First Isolation and Multilocus Sequence Typing of Brucella canis from a Subclinically Infected Pet Dog in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Sample
2.2. Serological Tests
2.3. Bacteriological Studies
2.4. DNA Extraction
2.5. Polymerase Chain Reaction Tests
2.6. MLST Genotyping
2.7. Analysis of MLST Data
3. Results
3.1. Serological and Bacteriological Tests
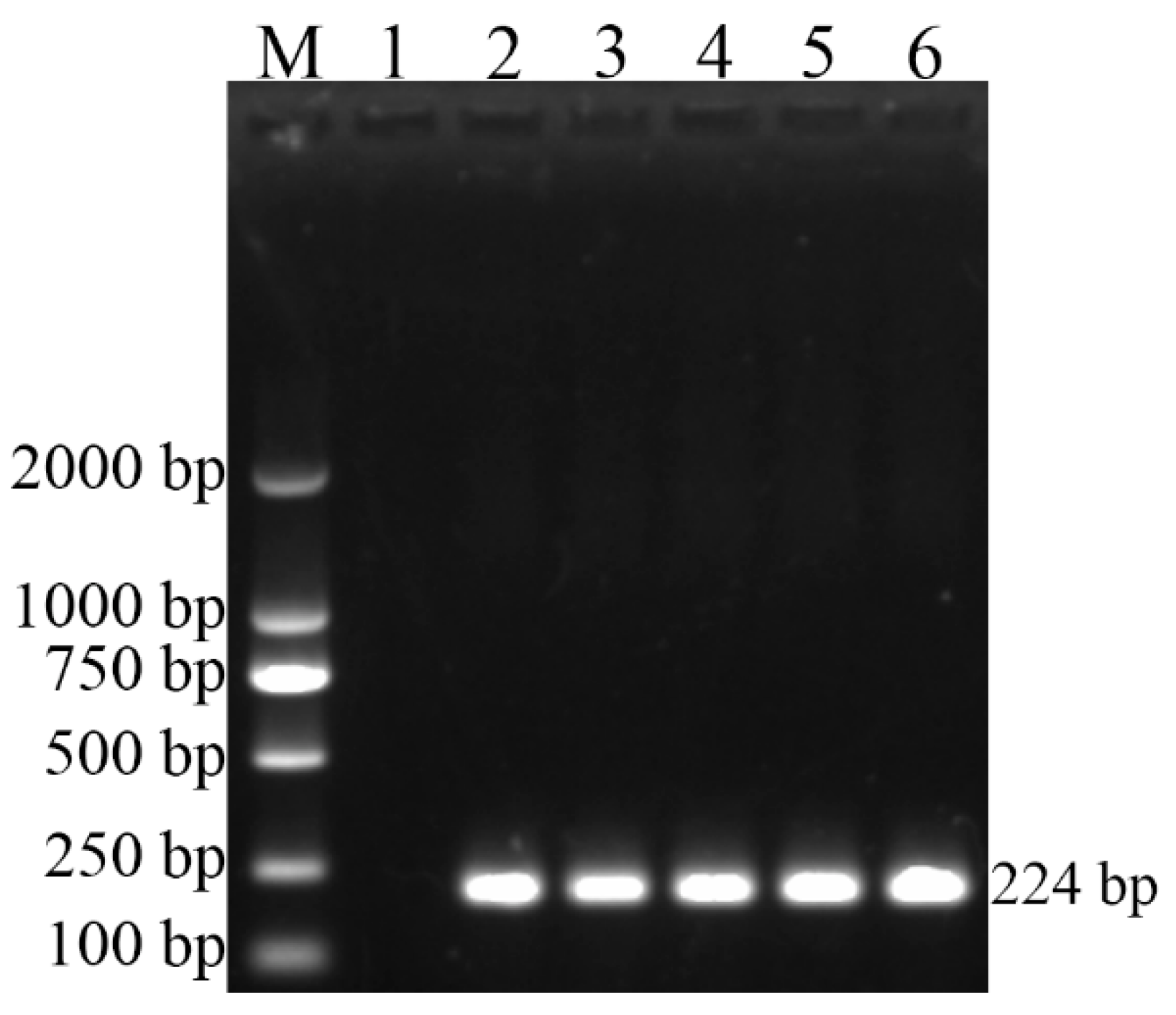
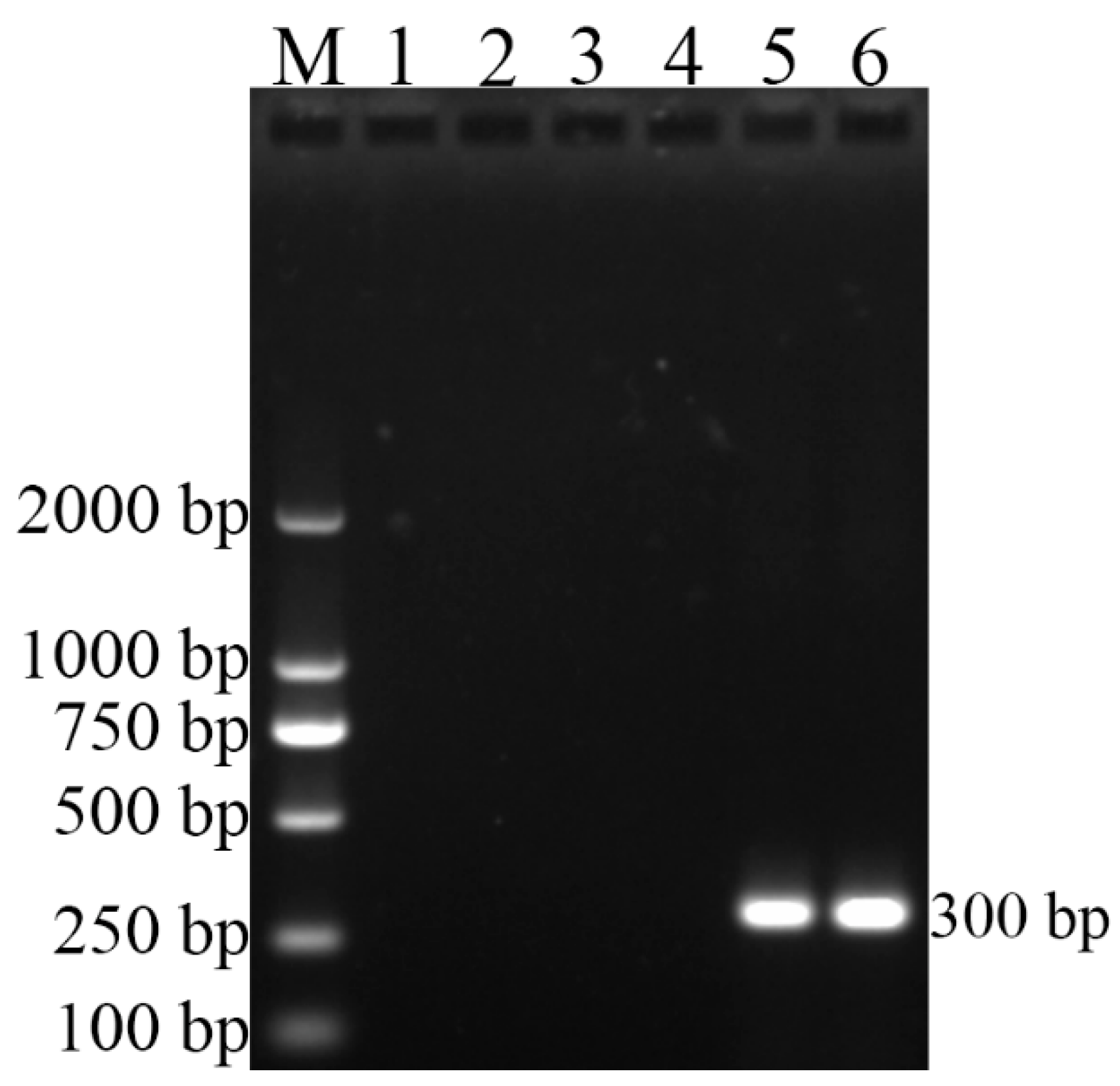
3.2. Polymerase Chain Reaction Tests
3.3. MLST Genotyping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, Z.; Yu, S.; Wang, X.; Dong, S.; Xu, J.; Wang, Y.; Chen, Z.; Ren, Z.; Peng, G. Human brucellosis in the People’s Republic of China during 2005–2010. Int. J. Infect. Dis. 2013, 17, e289–e292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, H. The Brucellae and Their Success as Pathogens. Crit. Rev. Microbiol. 2007, 33, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Hensel, M.E.; Negron, M.; Arenas-Gamboa, A.M. Brucellosis in Dogs and Public Health Risk. Emerg. Infect. Dis. 2018, 24, 1401–1406. [Google Scholar] [CrossRef] [Green Version]
- Deqiu, S.; Donglou, X.; Jiming, Y. Epidemiology and control of brucellosis in China. Veter-Microbiology 2002, 90, 165–182. [Google Scholar] [CrossRef]
- Zhong, Z.; Tu, R.; Wang, X.; Geng, Y.; Xiao, Q.; Tian, Y.; Wei, B.; Dan, J.; Wang, Y.; Peng, G. First Isolation of Brucella canis from Pet Dogs in Sichuan Province, China: Molecular Characterization, Pathogenicity and Antigen Location Analysis. Pak. J. Zool. 2019, 52, 283–291. [Google Scholar] [CrossRef]
- Piao, D.; Wang, H.; Di, D.; Tian, G.; Luo, J.; Gao, W.; Zhao, H.; Xu, W.; Fan, W.; Jiang, H. MLVA and LPS Characteristics of Brucella canis Isolated from Humans and Dogs in Zhejiang, China. Front. Veter-Sci. 2017, 4, 223. [Google Scholar] [CrossRef] [Green Version]
- Munford, R.S.; E Weaver, R.; Patton, C.; Feeley, J.C.; A Feldman, R. Human disease caused by Brucella canis. A clinical and epidemiologic study of two cases. JAMA 1975, 231, 1267–1269. [Google Scholar] [CrossRef]
- Dentinger, C.M.; Jacob, K.; Lee, L.V.; Mendez, H.A.; Chotikanatis, K.; McDonough, P.L.; Chico, D.M.; De, B.K.; Tiller, R.V.; Traxler, R.M.; et al. Human Brucella canis Infection and Subsequent Laboratory Exposures Associated with a Puppy, New York City, 2012. Zoonoses Public Health 2014, 62, 407–414. [Google Scholar] [CrossRef]
- Swenson, R.M.; Carmichael, L.E.; Cundy, K.R. Human Infection with Brucella canis. Ann. Intern. Med. 1972, 76, 435–438. [Google Scholar] [CrossRef]
- Lucero, N.E.; Corazza, R.; Almuzara, M.N.; Reynes, E.; Escobar, G.I.; Boeri, E.; Ayala, S.M. Human Brucella canis outbreak linked to infection in dogs. Epidemiol. Infect. 2009, 138, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Nomura, A.; Imaoka, K.; Imanishi, H.; Shimizu, H.; Nagura, F.; Maeda, K.; Tomino, T.; Fujita, Y.; Kimura, M.; Stein, G.H. Human Brucella canis Infections Diagnosed by Blood Culture. Emerg. Infect. Dis. 2010, 16, 1183–1185. [Google Scholar] [CrossRef]
- Tosi, M.F.; Nelson, T.J. Brucella canis infection in a 17-month-old child successfully treated with moxalactam. J. Pediatr. 1982, 101, 725–727. [Google Scholar] [CrossRef]
- Kang, S.-I.; Lee, S.-E.; Kim, J.-Y.; Lee, K.; Kim, J.-W.; Lee, H.-K.; Sung, S.-R.; Heo, Y.-R.; Jung, S.C.; Her, M. A new Brucella canis species-specific PCR assay for the diagnosis of canine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hua, X.; Cui, L. Characterization of microbiota diversity of engorged ticks collected from dogs in China. J. Veter-Sci. 2021, 22, e37. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; Perrett, L.L.; Macmillan, A.P.; Whatmore, A.M.; Perrett, L.L.; Macmillan, A.P. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 2007, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Ke, Y.; Wang, Y.; Yuan, X.; Zhou, X.; Jiang, H.; Wang, Z.; Zhen, Q.; Yu, Y.; Huang, L.; et al. Changes of predominant species/biovars and sequence types of Brucella isolates, Inner Mongolia, China. BMC Infect. Dis. 2013, 13, 514. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Shang, Y.; Liu, Y.; Li, Z.; Jing, Z. Genetic Characterization of Animal Brucella Isolates from Northwest Region in China. BioMed Res. Int. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-G.; Wang, M.; Zhao, H.-Y.; Piao, D.-R.; Jiang, H.; Li, Z.-J. Investigation of the molecular characteristics of Brucella isolates from Guangxi Province, China. BMC Microbiol. 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Neubauer, H.; Melzer, F.; Khan, I.; Akhter, S.; Jamil, T.; Umar, S. Molecular Identification of Bovine Brucellosis Causing Organisms at Selected Private Farms in Pothohar Plateau, Pakistan. Pak. J. Zool. 2017, 49, 1111–1114. [Google Scholar] [CrossRef]
- Alton, G.G.; Jones, L.M.; Pietz, D.E. Laboratory Techniques in Brucellosis. Monogr. Ser. World Health Organ 1975, 55, 1–163. [Google Scholar]
- Al Dahouk, S.; Tomaso, H.; Nöckler, K.; Neubauer, H.; Frangoulidis, D. Laboratory-based diagnosis of brucellosis--a review of the literature. Part I: Techniques for direct detection and identification of Brucella spp. Clin. Lab. 2003, 49, 487–505. [Google Scholar]
- Imaoka, K.; Kimura, M.; Suzuki, M.; Kamiyama, T.; Yamada, A. Simultaneous detection of the genus Brucella by combinatorial PCR. Jpn. J. Infect. Dis. 2007, 60, 137–139. [Google Scholar] [PubMed]
- Kauffman, L.K.; Petersen, C.A. Canine Brucellosis. Veter-Clin. N. Am. Small Anim. Pract. 2019, 49, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Wang, M.; Li, Z. Investigation of the molecular epizootiological characteristics and tracking of the geographical origins of Brucella canis strains in China. Transbound. Emerg. Dis. 2019, 67, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Wei, H.; Zhao, J.; Zhou, G.; Mao, K.; Cheng, J.; Shi, Z. Seroprevalence of Canine Brucellosis in Beijing, China. Chin. J. Vet. Med. 2009, 45, 64–65. (In Chinese) [Google Scholar]
- Wang, N.; Han, J.; Lv, Y.; Wu, Q. Isolation and Identification of Brucella from Pet Dogs in Beijing, China. Chin. J. Pre. Vet. Med. 2014, 36, 490–492. (In Chinese) [Google Scholar]
- Zhou, G.; I, X.L.; Wu, Q.; Cheng, J.; Wang, N.; Ding, J.; Mao, K.; Wei, H.; Xue, S.; Zhu, J. Isolation and Identification of a Brucella Canis Strain. Chin. J. Vet. Drug 2013, 47, 11–13. (In Chinese) [Google Scholar]
- Whatmore, A.M.; Koylass, M.S.; Muchowski, J.; Edwards-Smallbone, J.; Gopaul, K.K.; Perrett, L.L. Extended Multilocus Sequence Analysis to Describe the Global Population Structure of the Genus Brucella: Phylogeography and Relationship to Biovars. Front. Microbiol. 2016, 7, 2049. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| PCR tests | ||
| BCSP31 | F:TGGCTCGGTTGCCAATATCAA | 224 |
| R:CGCGCTTGCCTTTCAGGTCTG | ||
| BcSS | F:CCAGATAGACCTCTCTGGA | 300 |
| R:TGGCCTTTTCTGATCTGTTCTT | ||
| MLST genotyping | ||
| gap | F:YGCCAAGCGCGTCATCGT | 589 |
| R:GCGGYTGGAGAAGCCCCA | ||
| aroA | F:GACCATCGACGTGCCGGG | 565 |
| R:YCATCAKGCCCATGAATTC | ||
| glk | F:TATGGAAMAGATCGGCGG | 475 |
| R:GGGCCTTGTCCTCGAAGG | ||
| dnaK | F:CGTCTGGTCGAATATCTGG | 470 |
| R:GCGTTTCAATGCCGAGCGA | ||
| gyrB | F:ATGATTTCATCCGATCAGGT | 469 |
| R:CTGTGCCGTTGCATTGTC | ||
| trpE | F:GCGCGCMTGGTATGGCG | 486 |
| R:CKCSCCGCCATAGGCTTC | ||
| cobQ | F:GCGGGTTTCAAATGCTTGGA | 422 |
| R:GGCGTCAATCATGCCAGC | ||
| int_hyp | F:CAACTACTCTGTTGACCCGA | 430 |
| R:GCAGCATCATAGCGACGGA | ||
| omp25 | F:ATGCGCACTCTTAAGTCTC | 490 |
| R:GCCSAGGATGTTGTCCGT |
| Isolate | CO2 Requirement | H2S Production | Hydrolysis of Urea | Agglutination in Sera | Growth on Dyes | Phage Lysis Tests | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | R | Thionin | Basic Fuchsin | Tb | BK2 | Wb | ||||
| CD3 | - 1 | - | + 2 | - | - | + | + | - | - | - | - |
| RM6/66 | - | - | + | - | - | + | + | - | - | - | - |
| Species | Strains | PCR Results | |
|---|---|---|---|
| BCSP31-PCR | BcSS-PCR | ||
| B. abortus | 544A | + 1 | - 2 |
| B. melitensis | 16M | + | - |
| B. suis | S2 | + | - |
| B. canis | RM6/66 | + | + |
| B. canis | CD3 | + | + |
| Strains | Host | Year | Gap | aroA | glk | dnaK | gyrB | trpE | cobQ | int_hyp | omp25 | ST |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WJ5 | dog (golden retriever) | 2015 | 1 | 6 | 4 | 1 | 5 | 3 | 5 | 4 | 5 | 21 |
| YA4 | dog (poodle) | 2016 | 1 | 6 | 4 | 1 | 5 | 3 | 5 | 4 | 5 | 21 |
| CD3 | dog (golden retriever) | 2019 | 1 | 6 | 4 | 1 | 5 | 3 | 5 | 4 | 5 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Pang, Z.; Hu, Y.; Zhou, Z.; Liu, H.; Luo, Y.; Ren, Z.; Ma, X.; Cao, S.; Shen, L.; et al. First Isolation and Multilocus Sequence Typing of Brucella canis from a Subclinically Infected Pet Dog in China. Vet. Sci. 2022, 9, 22. https://doi.org/10.3390/vetsci9010022
Yan G, Pang Z, Hu Y, Zhou Z, Liu H, Luo Y, Ren Z, Ma X, Cao S, Shen L, et al. First Isolation and Multilocus Sequence Typing of Brucella canis from a Subclinically Infected Pet Dog in China. Veterinary Sciences. 2022; 9(1):22. https://doi.org/10.3390/vetsci9010022
Chicago/Turabian StyleYan, Guangwen, Zidong Pang, Yan Hu, Ziyao Zhou, Haifeng Liu, Yan Luo, Zhihua Ren, Xiaoping Ma, Suizhong Cao, Liuhong Shen, and et al. 2022. "First Isolation and Multilocus Sequence Typing of Brucella canis from a Subclinically Infected Pet Dog in China" Veterinary Sciences 9, no. 1: 22. https://doi.org/10.3390/vetsci9010022
APA StyleYan, G., Pang, Z., Hu, Y., Zhou, Z., Liu, H., Luo, Y., Ren, Z., Ma, X., Cao, S., Shen, L., Wang, Y., Gou, L., Cai, D., Zhu, Y., Zhong, Y., Li, W., Shi, X., Peng, G., & Zhong, Z. (2022). First Isolation and Multilocus Sequence Typing of Brucella canis from a Subclinically Infected Pet Dog in China. Veterinary Sciences, 9(1), 22. https://doi.org/10.3390/vetsci9010022









