Abstract
Control measures against common cattle tick Rhipicephalus microplus are of the upmost importance because of considerable, deleterious impact on a farm’s economy. Due to resistance phenomena to synthetic acaricides being a constraint in affected farms, the search for plant derivatives as acaricides has increased dramatically in recent years. In this work, essential oils obtained from two Ecuadorian plants, Ambrosia peruviana and Lepechinia mutica (EOAp, EOLm), traditionally used as insecticides in indigenous communities, were studied on larvae and engorged females at the parasitic stages of R. microplus. Larvae and females were treated with five (0.0625, 0.125, 0.25, 0.50 and 1%) and six concentrations (0.125, 0.25, 0.50, 1, 2 and 4%), respectively, of each EOsAp/Lm. A 98–99% larval mortality was achieved with 0.5% of both EOsAp/Lm. EOAp inhibited oviposition and egg hatching up to 82% and 80%, respectively, and had an overall efficacy of 93.12%. Efficacy of EOLm was 72.84%, due to the low influence of EOLm on reproductive parameters. By steam distillation and GC-MS analysis, γ-Curcumene was identified as the main constituent (52.02%) in the EOAp and Shyobunol (10.80%) in EOLm. The results suggest that major components of both essential oils should be further studied as promissory acaricides against R. microplus.
1. Introduction
The cattle tick Rhipicephalus microplus Canestrini, 1888 (Acari: Ixodidae) is well known in tropical and semitropical regions because of the harmful effects over the cattle production. These harmful effects result in anemia, decreased productivity, depression of the immune system, damage to hides, overspending due to tick control measures and the morbidity and mortality caused by tick-borne diseases (e.g., babesiosis due to Babesia bigemina, Babesia bovis) [1]. The life cycle of R. microplus includes four developmental stages: egg, larva, nymph and adult. The newly hatched larvae creep upon plants or grass to access the host, seek the flanks, thighs, forelegs and udders for attachment. On a typical host, R. microplus spends the rest of the life cycle in the same animal; only the engorged female will leave the host to lay her eggs on the ground. In Ecuador, unlimited use of tick-controlling chemicals has resulted in environmental pollution, milk and meat contamination; resistance development in the tick species has been expressed by veterinarians (no published data) and in published reports [2]. Annual potential losses due to R. microplus infections were estimated to be USD 3.24 billion in Brazil [3] and USD 573.6 million in Mexico [4]. Industrial, synthetic acaricides have long been used to effectively control the parasitic stages of the tick; however, these molecules have several disadvantages, including development of tick resistance, destruction of saprophytic species and permanence of residues in the animals and the environment with risks to animal and human health [5]. The intensification of the livestock production requires the use of environmentally friendly compounds to control tick infections.
At the present time, Ecuador is considered to be one of the countries possessing the highest rates of biodiversity in the world; 30% of the Ecuadorian populations are indigenous communities, which maintain their ancestral traditions in the use of natural remedies related to medical applications of plants. It makes this country an ideal place to discover new secondary metabolites, as a result of the chemical investigation of living organisms [6]. Plants accumulate organic substances in significant quantities and concentrations and are a renewable source of these substances; hence, they could be exploited economically and sustainably [7]. The use of plant derivatives in the control of veterinary ectoparasites is an area that holds considerable potential for the future and research into their use in vivo is just beginning [8]. Antimicrobial, insecticide and acaricidal effects of essential oils (EOs) have been exhaustively demonstrated [9,10,11]; numerous studies have confirmed the effect of herbal products on ticks, as larval mortality, reductions in weight, egg-laying, fecundity, and egg viability [12,13,14,15]. Compared to synthetic acaricides, phytoacaricides have several advantages: they are eco-friendly, biodegradable and resistance tends to develop slowly because they are a mixture of several active agents with different mechanisms of action [16]. In addition, plant oils enhanced the toxicity of permethrin when applied in combination with a discrete dose of the synthetic pyrethroid [17]. Hence, investigations on plant derivatives constitute a useful approach because EOs are promising sources of naturally occurring bioactive compounds that show acaricide/insecticidal activities. However, although the cytotoxicity caused by essential oils may be advantageous for killing ticks, the oils are not always harmless. For example, essential oils, including those from Salvia sclarea and Melaleuca quinquenervia, provoke estrogen secretions, which can induce estrogen-dependent cancers. Some essential oils contain photosensitizing molecules (flavins, cyanin, porphyrins and hydrocarbures), which can cause skin erythema or cancer [18].
Ambrosia peruviana Willd belongs to the largest family Asteraceae (Compositae), with more than 1620 genera and 23,600 species of herbaceous plants, shrubs, and trees distributed throughout the world. Ambrosia peruviana, also known as Peruvian ragweed or “marco”,”altamisa”, “artemisa” in Ecuador and Peru, is widely used in indigenous traditional medicine to treat rheumathism, menstruation disorders, neurological disturbances and as vermifuge and insecticide [19]. There are 35 natural activities of Ambrosia peruviana reported from the Phytochemical Interactions Data Base [20].
Lepechinia mutica Benth, also known as “Shalshon”, “turullante”, “casa-casa” by Saraguro communities, is endemic to Loja Province and belongs to the Lamiaceae family that comprises approximately 224 genera; the Lepechinia genus includes nine species, among which four are endemic to Ecuador [21,22]. Several species are valued in the horticultural trade and used in folk medicine for the treatment of uterine tumors, stomach ailments, diabetes mellitus control and diarrhea treatment. In particular, the leaves of L. mutica are believed to relieve headache and nervous affections and Saraguro reported to be used in veterinary medicine [23]. Lepechinia mutica essential oil exhibited moderate in vitro activity against five fungal strains, being especially effective against Microsporum canis [23,24].
Until now, there have been no reports about the effect of A. peruviana or L. mutica derivatives on ticks. This work aimed at evaluating the acaricidal effect of the EOs obtained from A. peruviana and L. mutica on parasitic stages, larvae and engorged adult females, of the common cattle tick R. microplus.
2. Materials and Methods
2.1. Obtention of Engorged Females of R. microplus
One-hundred eighty engorged females of Rhipicephalus microplus were collected from a bovine in Punzara location at Loja City, Loja Province, Ecuador (04°02′00″ S, 79°14′00″ W) with unpublished reports on shortening of acaricide application times. Throughout the tick sampling, it was difficult to find animals (a) from the same geographical area under the same tick control management, (b) that were not treated with acaricides for more than 30 days and (c) parasitized by an appropriate number of engorged females. Specimens were kept in plastic flasks with small holes until assays, no more than two days after collection. Rhipicephalus microplus females were identified by standard keys [25] and used for the assays.
2.2. Larval Package Test (LPT)
Ten adult engorged females were stuck to the lid of a glass Petri dish with double-sided sticky tape and maintained in an incubator at 29 ± 1 °C and relative humidity (RH) 80% for 20 days to obtain eggs and larvae as by Rey-Valeirón et al. [13]. One hundred larvae of 21 days old were used for LPT as by Stone and Haydock [26] with some modifications reported previously [13]. Five concentrations of A. peruviana EO (EOAp) and L. mutica EO (EOLm) were used (0.0625; 0.125; 0.25; 0.50 and 1%) diluted in 2% Tween40 (v/v). The positive control groups (most used commercial acaricides in Ecuador, cypermethrin 15% and amitraz 12.5%), were prepared in distilled water at dilution 1:1000 as recommended by manufacturer. The negative control consisted of distilled water. The percentage of mortality was calculated as Abbott [27]. Mortality (%) in the control group was calculated as number of dead larvae/total number of larvae × 100. The assays were repeated 10 times.
2.3. Adult Immersion Test (AIT)
AIT was carried out as by Drummond et al. [28] with some modifications as by Rey-Valeirón et al. [13]. Groups of eight engorged adult females were weighed and immersed for 3 min in 25 mL of each EOAp and EOLm dilution (0.125, 0.25, 0.50, 1, 2 and 4% in 2% Tween 40, v/v). Deionized water was used in negative control group. The positive control groups (cypermethrin 15% and amitraz 12.5%) were prepared in distilled water at dilution 1:1000 as recommended by the manufacturer.
2.4. Estimation of Efficacy
To evaluate the acaricidal effect of the EOAp, EOLm and synthetic acaricides on engorged females and to estimate efficacy, the following formulas were used [29]:
- (a)
- Survival period: number of days the ticks were able to survive after each treatment;
- (b)
- Egg hatching (% EH) = (number of larvae)/(total number of unhatched eggs and larvae) × 100;
- (c)
- Inhibition of oviposition (IOv) = (weight of treated females/weight of control females) − (weight of eggs laid in treated group/weight of eggs laid in control group);
- (d)
- Reproductive efficiency (% RE) = (weight of eggs/weight of females) × egg hatching
- (e)
- Efficacy of essential oil/synthetic acaricides = (RE control group − RE treated group/RE control group) × 100.
2.5. Statistical Analysis
Data obtained from the experimental design in LPT and AIT were evaluated by analysis of variance (ANOVA) (α = 0.05). The effects included in the ANOVA were EOs, concentrations and the interactions EO × concentration for each parameter.
LC50 y LC90 (concentration necessary for 50% and 90% larval lethality, respectively) of essential oils were obtained by a non-linear regression analysis of percentage of larval mortality versus log concentration. The model established by the program (Prism v9.3.0 for Windows, GraphPad Software, San Diego, CA, USA) was based on equation Y = basal mortality value + (maximal mortality value/(1 + 10^ ((LogLC-X) × HillSlope)).
2.6. Obtention and Characterization of Essential Oils
2.6.1. Plant Material
The collection of A. peruviana was performed in Los Operadores (4°00′09.8″ S, 79°15′02.2″ W), canton Loja and L. mutica in the canton Quilanga (4°17′43.7″ S, 79°23′59.7″ W) both in the province of Loja, Ecuador. The botanical specimens were identified by Dr. Bolivar Merino, at the herbarium of the Universidad Nacional de Loja, Ecuador. Voucher specimens are preserved in the Herbarium of the Universidad Técnica Particular de Loja, Ecuador, under codes HUTPL-5132 for A. peruviana and PPN-LA-005 for L. mutica, respectively.
2.6.2. Distillation of the Volatile Fraction
The fresh aerial parts of A. peruviana (9 kg) and leaves (5 kg) of L. mutica were steam distilled immediately after collection in a separate stainless steel Clevenger-type apparatus for 4 h. After distillation, each organic layer was separated from the aqueous phase, dried over anhydrous sodium sulfate and weighted.
2.6.3. Physical Properties of EOs
The density and refraction index of essential oils were determined according to the standard AFNOR NF T 75-111 and AFNOR NF T 75-112, respectively, as by Ramirez et al. [23]. The procedures were repeated three times and all measurements were performed at 20 °C.
2.6.4. Qualitative Analysis of the EOs
The qualitative analysis of the EOs was performed by GC-MS by injecting 1 µL of each distilled fraction, 1% (v/v) diluted in cyclohexane. The injector was kept at 220 °C, operating in split mode with a split ratio of 40:1. The carrier gas (He) was set at a constant flow of 1 mL/min. The analysis was performed in thermal gradient conditions, with the following temperature program: 60 °C for 5 min, increased to 110 °C at a rate of 5 °C/min, then to 148 °C at a rate of 2 °C/min. and to 250 °C at a rate of 20 °C/min, then hold at 250 °C for 2.4 min. The MS was operated in SCAN mode, with a scan rate of 2 scan/s within a mass range of 40–350 m/z at 70 eV. For each chromatographic peak, the corresponding linear retention index (LRI) was calculated according to Van den Dool and Kratz [30], with reference to a mixture of a homologous series of n-alkanes, from nonane to heptadecane and a homologous series of hydrocarbons C10-C25 (TPH-6RPM of CHEM SERVICE), which were analyzed by GC under the same conditions for essential oil. A non-polar capillary column, DB-5 ms 5%-phenyl-methylpolysiloxane, 30 m × 0.25 mm, thickness 0.25 μm film, was used. Samples were dissolved in dichloromethane.
2.6.5. Quantitative Analysis of the EOs
The quantitative analysis of the EOs was performed by GC-FID (Agilent Technologies chromatograph, model 6890N series), with the same instrumental configuration and method of the qualitative analysis. The constituents were quantified by external calibration, using n-nonane as the internal standard. An isomer was used to quantify isomeric metabolites; if an isomer was not available, a structurally close related terpene was selected [31]. Hence, the following terpenoids (purity > 98%) were used as calibration standards: limonene for aliphatic monoterpene hydrocarbons (R2 = 0.9962), p-cymene for aromatic monoterpene hydrocarbons (R2 = 0.9986), linalool for monoterpene alcohols (R2 = 0.9956), carvone for monoterpene ketones (R2 = 0.9958), cedrene for sesquiterpene hydrocarbons (R2 = 0.9998) and nerolidol for sesquiterpene alcohols (R2 = 0.9997). All calibration curves were built on six points. Quantitative results were reported as main values and standard deviations of three distillations of each plant. The percentage content of each oil component was computed from the corresponding GC-FID peak area without applying any correction factor. The analytical parameters were the same as the GC-MS analysis [23].
3. Results
3.1. Effect of EOsAp/Lm on Biological and Reproductive Parameters of R. microplus
3.1.1. Effects on Larvae
The highest value of larval mortality (100%) was achieved with 0.5% EOAp and 1% EOLm (Figure 1). At low concentrations (0.0625% and 0.125%), EOLm caused higher levels of mortality (46% and 95%) compared with EOAp (24% and 51%); however, a non-lineal trend was observed in both EOs. Statistical differences were not found between 0.25, 0.50 and 1% of EOLm, (p > 0.05) in contrast to EOAp in which significant differences were obtained between all doses. EOs and control groups were also different (p < 0.05). The LC50 and LC90 values obtained from the results in LPT with EOAp were 0.118% and 0.388% (R2 = 0.623) and with EOLm, 0.063% and 0.118% (R2 = 0.592), respectively (Figures S1 and S2). Means and standard errors are shown in Table S1.
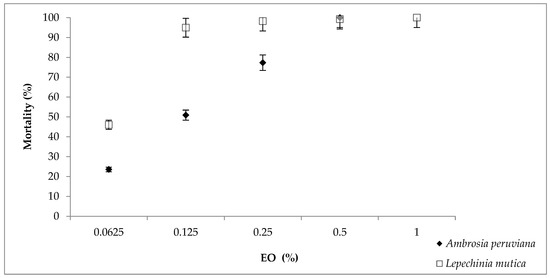
Figure 1.
Mortality of Rhipicephalus microplus larvae treated with essential oils of Ambrosia peruviana or Lepechinia mutica. Results are presented as mortality values of 10 replicates as Abbott [27] ±5% error.
Cypermethrin and amitraz (positive controls) in dilution 1:1000 caused 17.59 and 51.41% larval mortality, respectively (p > 0.05). The mortality value in control group treated with water was 0.23%.
3.1.2. Survival Period of Engorged Females
The average survival period of engorged females treated with EOs was dependent on the EOAp/Lm concentration (Table 1). None of the EOAp/Lm killed the ticks immediately after the treatment. However, at 4% concentration of EOAp, all the ticks died around four days (4 ± 1.69); with identical concentration of EOLm, the survival period was 9 ± 4.59; with the lower concentration (0.125%) of both EOs, the value was comparable to that of the control group. In general, the ticks survived longer in the group treated with EOLm. Ticks treated with amitraz had a similar survival period (13 ± 8.07) to the 1% EOLm-treated group (13 ± 5.33), but in those treated with cypermethrin (17 ± 3.78), this period was analogous to that of the control group (17 ± 2.53).

Table 1.
Survival period of Rhipicephalus microplus females treated with essential oil of Ambrosia peruviana or Lepechinia mutica.
3.1.3. Effects on Reproductive Parameters of Engorged Females of R. microplus
The effects of EOAp/Lm on reproductive parameters of R. microplus are shown in Table 2. The inhibition of oviposition (IOv) values ranked between 26.71 (0.125%) and 82.27% (4%) in groups treated with EOAp. The optimal value of IOv (82.27%) influenced the lowest value of reproductive efficiency (RE) (3.47%).

Table 2.
Reproductive parameters in treated groups and efficacy of Ambrosia peruviana and Lepechinia mutica essential oils on engorged females of Rhipicephalus microplus.
The 4% EOLm inhibited oviposition in 37.62% and RE resulted in 18.97% (p < 0.05); concentrations below 4%, although with significant differences (p < 0.05), had no relevant effect. Cypermethrin inhibited oviposition in 43.33% and amitraz in 79.58% (p < 0.05).
The efficacy of the EOAp/Lm on the egg hatchability (EH) of R. microplus was also evaluated (Table 2). EH (%) with EOAp ranged between 19.90 (4%) and 88.52 (0.125%), which means that hatching was inhibited up to 80% with 4% of EOAp.
EH (%) ranged between 60.18 and 91.22 with EOLm. In the control group, the EH value was 94.85%. Results with EOAp were significantly different to those of the negative control (p < 0.05), but not with EOLm, except for the group treated with the concentration at 4%. The EH (%) value with amitraz was 25.60 and with cypermethrin 54.29% (p > 0.05).
As a consequence of the IOv and mortality in EOs groups, RE decreased substantially (Table 2). Lowest RE values (3.47 and 18.97%) were achieved with 4% EOAp and EOLm, respectively. The RE of control group was 50.44%. No significant differences in RE values were found between concentrations of 0.125, 0.25 and 2% of EOLm and the control group. While remarkably low RE values were evidenced with both EOs at 4%, the RE was noticeably affected with EOAp if compared to an identical concentration of the EOLm (3.47 versus 18.97). Regarding the synthetic acaricides, no statistical differences were found (p > 0.05).
The highest efficacy (93.12%) on engorged females was achieved with 4% EOAp; a moderate value was also obtained with 2% EOAp (70.67%). The efficacy of EOLm was not as good as those with EOAp. At the higher concentration (4%), EOLm efficacy was 72.84%; the other concentrations gave negligible values (Table 2). Significant differences were found between both EOs (p < 0.05).
3.2. Chemical Analysis of Essential Oils from A. peruviana and L. mutica
3.2.1. Physical Properties of Essential Oils
The EOAp was a viscous liquid of subjective color light yellow. The EOAp yield was 0.06 ± 0.01% (w/w), referred to fresh plant material. The essential oil relative density was d20 = 0.8872 ± 0.0020 and refraction index was n20 = 1.4963 ± 0.0011. In the leaves of EOLm, d20 = 0.916 ± 0.026, n20 = 1.4867 ± 0.0009, [α] D20 = −5.8 (neat). The EOLm yield was 0.40 ± 0.12% (w/w).
3.2.2. Chemical Analysis of the Volatile Fraction
The qualitative and percent compositions are reported in Appendix A (Table A1). Aromatic sesquiterpene hydrocarbons (57.08%) dominated the characterization of the 41 individual compounds found in the essential oil of A. peruviana, with a low amount of aliphatic sesquiterpene hydrocarbons (14.03%), aliphatic monoterpene hydrocarbons (10.83%) and monoterpene ketones (5.57%) to account 92.08% of the total essential oil. The main constituents of the oil were γ-Curcumene (52.02%), Chrysanthenone (5.57%) and ar-curcumene (5.06%).
In the essential oil distilled from leaves of L. mutica, 78 compounds, representing 92.14% the total oil sample. The principal groups of compounds belong to aliphatic sesquiterpene hydrocarbons (35.80%) and aliphatic monoterpene hydrocarbons (24.5%). The most abundant components were Shyobunol (10.80%), δ-3-Carene (8.69%), δ-Cadinene (6.96%), Globulol (5.91%), (E)-Caryophyllene (4.55%), limonene (3.79%) and β-Pinene (3.78%).
4. Discussion
The findings in LPT revealed the acaricidal effect of EOs from A. peruviana and L. mutica on larvae of R. microplus. This is the first report of such effect of EOs obtained of both species. The results in the larval package test are of the utmost importance because larvae are considered as strategic targets in the tick control systems. Several authors reported high levels of larval mortality with low concentrations of EOs and it is known that larvae are more susceptible than adults. Mexican oregano (Lippia graeolens Kunth) and garlic (Allium sativum) EOs at concentration of 1.25% produced high mortality (90–100%) on 10-d-old R. microplus tick larvae [16]; cumin seeds (Cuminum cyminum) also produced 100% mortality with identical concentration [32]. EOs from Bursera graveolens and Schinus molle caused 100% mortality of R. microplus larvae at 2.5% [14]. However, not all the EOs are effective at low concentrations; for example, Tetraenia riparia at 25% or 10% of Eucalyptus staigeriana were necessary to achieve a larval mortality of 100% [33,34]. The acaricidal effect may vary because it depends not only on the tick stage but also on the compounds present in the essential oils, which in turn, may show an antagonistic or synergistic effect on potential targets in the arthropod.
An acaricide that kills females before the third day can prevent egg-laying, disrupting the tick’s life cycle [35] because engorged females begin laying eggs on day 3 or 4 after dropping from the host. In this work, none of the EOAp/Lm killed the female ticks immediately after the treatment; however, all the ticks died around day 4 (±1.69) in 4% EOAp. Essential oil from Citrus limonum exhibited 90% of mortality on engorged females tested after 48 h, reaching 100% at day 16, but these mortality values were obtained with a higher concentration, 10% [36].
When the essential oil presents a worthy effect on egg production or hatching, it means a reduction of the RE and a better efficacy when compared to the control groups. In this work, EOAp inhibited the oviposition more than 80%. Ribeiro et al. [37] found that EO from Hesperozygis ringens (which has a content of 86.0% oxygenated monoterpene Pulegone), inhibited egg laying by 76.4% at concentration of 5%; Piper nigrum and Pelargonium roseum EOs at identical concentration inhibited oviposition by 83% and 87.5%, respectively [35]. Those results suggest that sub-lethal doses of essential oils may have an effect on tick fecundity.
Inhibition of EH by EOs has been reported previously. Inside the female tick, the newly formed eggs were not in contact with the EO but there should be a different mechanism of action on the treated engorged female that impairs the viability of the eggs and the subsequent hatching of the larva. Vendramini et al. [38] reported that the andiroba (Carapa guianensis) oil acted as an inhibitor of the synthesis and/or incorporation of protein in oocytes of Rhipicephalus sanguineus. Because protein is needed to assure viability of the embryo, a reduction in the protein content will lead to the impairment of the embryonic development. Ovicidal effects would reduce the infection of the pastures with newly hatched larvae and thereby decrease cattle infections with the tick.
The results obtained with synthetic, industrial acaricides require further revision due to the low impact on larval mortality and the moderate efficacy on reproductive parameters. Both cypermethrin and amitraz are widely used in cattle farms in Ecuador; resistance values of 67% to amitraz and 50% to alpha-cypermethrin were found in 12 cattle farms [2].
In this work, aromatic sesquiterpene hydrocarbons dominated the characterization of the compounds in the EOAp. Both γ-Curcumene and ar-curcumene accounted for more than 57% of the total compounds. Such high percentages of curcumene has only been found in the genus Senecio (Asteraceae) [39]. Curcumene as major compound of EOs has been reported as larvicide against two-spotted spider mite, Tetranychus urticae [40] and in sand fly tick, Ornithodoros savignyi [41]. Shyobunol was the major constituent of the EOLm; the compound has been reported as an important element of the essential oil of Schinus molle leaves in Mexico and Tunisia [42], but is not a common major component in plant derivatives.
Several molecular studies have revealed the action mechanisms of plant-derived EOs that show the efficiency of pesticidal and insect repellent activity and identification of terpenoids as responsible for most of these biological properties [43]. In arthropods, the group of biogenic amine messengers consists of dopamine, tyramine, octopamine, serotonin and histamine; octopamine is an important hormone associated with the nervous system of insects and functions as a neurotransmitter and as a neuromodulator. The inhibition of octopamine will cause the impairment of physiological modulation associated with muscle juncture. Terpenoids are agonists of all types of octopamine and tyramine receptors [43,44]; the study of the effects of EOs on insect-specific octopamin receptor and other potential targets in the tick nervous system should be considered in the quest of candidates for the control of R. microplus.
Together, the results obtained in the present study show promising research in acaricidal effects of components mainly based on EOAp; evaluation of the major components of EOLm should not be discarded because of a lower LC50/90. However, due to the limited number of engorged females used in the present study, it is necessary to confirm the results obtained with both EOs by immersion tests. As the cattle remains infected by ticks at different life stages, the best compound would be that which is effective against all of them.
The use of botanically-based compounds to control R. microplus seems to be a viable alternative, given the number of plants with compounds with activity against the ticks that have already been found, but many factors have to be considered before obtaining a competitive acaricide from A. peruviana or L. mutica. Although botanicals tend to be less toxic to mammals and other non-target organisms, and their residues are rapidly biodegraded, field validation of experimental formulations and the availability of an adequate delivery system are some of the hurdles for their commercialization [45]. Regardless of all the observations that can be made with the use of essential oils at the field, the excellent level of efficacy obtained with EOAp at a concentration of 4% cannot be ruled out. This oil deserves further investigation. Both A. peruviana and L. mutica plants are widely distributed in Loja Province, Ecuador, in areas where there is neither arable soil nor cattle breeding, so these lands are not used for food crops.
Before the advent of synthetic insecticides, nicotine, pyrethrum, and inorganic chemistries dominated the domestic and agricultural pest control arsenal. Pyrethrum, the oleoresin extracted from the dried flowers of the pyrethrum daisy, Tanacetum cinerariifolium, led to the synthesis of permethrin, one of the most used acaricidal compounds in the control of cattle tick. Research on plant bioactive compounds can lead to the identification of new molecules presenting different modes of action, biological targets and synergy in comparison to current commercial products [46]. Major compounds could be a reliable alternative to avoid the high costs of EOs extraction and to develop new acaricides. The isolated compound may act as a lead compound or prototype for the synthesis or semi synthesis of pesticide derivatives, which may result in more effective and safer products. However, it should be noted that the isolated compound may promote no activity at all because EO is more effective due to the synergic effect of compound mixture [47].
Finally, the results obtained in the present work contribute to the growing list of scientific evidence about the therapeutic efficacy and absence of toxicity in Ecuadorian medicinal plants and their products, as reported recently [48].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci9010023/s1. Figure S1. Graph and data for calculation of LC50 and LC90 in larval package test with essential oil of Ambrosia peruviana.Figure S2. Graph and data for calculation of LC50 and LC90 in larval package test with essential oil of Lepechinia mutica. Table S1. Mean and standard error (SE) of larval mortality values obtained by larval package test with essential oils of Ambrosia peruviana and Lepechinia mutica.
Author Contributions
L.G., J.R., G.G. and E.V.: conceptualization, supervision, formal analysis, investigation, writing of original draft. C.R.-V.: conceptualization, formal analysis, supervision, methodology, investigation, writing of original draft, review and editing. J.L.M., J.C. and D.H.: methodology, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Técnica Particular de Loja, Ecuador.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
To Omar Verde, Faculty of Veterinary Sciences, Universidad Central de Venezuela, for his invaluable support and advice in the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Chemical composition of the essential oil from Ambrosia peruviana and Lepechinia mutica.
Table A1.
Chemical composition of the essential oil from Ambrosia peruviana and Lepechinia mutica.
| LRI a | LRI b | Compounds | Ambrosia peruviana | Lepechinia mutica | Type | ||
|---|---|---|---|---|---|---|---|
| % | σ | % | σ | ||||
| 906 | 906 | Santolina triene | 1.22 | 0.22 | - | - | AMH |
| 921 | 926 | Tricyclene | - | - | Trace | - | AMH |
| 924 | 924 | Thujene <α-> | 0.35 | 0.03 | Trace | - | AMH |
| 931 | 932 | Pinene <α-> | 0.50 | 0.04 | 1.23 | 0.89 | AMH |
| 946 | 949 | Camphene | - | - | 0.75 | 0.80 | AMH |
| 948 | 945 | Fenchene <α-> | 1.60 | 0.35 | - | - | AMH |
| 971 | 969 | Sabinene | - | - | 0.24 | 0.15 | AMH |
| 974 | 983 | Oct-3-en-1-ol | - | - | Trace | - | OTH |
| 976 | 974 | Pinene <β-> | - | - | 3.78 | 1.76 | AMH |
| 988 | 988 | Myrcene | 2.91 | 0.85 | 0.52 | 0.28 | AMH |
| 1003 | 1003 | Mentha-1(7),8-diene <p-> | - | - | 0.16 | 0.13 | AMH |
| 1006 | 1002 | Phellandrene <α-> | 1.40 | 0.31 | 3.8 | 1.70 | AMH |
| 1008 | 1008 | Carene <δ-3-> | 8.69 | 4.24 | AMH | ||
| 1016 | 1014 | Terpinene <α-> | - | - | 0.11 | 0.07 | AMH |
| 1019 | 1020 | Cymene <p-> | 0.24 | 0.16 | 0.10 | 0.06 | ARM |
| 1025 | 1023 | Sylvestrene | - | - | 0.29 | 0.18 | AMH |
| 1029 | 1024 | Limonene | 0.75 | 0.27 | 3.79 | 2.18 | AMH |
| 1026 | 1026 | Cineole <1,8-> | 0.31 | 0.18 | - | - | OTH |
| 1029 | 1030 | Phellandrene <β-> | 0.15 | 0.01 | - | - | AMH |
| 1045 | 1044 | Ocimene <(E)-β-> | 0.66 | 0.02 | - | AMH | |
| 1052 | 1054 | Terpinene <γ-> | 0.38 | 0.04 | 0.23 | 0.12 | AMH |
| 1065 | 1071 | cis-Sabinene hydrate | - | - | Trace | - | MOH |
| 1080 | 1085 | Mentha-2,4(8)-diene <p-> | - | - | 0.35 | 0.18 | AMH |
| 1084 | 1086 | Terpinolene | 0.91 | 0.59 | 0.60 | 0.33 | AMH |
| 1084 | 1086 | trans-Linalool oxide | - | - | Trace | - | MOH |
| 1095 | 1102 | Linalool | - | - | 0.20 | 0.09 | MOH |
| 1110 | 1109 | Oct-1-en-3-yl acetate | 1.37 | 0.60 | OTH | ||
| 1124 | 1124 | Chrysanthenone | 5.57 | 1.88 | - | - | MKE |
| 1141 | 1145 | Camphor | - | - | Trace | - | MKE |
| 1165 | 1172 | Borneol | - | - | 0.25 | 0.05 | MOH |
| 1174 | 1180 | 4-Terpineol | - | - | 0.14 | 0.02 | MOH |
| 1194 | 1186 | Terpineol <α-> | - | - | 0.11 | 0.02 | MOH |
| 1283 | 1284 | Bornyl acetate | 1.59 | 0.42 | 2.20 | 1.04 | OTH |
| 1335 | 1328 | Elemene <δ-> | Trace | - | Trace | - | ASH |
| 1345 | 1345 | Cubebene <α-> | 0.47 | 0.26 | 0.57 | 0.08 | ASH |
| 1373 | 1373 | Ylangene <α-> | - | - | 0.15 | 0.05 | ASH |
| 1374 | 1362 | Isoledene | 0.33 | 0.11 | - | - | ASH |
| 1374 | 1367 | Copaene <α-> | - | - | 1.46 | 0.23 | ASH |
| 1381 | 1387 | Bourbonene <β-> | - | - | 0.47 | 0.25 | ASH |
| 1385 | 1382 | Modheph-2-ene | - | - | - | - | ASH |
| 1392 | 1387 | Cubebene <β-> | 0.56 | 0.15 | 0.15 | 0.04 | ASH |
| 1405 | 1410 | Cedrene <α-> | 0.11 | 0.04 | - | - | ASH |
| 1407 | 1409 | Gurjunene <α-> | - | - | 1.94 | 0.37 | ASH |
| 1407 | 1418 | Longifolene | - | - | 0.15 | 0.07 | ASH |
| 1417 | 1411 | Funebrene <2-epi-β-> | - | - | Trace | - | ASH |
| 1417 | 1412 | (E)-Caryophyllene | 2.01 | 0.11 | 4.55 | 2.16 | ASH |
| 1419 | 1429 | Thujopsene <cis-> | 0.26 | 0.01 | - | - | ASH |
| 1424 | 1431 | Copaene <β-> | - | - | 0.50 | 0.08 | ASH |
| 1431 | 1431 | Gurjunene <β-> | 0.73 | 0.89 | 1.47 | 0.78 | ASH |
| 1436 | 1440 | Farnesene <(Z)-β-> | 0.54 | 0.13 | - | - | ASH |
| 1439 | 1449 | Aromadendrene | 0.37 | 0.19 | 0.56 | 0.10 | ASH |
| 1446 | 1448 | Muurola-3,5-diene <cis-> | - | - | 0.45 | 0.36 | ASH |
| 1453 | 1452 | Humulene <α-> | 0.48 | 0.12 | 1.20 | 0.47 | ASH |
| 1461 | 1452 | cis-Cadina-1(6),4-diene | - | - | 0.99 | 1.36 | ASH |
| 1471 | 1463 | Dauca-5,8-diene | - | - | 0.38 | 0.09 | ASH |
| 1475 | 1466 | trans-Cadina-1(6),4-diene | - | - | 0.99 | 0.12 | ASH |
| 1478 | 1479 | Amorpha-4,7(11)-diene | - | - | 0.15 | 0.07 | ASH |
| 1479 | 1479 | Curcumene <ar-> | 5.06 | 2.01 | - | - | ARS |
| 1482 | 1478 | Muurolene <γ-> | 0.41 | 0.01 | 0.92 | 0.23 | ASH |
| 1481 | 1481 | Curcumene <γ-> | 52.02 | 8.62 | - | - | ARS |
| 1484 | 1484 | Germacrene D | 0.38 | 0.27 | - | - | ASH |
| 1486 | 1489 | Selinene <β-> | 0.85 | 0.14 | - | - | ASH |
| 1492 | 1481 | cis-β-Guaiene | - | - | 0.71 | 0.11 | ASH |
| 1493 | 1486 | Bicyclogermacrene | - | - | 4.62 | 0.58 | ASH |
| 1493 | 1492 | Selinene <δ-> | - | - | 0.81 | 0.08 | ASH |
| 1503 | 1505 | Farnesene <(E,E)-α-> | 2.15 | 0.10 | 0.83 | 0.25 | ASH |
| 1510 | 1500 | Muurolene <α-> | - | - | 0.91 | 0.17 | ASH |
| 1513 | 1505 | Cadinene <γ-> | 0.35 | 0.09 | 2.86 | 0.37 | ASH |
| 1514 | 1508 | Cubebol | - | - | 0.36 | 0.21 | SOH |
| 1521 | 1512 | trans-Calamenene | - | - | 0.15 | 0.04 | ARS |
| 1522 | 1511 | Cadinene <δ-> | 0.77 | 0.88 | 6.96 | 0.99 | ASH |
| 1521 | 1521 | Sesquiphellandrene <β-> | 0.16 | 0.07 | - | - | ASH |
| 1533 | 1523 | trans-Cadina-1,4-diene | - | - | 0.37 | 0.10 | ASH |
| 1534 | 1537 | Cadinene <α-> | - | - | 0.39 | 0.12 | ASH |
| 1538 | 1545 | Selina-3,7(11)-diene | - | - | 0.14 | 0.04 | ASH |
| 1567 | 1559 | Germacrene B | 3.10 | 2.29 | 0.18 | 0.06 | ASH |
| 1574 | 1567 | Germacrene D-4-ol | - | - | 1.46 | 0.40 | SOH |
| 1582 | 1569 | Caryophyllene oxide | - | - | 0.29 | 0.24 | OTH |
| 1577 | 1577 | Spathulenol | 0.62 | 0.23 | - | - | SOH |
| 1590 | 1584 | Globulol | 0.43 | 0.49 | 5.91 | 2.61 | SOH |
| 1592 | 1592 | Viridiflorol | 0.25 | 0.15 | 1.29 | 0.45 | SOH |
| 1594 | 1594 | Carotol | 0.89 | 0.49 | - | - | SOH |
| 1618 | 1617 | 1,10-di-epi-Cubenol | - | - | 0.27 | 0.11 | SOH |
| 1618 | 1623 | Junenol | - | - | 1.39 | 0.42 | SOH |
| 1629 | 1622 | Eudesmol <10-epi-γ-> | - | - | 0.54 | 0.15 | SOH |
| 1636 | 1639 | Acorenol <β-> | - | - | 0.47 | 0.81 | SOH |
| 1639 | 1632 | Acorenol <α-> | - | - | Trace | - | SOH |
| 1649 | 1644 | Eudesmol <β-> | 0.24 | 0.05 | 4.47 | 1.93 | SOH |
| 1688 | 1681 | Shyobunol | - | - | 10.80 | 5.91 | SOH |
| Aliphatic monoterpene hydrocarbons (AMH) | 10.83 | 24.50 | |||||
| Aromatic monoterpene hydrocarbons(ARM) | 0.24 | 0.10 | |||||
| Monoterpene alcohols (MOH) | - | 0.70 | |||||
| Monoterpene ketones (MKE) | 5.57 | - | |||||
| Aliphatic sesquiterpene hydrocarbons (ASH) | 14.03 | 35.80 | |||||
| Aromatic sesquiterpene hydrocarbons(ARS) | 57.08 | 0.15 | |||||
| Sesquiterpene alcohols (SOH) | 2.43 | 27.00 | |||||
| Other compounds (OTH) | 1.90 | 3.86 | |||||
| Total identified | 92.08 | 92.10 | |||||
a Calculated retention indices according to van den Dool and Kratz [30]. b Reference retention indices according to Adams [31].
References
- Grisi, L.; Leite, R.C.; Martins, J.R.D.S.; De Barros, A.T.M.; Andreotti, R.; Cançado, P.H.D.; De León, A.A.P.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Garcés-Carrera, S.; Vanwambeke, S.O.; Madder, M.; Benítez-Ortiz, W. The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 2017, 12, e0174652. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vivas, R.I.; Jonsson, N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.; Aguilar-Caballero, A.J.; Rodriguez-Vivas, R.; Borges-Argaez, R.; Garcia-Vazquez, Z.; Mendez-Gonzalez, M. Acaricidal activity of extracts from Petiveria alliacea (Phytolaccaceae) against the cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2010, 168, 299–303. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Guauque, M.D.P.; Castaño, J.C.; Gómez, M. Detección de metabolitos secundarios en Ambrosia peruviana Willd y determinación de la actividad antibacteriana y antihelmíntica. Infectio 2010, 14, 186–194. [Google Scholar] [CrossRef][Green Version]
- Malagón, O.; Ramírez, J.; Andrade, J.M.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [CrossRef]
- Barbosa, C.D.S.; Borges, L.M.F.; Nicácio, J.; Alves, R.D.; Miguita, C.H.; Violante, I.M.P.; Hamerski, L.; Garcez, W.S.; Garcez, F.R. In vitro activities of plant extracts from the Brazilian Cerrado and Pantanal against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp. Appl. Acarol. 2013, 60, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ellse, L.; Wall, R. The use of essential oils in veterinary ectoparasite control: A review. Med. Vet. Entomol. 2014, 28, 233–243. [Google Scholar] [CrossRef]
- Andreotti, R.; Garcia, M.V.; Cunha, R.C.; Barros, J. Protective action of Tagetes minuta (Asteraceae) essential oil in the control of Rhipicephalus microplus (Canestrini; 1887) (Acari: Ixodidae) in a cattle pen trial. Vet. Parasitol. 2013, 197, 341–345. [Google Scholar] [CrossRef]
- Martins, M.D.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Wanzala, W.; Hassanali, A.; Mukabana, W.R.; Takken, W. Repellent Activities of Essential Oils of Some Plants Used Traditionally to Control the Brown Ear Tick, Rhipicephalus appendiculatus. J. Parasitol. Res. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Rey-Valeirón, C.; Pérez, K.; Guzmán, L.; López-Vargas, J.; Valarezo, E. Acaricidal effect of Schinus molle (Anacardiaceae) essential oil on unengorged larvae and engorged adult females of Rhipicephalus sanguineus (Acari: Ixodidae). Exp. Appl. Acarol. 2018, 76, 399–411. [Google Scholar] [CrossRef]
- Rey-Valeirón, C.; Guzmán, L.; Saa, L.R.; López-Vargas, J.; Valarezo, E. Acaricidal activity of essential oils of Bursera graveolens (Kunth) Triana & Planch and Schinus molle L. on unengorged larvae of cattle tick Rhipicephalus (Boophilus) microplus (Acari:Ixodidae). J. Essent. Oil Res. 2017, 29, 344–350. [Google Scholar] [CrossRef]
- Oh, J.; Bowling, J.J.; Carroll, J.F.; Demirci, B.; Baser, K.H.C.; Leininger, T.D.; Bernier, U.R.; Hamann, M.T. Natural product studies of U.S. endangered plants: Volatile components of Lindera melissifolia (Lauraceae) repel mosquitoes and ticks. Phytochemistry 2012, 80, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Velazquez, M.; Rosario-Cruz, R.; Castillo-Herrera, G.; Flores-Fernandez, J.M.; Alvarez, A.H.; Lugo-Cervantes, E. Acaricidal Effect of Essential Oils From Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) Against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). J. Med. Entomol. 2011, 48, 822–827. [Google Scholar] [CrossRef]
- Borges, L.M.F.; De Sousa, L.A.D.; Barbosa, C.D.S. Perspectives for the use of plant extracts to control the cattle tick Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 2011, 20, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Norris, E.J.; Gross, A.D.; Bartholomay, L.C.; Coats, J.R. Plant essential oils synergize various pyrethroid insecticides and antagonize malathion in Aedes aegypti. Med. Vet. Entomol. 2019, 33, 453–466. [Google Scholar] [CrossRef]
- PhytoChemical Interactions Data Base (PCIDB). Available online: https://www.genome.jp/db/pcidb/kna_species/17816#natural (accessed on 9 November 2021).
- Drew, B.T.; Sytsma, K.J. The South American radiation ofLepechinia (Lamiaceae): Phylogenetics, divergence times and evolution of dioecy. Bot. J. Linn. Soc. 2013, 171, 171–190. [Google Scholar] [CrossRef]
- Moscoso, A.; Montúfar, R.; Tye, A.; Lepechinia, M. Libro Rojo de Plantas Endémicas del Ecuador; León-Yánez, S., Valencia, R., Pitmam, N., Endara, L., Ulloa, C., Ulloa, Y., Navarrete, H., Eds.; Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2017; Available online: https://bioweb.bio/floraweb/librorojo/FichaEspecie/Lepechinia%20mutica (accessed on 9 November 2021).
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.L.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical Composition, Enantiomeric Analysis, AEDA Sensorial Evaluation and Antifungal Activity of the Essential Oil from the Ecuadorian PlantLepechinia muticaBenth (Lamiaceae). Chem. Biodivers. 2017, 14, e1700292. [Google Scholar] [CrossRef]
- Ramírez, J.; Gilardoni, G.; Ramón, E.; Tosi, S.; Picco, A.M.; Bicchi, C.; Vidari, G. Phytochemical Study of the Ecuadorian Species Lepechinia mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia oryzae. Pharmaceuticals 2018, 11, 33. [Google Scholar] [CrossRef]
- Nava, S.; Mangold, A.J.; Simonato, G.E.; Puntin, E.; Sproat, M.C. Guía Para La Identificación De Las Principales Especies De Garrapatas Que Parasitan A Los Bovinos En La Provincia de Entre Ríos, Argentina, 1st ed.; Ediciones INTA: Buenos Aires, Argentina, 2019. [Google Scholar]
- Stone, B.F.; Haydock, K.P. A method for measuring the acaricide-susceptibility of the cattle tick Boophilus microplus (Can.). Bull. Entomol. Res. 1962, 53, 563–578. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Drummond, R.O.; Ernst, S.E.; Trevino, J.L.; Gladney, W.J.; Graham, O.H. Boophilus annulatus and B. microplus: Laboratory Tests of Insecticides13. J. Econ. Entomol. 1973, 66, 130–133. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Resistance Management and Integrated Parasites Control in Ruminants. Guidelines, Module 1: Ticks Acaricide Resistance; Diagnosis; Management and Prevention. Available online: http://www.fao.org/3/ag014e/ag014e.pdf (accessed on 9 July 2021).
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Martínez-Velázquez, M.; Castillo-Herrera, G.A.; Rosario-Cruz, R.; Flores-Fernandez, J.M.; Lopez-Ramirez, J.; Hernandez-Gutierrez, R.; Lugo-Cervantes, E.D.C.; Rosario-Cruz, R. Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol. Res. 2010, 108, 481–487. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Demarchi, I.G.; Lonardoni, M.V.C.; Amorim, A.C.L.; Hovell, A.M.C.; Rezende, C.M.; Ferreira, G.A.; de Lima, E.L.; de Cosmo, F.A.; Cortez, D.A.G. Acaricidal activity of the essential oil from Tetradenia riparia (Lamiaceae) on the cattle tick Rhipicephalus (Boophilus) microplus (Acari; Ixodidae). Exp. Parasitol. 2011, 129, 175–178. [Google Scholar] [CrossRef]
- Chagas, A.C.D.S.; Passos, W.M.; Prates, H.T.; Leite, R.C.; Furlong, J.; Fortes, I.C.P. Efeito acaricida de óleos essenciais e concentrados emulsionáveis de Eucalyptus spp em Boophilus microplus. Braz. J. Vet. Res. Anim. Sci. 2002, 39, 247–253. [Google Scholar] [CrossRef]
- Vinturelle, R.; Mattos, C.; Meloni, J.; Lamberti, H.D.; Nogueira, J.; Júnior, I.D.S.V.; Rocha, L.; Lione, V.; Folly, E. Evaluation of essential oils as an ecological alternative in the search for control Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100523. [Google Scholar] [CrossRef]
- Vinturelle, R.; Mattos, C.; Meloni, J.; Nogueira, J.; Nunes, M.J.; Vaz, I.S.; Rocha, L.; Lione, V.; Castro, H.C.; Das Chagas, E.F. In Vitro Evaluation of Essential Oils Derived from Piper nigrum (Piperaceae) and Citrus limonum (Rutaceae) against the Tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Biochem. Res. Int. 2017, 2017, 5342947. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.L.S.; Dos Santos, J.C.; Bordignon, S.A.; Apel, M.A.; Henriques, A.T.; Von Poser, G.L. Acaricidal properties of the essential oil from Hesperozygis ringens (Lamiaceae) on the cattle tick Riphicephalus (Boophilus) microplus. Bioresour. Technol. 2010, 101, 2506–2509. [Google Scholar] [CrossRef]
- Vendramini, M.C.R.; Mathias, M.I.C.; De Faria, A.U.; Furquim, K.C.S.; De Souza, L.P.; Bechara, G.H.; Roma, G.C. Action of andiroba oil (Carapa guianensis) onRhipicephalus sanguineus(Latreille, 1806) (Acari: Ixodidae) semi-engorged females: Morphophysiological evaluation of reproductive system. Microsc. Res. Tech. 2012, 75, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Üçüncü, O.; Kahriman, N.; Terzioğrlu, S.; Karaoğrlu, Ş.A.; Yaylı, N. Composition and Antimicrobial Activity of the Essential Oils from Flowers of Senecio othonnae, S. racemosus, and S. nemorensis. Nat. Prod. Commun. 2010, 5, 831–834. [Google Scholar] [CrossRef]
- Antonious, G.F.; Snyder, J.C. Natural Products: Repellency and Toxicity of Wild Tomato Leaf Extracts to the Two-Spotted Spider Mite, Tetranychus urticae Koch. J. Environ. Sci. Health Part B 2006, 41, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, S.; Alanazi, A.D.; Gabr, H.S.M.; Allam, A.M.; Abou-Zeina, H.A.A.; Masoud, R.A.; Soliman, D.E.; Alshahrani, M.Y. Efficacy and safety of ethanolic Curcuma longa extract as a treatment for sand tampan ticks in a rabbit model. Vet. World 2020, 13, 812–820. [Google Scholar] [CrossRef]
- Abir, K.; Majdi, H.; Manef, A.; Sameh, A. Schinus molle: Chemical analysis, phenolic compounds and evaluation of its antioxidant activity. J. Chem. Pharma Res. 2016, 8, 93–101. [Google Scholar]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant essential oils and formamidines as insecticides/acaricides: What are the molecular targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Agwunobi, D.O.; Pei, T.; Wang, K.; Yu, Z.; Liu, J. Effects of the essential oil from Cymbopogon citratus on mortality and morphology of the tick Haemaphysalis longicornis (Acari: Ixodidae). Exp. Appl. Acarol. 2020, 81, 37–50. [Google Scholar] [CrossRef]
- Nwanade, C.; Wang, M.; Wang, T.; Yu, Z.; Liu, J. Botanical acaricides and repellents in tick control: Current status and future directions. Exp. Appl. Acarol. 2020, 81, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.; Sylvestre, M.; Tölke, E.; Tavares, J.; Barbosa-Filho, J.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).