Disseminated Toxoplasma gondii Infection in an Adult Osprey (Pandion haliaetus)
Abstract
:1. Introduction
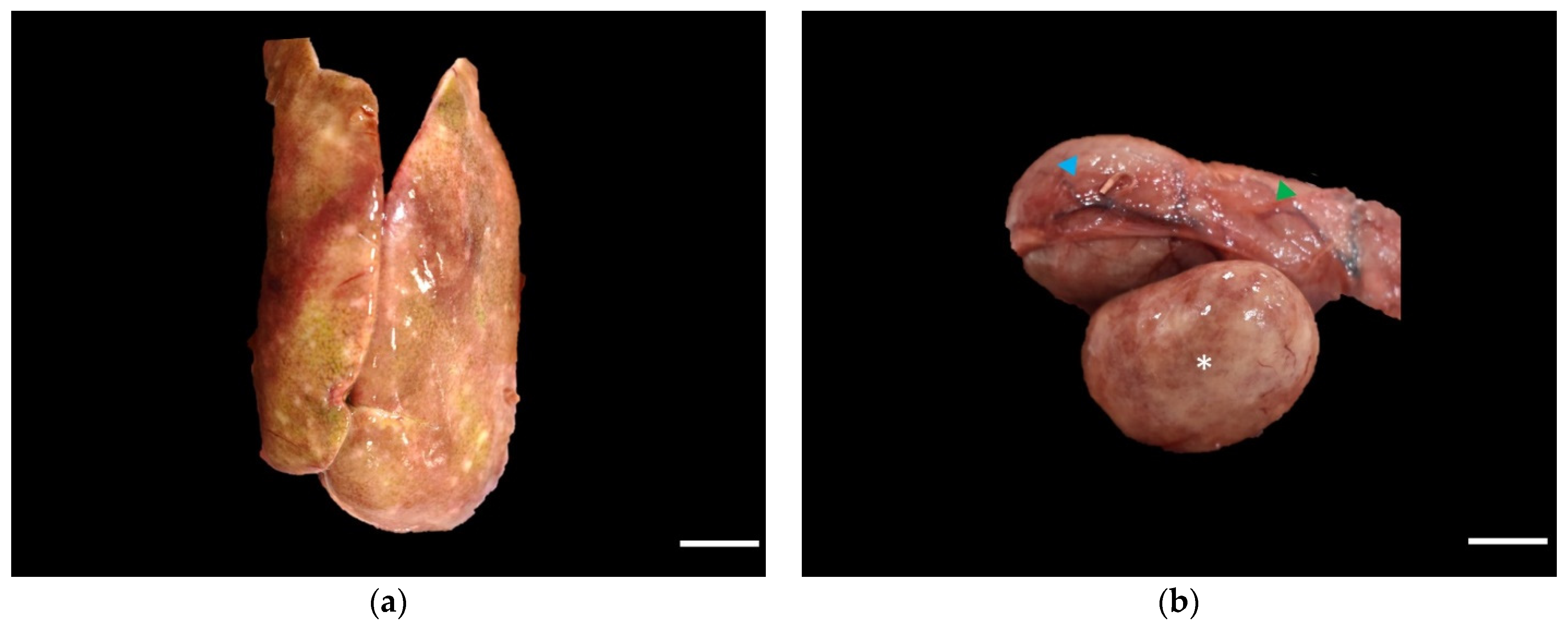
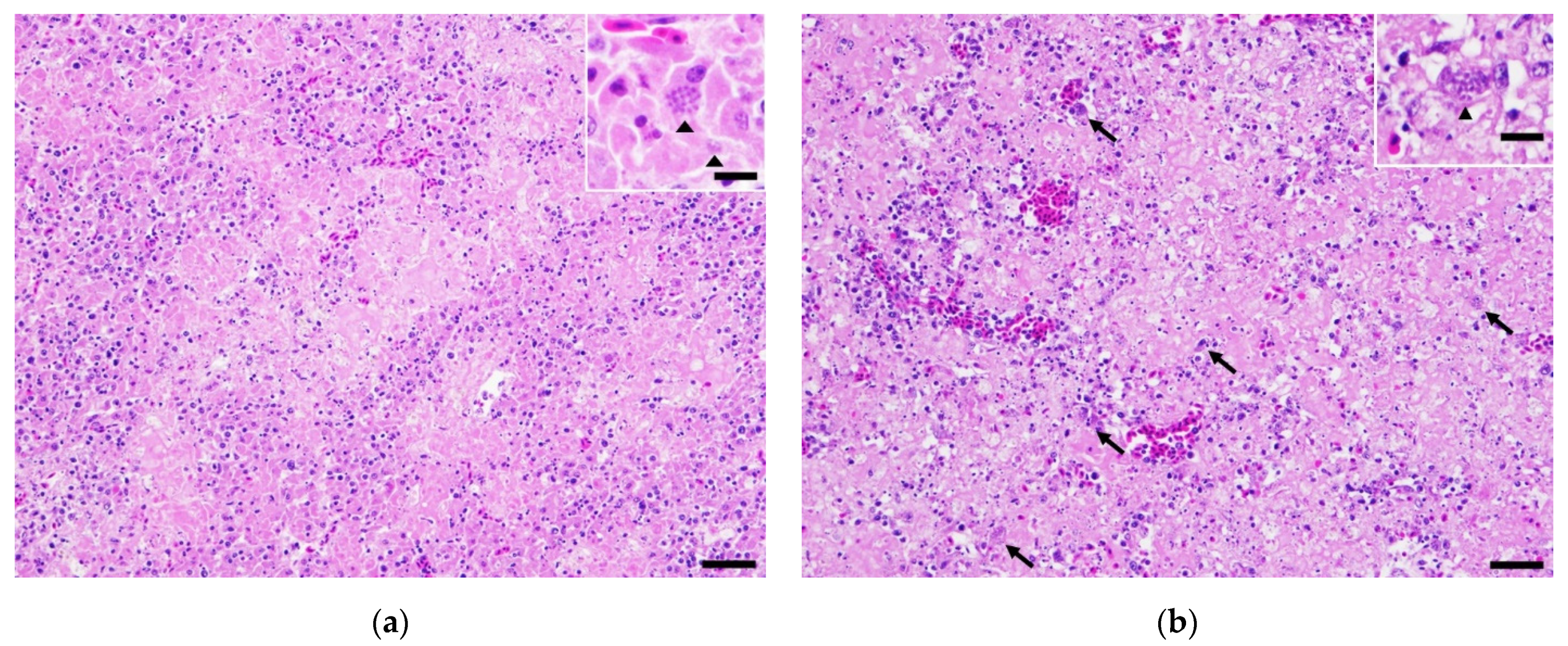
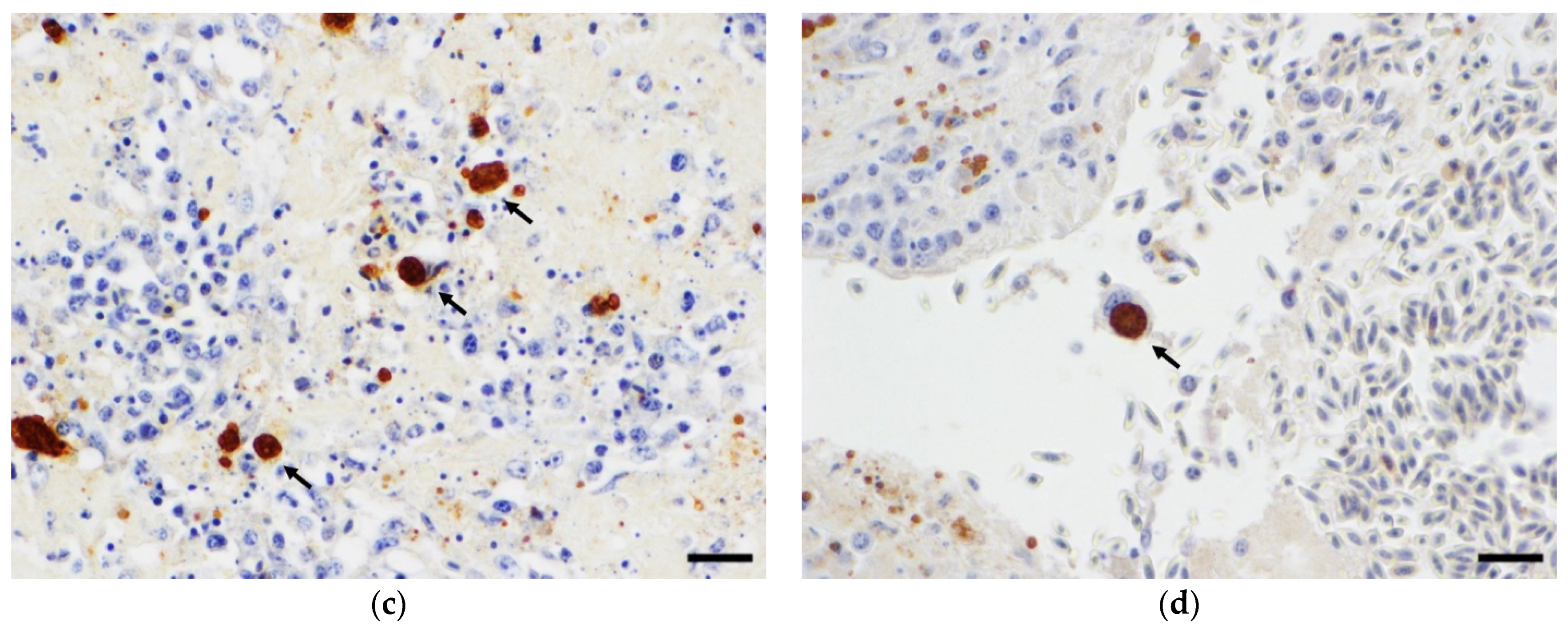
2. Case History
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Odening, K. Toxoplasmosis and Related Infections; Iowa State University Press: Ames, IA, USA, 2001. [Google Scholar]
- Dubey, J.P.; Felix, T.A.; Kwok, O.C. Serological and parasitological prevalence of Toxoplasma gondii in wild birds from Colorado. J. Parasitol. 2010, 96, 937–939. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [Green Version]
- Blader, I.J.; Coleman, B.I.; Chen, C.T.; Gubbels, M.J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Wang, Z.D.; Liu, H.H.; Ma, Z.X.; Ma, H.Y.; Li, Z.Y.; Yang, Z.B.; Zhu, X.Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 2017, 8, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.A.; Ouellette, C.; Chen, K.; Moretto, M. Toxoplasma: Immunity and Pathogenesis. Curr. Clin. Microbiol. Rep. 2019, 6, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Rougier, S.; Montoya, J.G.; Peyron, F. Lifelong Persistence of Toxoplasma Cysts: A Questionable Dogma? Trends Parasitol. 2017, 33, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Chen, T.C.; Kuo, T.T.; Tseng, C.C.; Tseng, C.P. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 2000, 38, 4121–4125. [Google Scholar] [CrossRef] [Green Version]
- Homan, W.L.; Vercammen, M.; De Braekeleer, J.; Verschueren, H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000, 30, 69–75. [Google Scholar] [CrossRef]
- Wright, D.K.; Manos, M.M. Sample Preparation from Paraffin-Embedded Tissues; Academic: San Diego, CA, USA, 1990. [Google Scholar]
- Lindsay, D.S.; Smith, P.C.; Hoerr, F.J.; Blagburn, B.L. Prevalence of encysted Toxoplasma gondii in raptors from Alabama. J. Parasitol. 1993, 79, 870–873. [Google Scholar] [CrossRef]
- Bierregaard, R. Osprey (Pandion haliaetus) Birds of the World. Cornell Laboratory of Ornithology; Ithaca: New York, NY, USA, 2020. [Google Scholar]
- Bierregaard, R. The Cornell Lab of Ornithology. Birds of the World. Available online: https://birdsoftheworld.org/bow/species/osprey/cur/introduction (accessed on 12 August 2021).
- Szabo, K.A.; Mense, M.G.; Lipscomb, T.P.; Felix, K.J.; Dubey, J.P. Fatal toxoplasmosis in a bald eagle (Haliaeetus leucocephalus). J. Parasitol. 2004, 90, 907–908. [Google Scholar] [CrossRef]
- Love, D.; Kwok, O.C.; Verma, S.K.; Dubey, J.P.; Bellah, J. Antibody Prevalence and Isolation of Viable Toxoplasma gondii from Raptors in the Southeastern USA. J. Wildl. Dis. 2016, 52, 653–656. [Google Scholar] [CrossRef]
- Mikaelian, I.; Dubey, J.P.; Martineau, D. Severe hepatitis resulting from toxoplasmosis in a barred owl (Strix varia) from Québec, Canada. Avian Dis. 1997, 41, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Cabezón, O.; García-Bocanegra, I.; Molina-López, R.; Marco, I.; Blanco, J.M.; Höfle, U.; Margalida, A.; Bach-Raich, E.; Darwich, L.; Echeverría, I.; et al. Seropositivity and risk factors associated with Toxoplasma gondii infection in wild birds from Spain. PLoS ONE 2011, 6, e29549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, D.S.; Dubey, J.P.; Blagburn, B.L. Toxoplasma gondii infections in red-tailed hawks inoculated orally with tissue cysts. J. Parasitol. 1991, 77, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Induced Toxoplasmosis in Owls. J. Zoo Wildl. Med. 1992, 23, 98–102. [Google Scholar]
- Dumètre, A.; Dardé, M.L. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiol. Rev. 2003, 27, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P. Toxoplasma gondii oocyst survival under defined temperatures. J. Parasitol. 1998, 84, 862–865. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 2009, 95, 1019–1020. [Google Scholar] [CrossRef]
- Dabritz, H.A.; Miller, M.A.; Gardner, I.A.; Packham, A.E.; Atwill, E.R.; Conrad, P.A. Risk factors for Toxoplasma gondii infection in wild rodents from central coastal California and a review of T. gondii prevalence in rodents. J. Parasitol. 2008, 94, 675–683. [Google Scholar] [CrossRef]
- Miller, M. Protozoan Parasites of Marine Mammals; CRC Press: Boca Raton, FL, USA, 2018; pp. 425–470. [Google Scholar]
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health 2019, 66, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massie, G.N.; Ware, M.W.; Villegas, E.N.; Black, M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010, 169, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Arkush, K.D.; Miller, M.A.; Leutenegger, C.M.; Gardner, I.A.; Packham, A.E.; Heckeroth, A.R.; Tenter, A.M.; Barr, B.C.; Conrad, P.A. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis). Int J. Parasitol. 2003, 33, 1087–1097. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Collins, M.V.; Mitchell, S.M.; Wetch, C.N.; Rosypal, A.C.; Flick, G.J.; Zajac, A.M.; Lindquist, A.; Dubey, J.P. Survival of Toxoplasma gondii oocysts in Eastern oysters (Crassostrea virginica). J. Parasitol. 2004, 90, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Krusor, C.; Smith, W.A.; Tinker, M.T.; Silver, M.; Conrad, P.A.; Shapiro, K. Concentration and retention of Toxoplasma gondii oocysts by marine snails demonstrate a novel mechanism for transmission of terrestrial zoonotic pathogens in coastal ecosystems. Environ. Microbiol. 2015, 17, 4527–4537. [Google Scholar] [CrossRef] [PubMed]
- Schott, K.C.; Krusor, C.; Tinker, M.T.; Moore, J.; Conrad, P.A.; Shapiro, K. Concentration and retention of Toxoplasma gondii surrogates from seawater by red abalone (Haliotis rufescens). Parasitology 2016, 143, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Omata, Y.; Umeshita, Y.; Murao, T.; Kano, R.; Kamiya, H.; Kudo, A.; Masukata, Y.; Kobayashi, Y.; Maeda, R.; Saito, A.; et al. Toxoplasma gondii does not persist in goldfish (Carassius auratus). J. Parasitol. 2005, 91, 1496–1499. [Google Scholar] [CrossRef]
- Sanders, J.L.; Zhou, Y.; Moulton, H.M.; Moulton, Z.X.; McLeod, R.; Dubey, J.P.; Weiss, L.M.; Kent, M.L. The zebrafish, Danio rerio, as a model for Toxoplasma gondii: An initial description of infection in fish. J. Fish. Dis. 2015, 38, 675–679. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Talbot, C.T.; Yin, J.-H.; Kalalah, A.A.; Wang, C.; Newton, J.C. Disseminated Toxoplasma gondii Infection in an Adult Osprey (Pandion haliaetus). Vet. Sci. 2022, 9, 5. https://doi.org/10.3390/vetsci9010005
Wang X, Talbot CT, Yin J-H, Kalalah AA, Wang C, Newton JC. Disseminated Toxoplasma gondii Infection in an Adult Osprey (Pandion haliaetus). Veterinary Sciences. 2022; 9(1):5. https://doi.org/10.3390/vetsci9010005
Chicago/Turabian StyleWang, Xiaobo, Charles T. Talbot, Ji-Hang Yin, Anwar A. Kalalah, Chengming Wang, and Joseph C. Newton. 2022. "Disseminated Toxoplasma gondii Infection in an Adult Osprey (Pandion haliaetus)" Veterinary Sciences 9, no. 1: 5. https://doi.org/10.3390/vetsci9010005
APA StyleWang, X., Talbot, C. T., Yin, J.-H., Kalalah, A. A., Wang, C., & Newton, J. C. (2022). Disseminated Toxoplasma gondii Infection in an Adult Osprey (Pandion haliaetus). Veterinary Sciences, 9(1), 5. https://doi.org/10.3390/vetsci9010005






