; Hemmi, H.; Knoop, K.A.; Kumar, N.; Sato, M.; et al. The Ets Transcription Factor Spi-B Is Essential for the Differentiation of Intestinal Microfold Cells. Nat. Immunol. 2012, 13, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Tahoun, A.; Mahajan, S.; Paxton, E.; Malterer, G.; Donaldson, D.S.; Wang, D.; Tan, A.; Gillespie, T.L.; O’Shea, M.; Roe, A.J.; et al. Salmonella Transforms Follicle-Associated Epithelial Cells into M Cells to Promote Intestinal Invasion. Cell Host Microbe 2012, 12, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial Crosstalk at the Microbiota–Mucosal Interface. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4607–4614. [Google Scholar] [CrossRef] [Green Version]
- Larsson, E.; Tremaroli, V.; Lee, Y.S.; Koren, O.; Nookaew, I.; Fricker, A.; Nielsen, J.; Ley, R.E.; Bäckhed, F. Analysis of Gut Microbial Regulation of Host Gene Expression Along the Length of the Gut and Regulation of Gut Microbial Ecology through Myd88. Gut 2012, 61, 1124. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Oka, A.; Liu, B.; Herzog, J.W.; Eun, C.S.; Fan, T.-J.; Bulik-Sullivan, E.; Carroll, I.M.; Hansen, J.J.; Chen, L.; et al. Microbiota Maintain Colonic Homeostasis by Activating Tlr2/Myd88/Pi3k Signaling in Il-10–Producing Regulatory B Cells. J. Clin. Investig. 2019, 129, 3702–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Takahashi, N.; Suda, K.; Nakamura, M.; Yamaki, M.; Ninomiya, T.; Kobayashi, Y.; Takada, H.; Shibata, K.; Yamamoto, M.; et al. Myd88 but Not Trif Is Essential for Osteoclastogenesis Induced by Lipopolysaccharide, Diacyl Lipopeptide, and Il-1α. J. Exp. Med. 2004, 200, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, F.R.M.; de Aquino, S.G.; Guimarães, M.R.; Cirelli, J.A.; Zamboni, D.S.; Silva, J.S.; Junior, C.R. Relevance of the Myeloid Differentiation Factor 88 (Myd88) on Rankl, Opg, and Nod Expressions Induced by Tlr and Il-1r Signaling in Bone Marrow Stromal Cells. Inflammation 2015, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Nakamura, Y.; Kobayashi, N.; Shiroguchi, K.; Kawakami, E.; Mutoh, M.; Takahashi-Iwanaga, H.; Yamada, T.; Hisamoto, M.; Nakamura, M.; et al. Osteoprotegerin-Dependent M Cell Self-Regulation Balances Gut Infection and Immunity. Nat. Commun. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer/Probe | Sequence (5′–3′) |
|---|---|---|
| GAPDH | Forward | GCCTTCCGTGTTCCTACCC |
| Reverse | TGCCTGCTTCACCACCTTC | |
| Spi-B | Forward | GGGGGCCTTGACTCTA |
| Reverse | CTCTGGGGGGTACACC | |
| GP2 | Forward | CCTGCGTTCTGACACTG |
| Reverse | GCCGTGCAGGTTATCA | |
| RANK | Forward | ATGCGAACCAGGAAAGT |
| Reverse | TGCCTGCATCACAGACT | |
| Tnfaip2 | Forward | GTGCAGAACCTCTACCCCAATG |
| Reverse | TGGAGAATGTCGATGGCCA | |
| CCL9 | Forward | GCCCAGATCACACATGCAAC |
| Reverse | AGGACAGGCAGCAATCTGAA | |
| TNF-α | Forward | TCAGTTCCATGGCCCAGAC |
| Reverse | GTTGTCTTTGAGATCCATGCCATT | |
| IL-1β | Forward | CCCTGAACTCAACTGTGAAATAGCA |
| Reverse | CCCAAGTCAAGGGCTTGGAA | |
| IL-6 | Forward | TAGTCCTTCCTACCCCAATTTCC |
| Reverse | TTGGTCCTTAGCCACTCCTTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, S.; Huang, X.; Yang, N.; Wang, C.; Zhao, J.; Jing, Z.; Willems, L.; Liu, G. MyD88 Mediates Colitis- and RANKL-Induced Microfold Cell Differentiation. Vet. Sci. 2022, 9, 6. https://doi.org/10.3390/vetsci9010006
Li Y, Yang S, Huang X, Yang N, Wang C, Zhao J, Jing Z, Willems L, Liu G. MyD88 Mediates Colitis- and RANKL-Induced Microfold Cell Differentiation. Veterinary Sciences. 2022; 9(1):6. https://doi.org/10.3390/vetsci9010006
Chicago/Turabian StyleLi, Yang, Shanshan Yang, Xin Huang, Ning Yang, Caiying Wang, Jing Zhao, Zhizhong Jing, Luc Willems, and Guangliang Liu. 2022. "MyD88 Mediates Colitis- and RANKL-Induced Microfold Cell Differentiation" Veterinary Sciences 9, no. 1: 6. https://doi.org/10.3390/vetsci9010006
APA StyleLi, Y., Yang, S., Huang, X., Yang, N., Wang, C., Zhao, J., Jing, Z., Willems, L., & Liu, G. (2022). MyD88 Mediates Colitis- and RANKL-Induced Microfold Cell Differentiation. Veterinary Sciences, 9(1), 6. https://doi.org/10.3390/vetsci9010006






