Effect of Intense Exercise on Plasma Macrominerals and Trace Elements in Lidia Bulls
Abstract
1. Introduction
2. Materials and Methods
3. Animals and Legal Regulations
4. Statistical Analysis
5. Results
6. Discussion
6.1. Macrominerals
6.2. Microminerals or Trace Elements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- BOE. Real Decreto 60/2001, de 26 de Enero, Sobre Prototipo Racial de la Raza Bovina de Lidia; Boletín Oficial del Estado, nº 38 de 13 de febrero de 2001; Government of Spain: Madrid, Spain, 2001; pp. 5255–5261.
- Kaneko, J.J. Carbohydrate metabolism and its diseases. In Clinical Biochemistry of Domestic Animals, 6th ed.; Bruss, J.J., Kaneko, J.W., Harvey, M.L., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 45–80. [Google Scholar]
- Hendrix, C.M.; Sirois, M. Laboratory Procedures for Veterinary Technicians; Mosby: St. Louis, MO, USA, 2007. [Google Scholar]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Saunders Ltd.: New York, NY, USA, 2006. [Google Scholar]
- González, F.H.D. Indicadores Sanguíneos do Metabolismo Mineral em Ruminantes. In Perfil Metabólico em Ruminantes: Seu Uso em Nutrição e Doenças Nutricionais; González, F.H., Barcellos, J.O., Ospina, H., Ribeiro, L.A., Eds.; Gráfica da Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2000; pp. 31–51. [Google Scholar]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CABI Head Office: Wallingford, UK, 2010. [Google Scholar]
- Rucker, R.B.; Fascetti, A.J.; Keen, C.L. Trace minerals. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 663–693. [Google Scholar]
- Assenza, A.; Bergero, D.; Congiu, F.; Tosto, F.; Giannetto, C.; Piccione, G. Evaluation of serum electrolytes and blood lactate concentration during repeated maximal exercise in horse. J. Equine Vet. Sci. 2014, 34, 1175–1180. [Google Scholar] [CrossRef]
- Goundasheva, D.; Sabev, S. Influence of exercise on acid-base, blood gas and electrolyte status in horses. Trakia J. Sci. 2011, 9, 63–67. [Google Scholar]
- Aguilera-Tejero, E.; Estepa, J.C.; López, I.; Bas, S.; Mayer-Valor, R.; Rodríguez, M. Quantitative analysis of acid–base balance in show jumpers before and after exercise. Res. Vet. Sci. 2000, 68, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Gugliandolo, E.; Nava, V.; Piccione, G.; Giannetto, C.; Licata, P. Bioaccumulation of mineral elements in different biological substrates of athletic horse from Messina, Italy. Animals 2020, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Hiney, K.M.; Nielsen, B.D.; Rosenstein, D.; Orth, M.W.; Marks, B.P. High-intensity exercise of short duration alters bovine bone density and shape. J. Anim. Sci. 2004, 82, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Allen, J.R.; Hodgson, D.R.; Stewart, J.H.; Chan, W. Responses to submaximal treadmill exercise and training in the horse: Changes in haematology, arterial blood gas and acid base measurements, plasma biochemical values and heart rate. Vet. Rec. 1983, 113, 612–618. [Google Scholar] [PubMed]
- Forero López, J.H.; Lozano Martínez, P.A.; Camargo Roncancio, B.O. Parámetros fisiológicos en caninos pre y post competencia de agility en Bogotá, Colombia. (Canine physiological parameters pre and post agility competition at Bogotá, Colombia). Rev. Med. Vet. 2006, 12, 57–72. [Google Scholar] [CrossRef]
- Ilkiw, J.E.; Davis, P.E.; Church, D.B. Hematologic, biochemical, blood-gas, and acid-base values in Greyhounds before and after exercise. Am. J. Anim. Vet. Res. 1989, 50, 583–586. [Google Scholar]
- de Aluja, A.S.; Bouda, J.; López, C.A.; Chavira, H.H. Valores bioquímicos en sangre de burros antes y después del trabajo. Vet. Mex. 2001, 32, 271–278. [Google Scholar]
- Knight, P.K.; Rose, R.J.; Evans, D.L.; Cluer, D.; Henckel, P.; Saltin, B. Metabolic responses to maximal intensity exercise in the racing camel. Acta Physiol. Scand. Suppl. 1994, 150, 61–77. [Google Scholar]
- Rose, R.J.; Evans, D.L.; Henckel, P.; Knight, P.K.; Cluer, D.; Saltin, B. Metabolic responses to prolonged exercise in the racing camel. Acta Physiol. Scand. Suppl. 1994, 617, 49–60. [Google Scholar] [PubMed]
- Agüera Buendía, E.; Rubio, M.D.; Vivo, R.; Escribano, B.M.; Muñoz, A.; Villafuerte, J.L.; Castejón, F. Adaptaciones fisiológicas a la lidia en el toro bravo. Parámetros plasmáticos y musculares. Vet. Mex 1998, 29, 399–403. [Google Scholar]
- Escalera-Valente, F.; González-Montaña, J.R.; Alonso de la Varga, M.E.; Lomillos-Perez, J.M.; Gaudioso-Lacasa, V.R. Influence of intense exercise on acid-base, blood gas and electrolyte status in bulls. Res. Vet. Sci. 2013, 95, 623–628. [Google Scholar] [CrossRef] [PubMed]
- García-Belenguer, S.; Gascón, M.; Purroy, A.; Aceña, C. Niveles de peroxidación en el toro bravo. ITEA 1989, Extra, 188–190. [Google Scholar]
- Jordán, D.; Villa, N.A.; Gutiérrez, M.; Gallego, Á.B.; Ochoa, G.A.; Ceballos, A. Indicadores bioquímicos sanguíneos en ganado de lidia mantenido en pastoreo en la Cordillera Central Colombiana. Rev. Colomb. Cienc. Pec. 2006, 19, 18–26. [Google Scholar]
- BOCyL. Decree 57/2008, of 21 August, General Taurino Regulation of Castilla and Leon Community; Regional Government of Castilla y León: León, Spain, 2008; Volume 165, Augus; pp. 17317–17333.
- BOE. Ley 32/2007, de 7 de Noviembre, Para el Cuidado de los Animales, en su Explotación, Transporte, Experimentación y Sacrificio; Boletín Oficial del Estado, nº 268 de 8 de noviembre de 2007; Government of Spain: Madrid, Spain, 2007; pp. 45914–45920.
- European-Union. Directive 63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union L 276, 1 March 2010; 33–79. [Google Scholar]
- BOE. Real Decreto 53/2013, de 1 de Febrero, Por el que se Establecen las Normas Básicas Aplicables Para la Protección de los Ani-males Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. Boletín Oficial del Estado, nº 34 de 8 de febrero de. 1 March 2013; 11370–11420. [Google Scholar]
- Carpintero Hervás, C.M.; Fernández Zapata, C.; Gómez Ballesteros, J.P.; Gómez Muñoz, S.; Gómez Pérez, J.A.; Martínez Carrillo, A.; Merchante Carrizo, J.; Morales Fernández, J.; Pizarro Díaz, M.; Urquía García, J.J.; et al. Estudio de las variaciones de ciertos parámetros hematológicos y bioquímicos sanguíneos del toro bravo tras la lidia. Vet. Madr. 1995, 34, 22–26. [Google Scholar]
- Alonso, M.E.; Sánchez, J.M.; Robles, R.; Zarza, A.M.; Gaudioso, V.R. Relation entre la fréquence des chutes et différents paramètres hématologiques chez le taureau de combat. Rev. Med. Vet. 1997, 148, 999–1004. [Google Scholar]
- Chaves, S.P.; García, G.J.; Miguel, R.J.; López, S.M. Estudio comparativo de los valores de calcio, glucosa, sodio, potasio y cortisol en ganado bravo de lidia ordinaria y en toros de suelta con referencia al ganado vacuno. In Proceedings of the V Simposium Nacional del Toro de Lidia, Zafra, Spain, 26 October 2001; pp. 222–225. [Google Scholar]
- Bartolomé, D.J.; Alonso, M.E.; Ferrero, R.; García, J.J.; Gaudioso, V.R. Correlación entre pH sanguíneo de reses de lidia y diversos parámetros hemáticos. In Proceedings of the V Congreso Mundial Taurino de Veterinaria, Valladolid, Spain, 18 May 2005; pp. 117–122. [Google Scholar]
- Diez, I.; De Vicente, J.; Montes, A.; Gonzalo, J.M. Estudio hematológico en bovinos de raza Avileña: Elementos morfológicos y proteinograma. In Proceedings of the I Congreso Nacional de Patología Bovina, León, España, 7–9 October 1982; pp. 255–259. [Google Scholar]
- Goicoa, A. Estudios de Distintos Parámetros Hemáticos y Séricos en Hembras de Raza Rubia Gallega Durante la Gestación y Primer mes de Puerperio. Ph.D. Thesis, University Santiago de Compostela, Lugo, España, 1989. [Google Scholar]
- Gutiérrez, C.; Orden, M.A.; Fernández, J.; Gonzalo, J.M. Parámetros séricos en bovinos de la raza Asturiana de los Valles. In Proceedings of the I Congreso Nacional de Patología Bovina, León, España, 7–9 October 1982; pp. 275–278. [Google Scholar]
- Hernández, J. Estudio de Distintos Parámetros Hematológicos y Séricos en Razas Bovinas (Bos taurus, Linnaeus 1758) Rústicas de Galicia. Ph.D. Thesis, Santiago de Compostela, Lugo, España, 1992. [Google Scholar]
- Prieto, F.; Diez, I.; Vicente, J.; Montes, A.; Gonzalo, J.M. Variaciones de los parámetros séricos en vacas retintas de diferentes edades. In Proceedings of the I Congreso Nacional de Patología Bovina, León, España, 7–9 October 1982; pp. 267–272. [Google Scholar]
- Underwood, E.J.; Suttle, N.F. Los Minerales en la Alimentación del Ganado, 3rd ed.; Acribia: Zaragoza, España, 2002. [Google Scholar]
- Araujo, F.O. La alimentación mineral del ganado. IV. Minerales trazas. Aerotécnico Fac. Agron. Univ. Zulia Venez. 2008, 24, 1–5. [Google Scholar]
- Herdt, T.H.; Hoff, B. The use of blood analysis to evaluate trace mineral status in ruminant livestock. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef]
- Rollin, F.; Guyot, H. Trace minerals management in cattle. In Proceedings of the XVIII Congreso Internacional ANEMBE de Medicina Bovina, Lérida, Spain, 24–26 April 2013; pp. 154–159. [Google Scholar]
- García-Belenguer, S. Estudio de Degeneraciones Musculares en Ganado Bravo y su Relación con la Fuerza Exhibida por los Animales Durante la Lidia. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, Spain, 1991. [Google Scholar]
- Mayland, H.F.; Schuman, G.E.; Booth, D.T.; Waggoner, J.W. Mineral concentrations in soil extracts, forages, and blood sera of cattle grazing on reclaimed uranium-mined land in Southeastern Wyoming. In Proceedings of the 10th Billings Land Reclamation Symposium, Billings, MT, USA, 4 June 2006. [Google Scholar]
- Subiyatno, A.; Mowat, D.N.; Yang, W.Z. Metabolite and hormonal responses to glucose or propionate infusions in periparturient dairy cows supplemented with chromium. J. Dairy Sci. 1996, 79, 1436–1445. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Lefebvre, L.E.; Cronrath, J.D.; Socha, M.T.; Johnson, A.B. Effect of dietary cobalt supplementation on cobalt metabolism and performance of dairy cattle. J. Dairy Sci. 2003, 86, 1405–1414. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Socha, M.T. Effect of cobalt Supplementation during late gestation and early lactation on milk and serum measures. J. Dairy Sci. 2007, 90, 1880–1886. [Google Scholar] [CrossRef]
- Spears, J.W.; Harvey, R.W.; Samsell, L.J. Effects of dietary nickel and protein on growth, nitrogen metabolism and tissue concentrations of nickel, iron, zinc, manganese and copper in calves. J. Nutr. 1986, 116, 1873–1882. [Google Scholar] [CrossRef]
- Hesketh, S.; Sassoon, J.; Knight, R.; Hopkins, J.; Brown, D.R. Elevated manganese levels in blood and central nervous system occur before onset of clinical signs in scrapie and bovine spongiform encephalopathy. J. Anim. Sci. 2007, 85, 1596–1609. [Google Scholar] [CrossRef]
- Kommisrud, E.; Osteras, O.; Vatn, T. Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet. Scand. 2005, 46, 229–240. [Google Scholar] [CrossRef]
- Gómez-Cárdenas, G.; Fernández-Gómez, M.; Mayer-Valor, R.; Sánchez-Morales, M.; Aguilera-Tejero, E. Efectos de la lidia sobre algunas constantes hemáticas. In Proceedings of the II Simposium Nacional del Toro de Lidia, Zafra, Badajoz, España, 9 June 1995; pp. 179–183. [Google Scholar]
- Kruljc, P.; Čebulj-Kadunc, N.; Frangež, R.; Svete, A.N. Changes in blood antioxidant, biochemical and haematological parameters in police horses on duty. Slov. Vet. Zb. 2014, 51, 119–129. [Google Scholar]
- Capen, C.C. Metabolic disorders of calcium-phosphorus homestasis. In Veterinary Pathophysiology, 1st ed.; Dunlop, R.H., Malbert, C.H., Eds.; Blackwell Publishing: Oxford, UK, 2004; pp. 422–443. [Google Scholar]
- Sánchez, J.M.; Castro, M.J.; Alonso, M.E.; Gaudioso, V.R. Adaptive metabolic responses in females of the fighting breed submitted to different sequences of stress stimuli. Physiol. Behav. 1996, 60, 1047–1052. [Google Scholar] [CrossRef]
- Malavolta, E. Elementos de Nutrição Mineral de Plantas; Agronômica Ceres São Paulo: São Paulo, Brazil, 1980. [Google Scholar]
- Chang, X.; Mowat, D.N. Supplemental chromium for stressed and growing feeder calves. J. Anim. Sci. 1992, 70, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.E.; Kröner, A.; Kluger, R.; Baron, R.; Steffan, I.; Engel, A. Effects of marathon running on the trace minerals chromium, cobalt, nickel, and molybdenum. J. Trace Elem. Med. Biol 2002, 15, 201–209. [Google Scholar] [CrossRef]
- Anderson, R.A. Essentiality of chromium in humans. Sci. Total Environ. 1989, 86, 75–81. [Google Scholar] [CrossRef]
- González-Montaña, J.R.; Escalera-Valente, F.; Alonso, A.J.; Lomillos, J.M.; Robles, R.; Alonso, M.E. Relationship between vitamin B12 and cobalt metabolism in domestic ruminant: An update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, M.E.; Fellner, V.; Spears, J.W. Influence of cobalt concentration on vitamin b-12 production and fermentation of mixed ruminal microorganisms grown in continuous culture flow-through fermentors. J. Anim. Sci. 2006, 84, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, M.E.; Spears, J.W. Differential responses to dietary cobalt in finishing steers fed corn-versus barley-based diets. J. Anim. Sci. 2005, 83, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- Stangl, G.I.; Kirchgessner, M. Nickel deficiency alters liver lipid metabolism in rats. J. Nutr. 1996, 126, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Stowe, H.D.; Herdt, T.H. Clinical assessment of selenium status of livestock. J. Anim. Sci. 1992, 70, 3928–3933. [Google Scholar] [CrossRef]
- Garcia-Belenguer, S.; Purroy, A.; Gascon, M. Relation entre les concentrations seriques en selenium et en vitamine e, la pathologie musculaire et le comportement des taureaux de combat pendant la corrida. Rec. Med. Vet. 1992, 168, 105–110. [Google Scholar]
- Hefnawy, A.E.G.; Tórtora-Pérez, J.L. The importance of selenium and the effects of its deficiency in animal health. Small Rumin. Res. 2010, 89, 185–192. [Google Scholar] [CrossRef]
- Oblitas, F.; Contreras, P.A.; Böhmwald, H.; Wittwer, F. Efecto de la suplementación con selenio sobre la actividad sanguínea de glutation peroxidasa (GSH-Px) y ganancia de peso en bovinos selenio deficientes mantenidos a pastoreo. Arch. Med. Vet. 2000, 32, 55–62. [Google Scholar] [CrossRef]
- Carlson, B.A.; Yoo, M.-H.; Sano, Y.; Sengupta, A.; Kim, J.Y.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol. 2009, 10, 57. [Google Scholar] [CrossRef]
- Aceña, M.C.; García-Belenguer, S.; Gascón, M.; Purroy, A. Modifications hématologiques et musculaires pendant la corrida chez le taureau de combat. Rev. Med. Vet. 1995, 146, 277–282. [Google Scholar]
- Escalera, F.; Alonso, M.E.; Lomillos, J.M.; Bartolomé, D.J.; García, J.J.; Gaudioso, V.R. Relación de las concentraciones plasmáticas de algunos oligoelementos con el estrés del toro durante la lidia. In Proceedings of the VI Congreso Mundial Taurino de Veterinaria, Murcia, Spain, 6–8 November 2008; pp. 199–205. [Google Scholar]
- Corrigall, W.; Dalgarno, A.C.; Ewen, L.A.; Williams, R.B. Modulation of plasma copper and zinc concentrations by disease states in ruminants. Vet. Rec. 1976, 99, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Naresh, R.; Dwivedi, S.K.; Dey, S.; Swarup, D. Zinc, copper and cobalt concentrations in blood during inflammation of the mammary gland in dairy cows. Asian-Australas J. Anim. Sci. 2001, 14, 564–566. [Google Scholar] [CrossRef]
- Cousings, R.B. Zinc. In Presented Knowledge in Nutrition, 7th ed.; Filer, L.J., Ziegler, E.E., Eds.; International Life Science Institute-Nutrition Foundation: Washington, DC, USA, 1997. [Google Scholar]
- Bonilla, J.F.; Narváez, R.; Chuaire, L. El deporte como estrés oxidativo y hemólisis (Sports as a cause of oxidative stress and hemolysis). Colomb. Med. 2005, 36, 275–280. [Google Scholar]
- Bonilla, J.F.; Palomino, F. Hemólisis inducida por el ejercicio: Relación entre el nivel de actividad de la glucosa-6-fosfato deshidrogenasa y el grado de hemólisis. Colomb. Med. 2008, 39, 126–134. [Google Scholar]
- O’Toole, M.L.; Hiller, W.; Roalstad, M.S.; Douglas, P.S. Hemolysis during triathlon races: Its relation to race distance. Med. Sci. Sports Exer. 1988, 20, 272–275. [Google Scholar] [CrossRef] [PubMed]
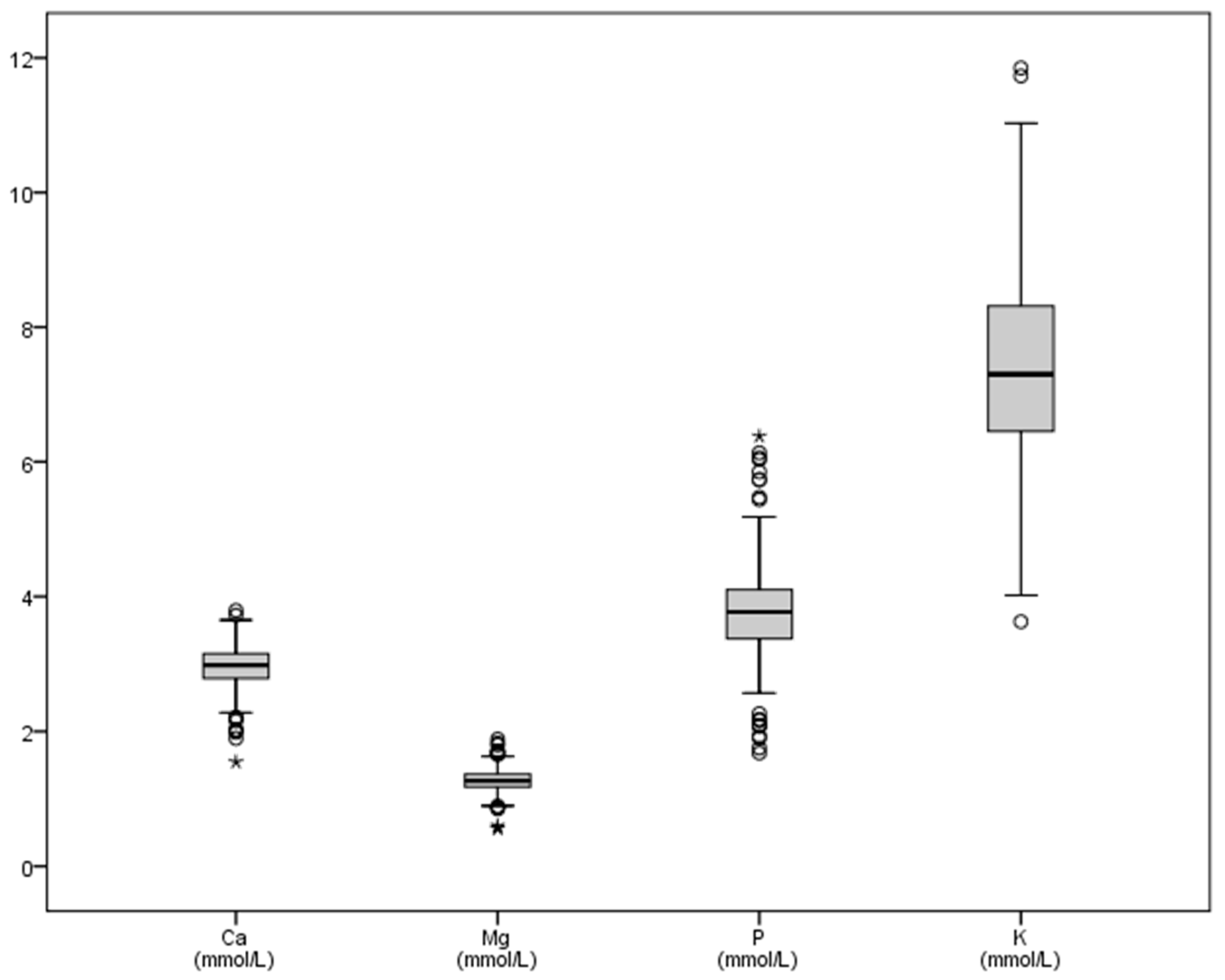
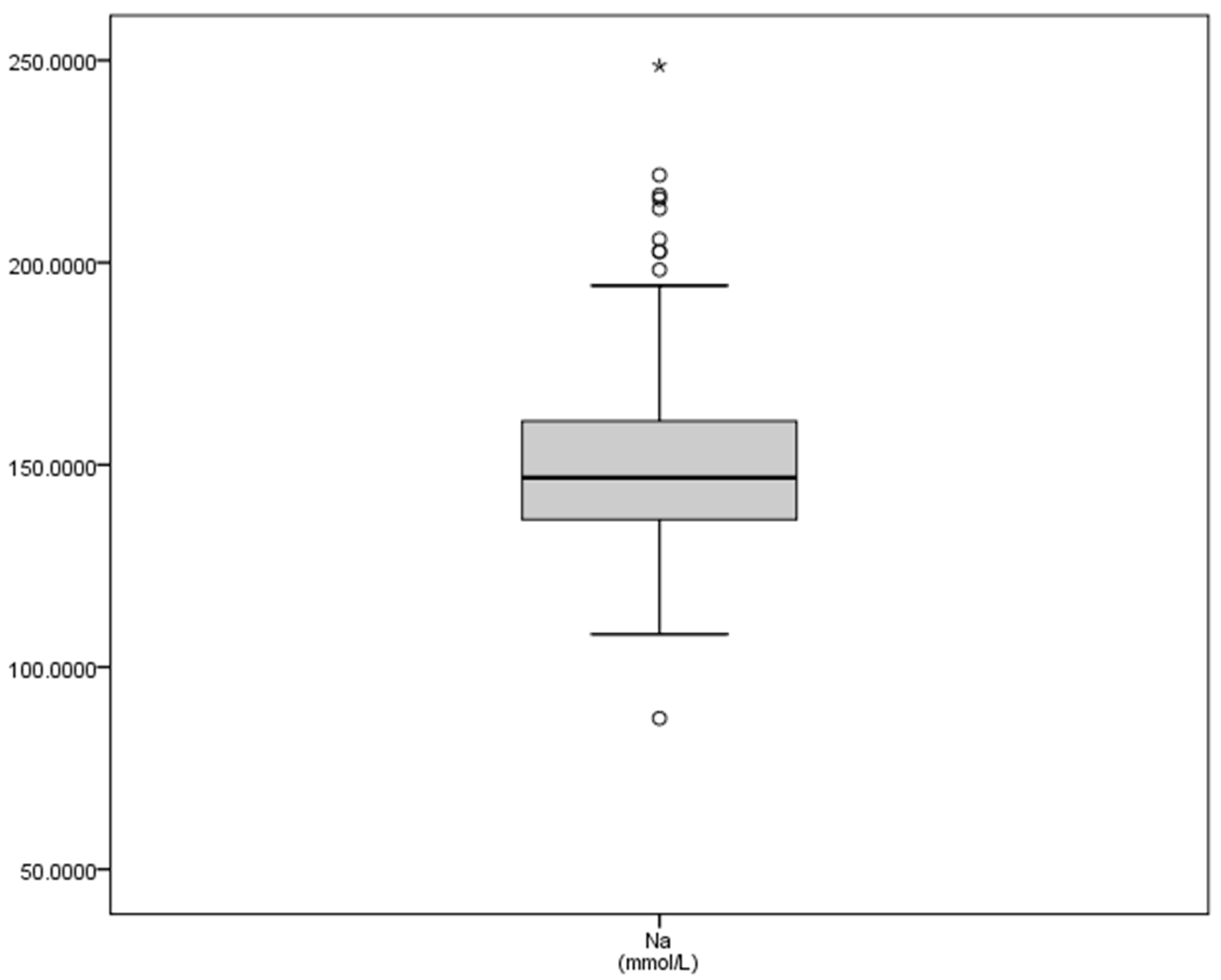
| Ca | Mg | P | K | Na | |
|---|---|---|---|---|---|
| N | 438 | 438 | 438 | 437 | 437 |
| Mean | 2.96 | 1.27 | 3.78 | 7.50 | 150.15 |
| SD | 0.31 | 0.17 | 0.65 | 1.58 | 19.59 |
| Minimum | 1.55 | 0.56 | 1.68 | 3.63 | 87.33 |
| Maximum | 3.79 | 1.88 | 6.38 | 18.30 | 248.68 |
| Sum | 1296.85 | 557.62 | 1656.22 | 3279.71 | 65,616.02 |
| Range | 2.25 | 1.33 | 4.70 | 14.67 | 161.35 |
| Some authors a | 2.00–2.43 | 0.70–1.23 | 1.45–2.03 | 3.3–5.8 | 132–155 |
| Radostits et al., 2006 [4] | 2.43–3.10 | 0.74–1.10 | 1.08–2.76 | 3.9–5.8 | 132–152 |
| Kaneko et al., 2008 [2] | 2.43–3.10 | 0.74–0.95 | 1.81–2.10 | 3.9–5.8 | |
| Carpintero et al., 1996 [27] b | 2.80–2.83 | 1.15–1.31 | 3.85–3.86 | — | |
| Alonso et al., 1997 [28] b | 3.15 | 1.19 | 4.01 | 8.10 | 162.8 |
| Chaves et al., 2001 [29] b | 2.61 | — | — | 9.49 | 160.1 |
| Bartolomé et al., 2005 [30] b | 2.87 | 1.07 | 3.18 | — | 141.8 |
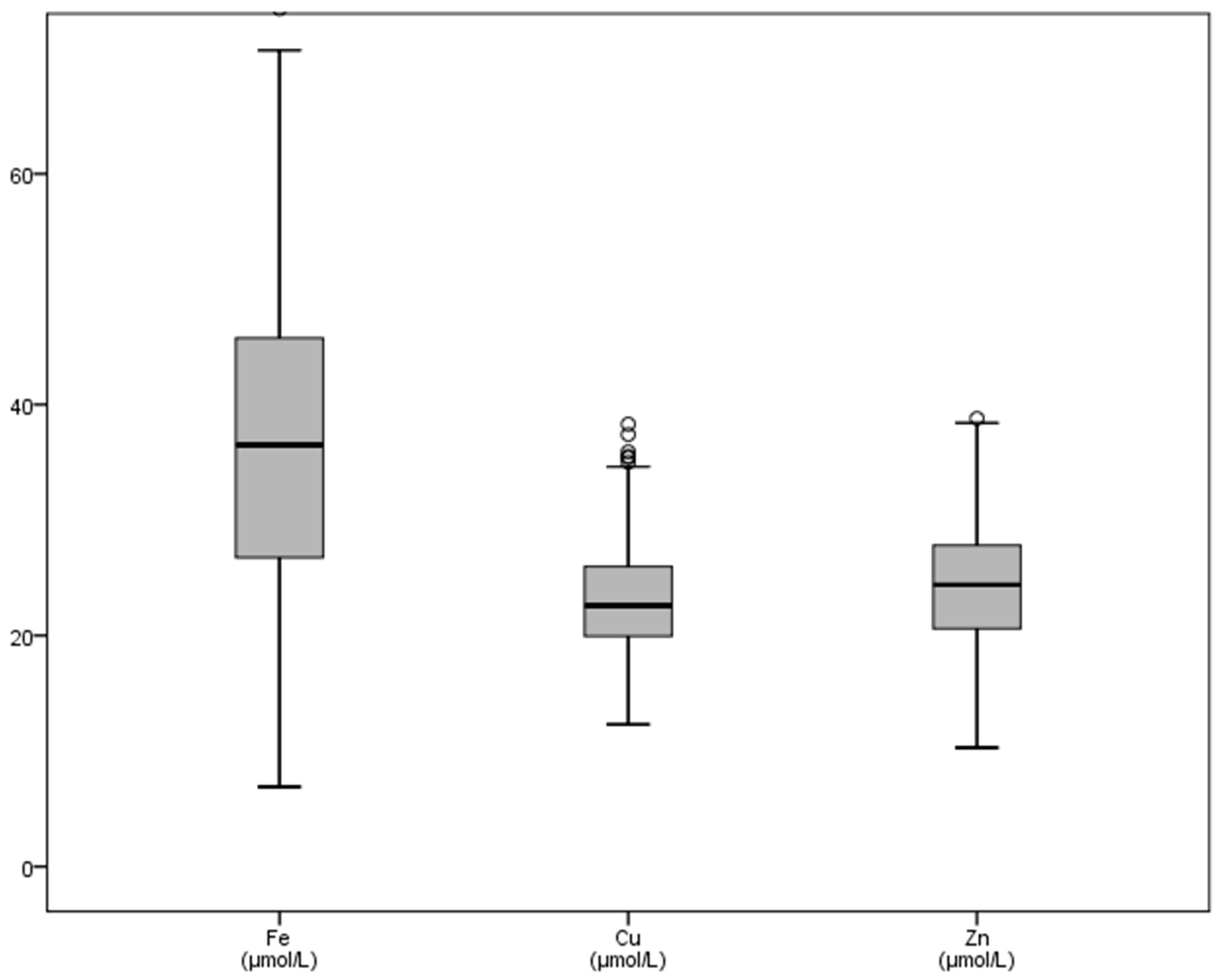
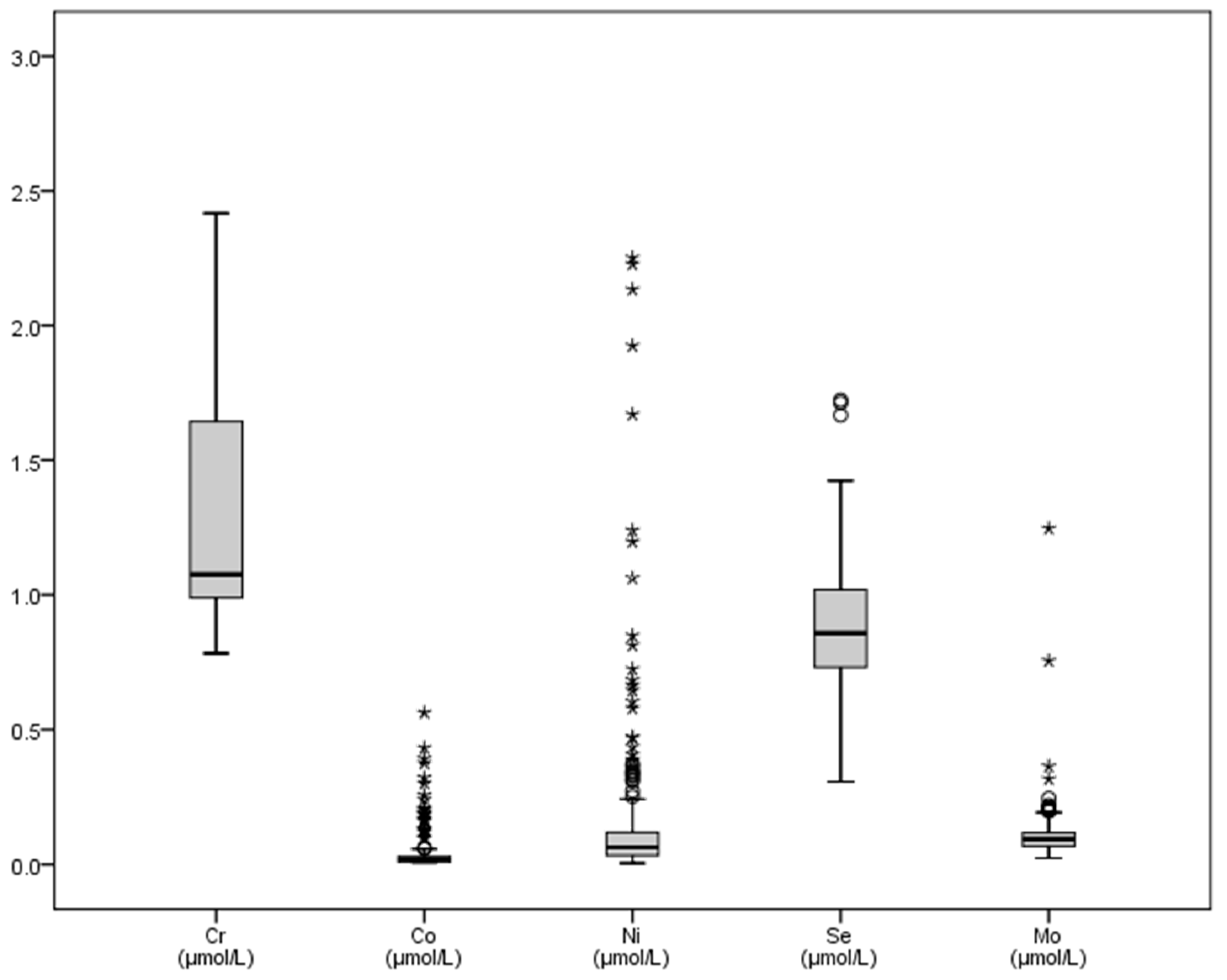
| Fe | Cr | Co | Ni | Cu | Zn | Se | Mo | |
|---|---|---|---|---|---|---|---|---|
| N | 327 | 427 | 255 | 427 | 427 | 427 | 427 | 427 |
| Mean | 36.87 | 1.24 | 0.041 | 0.249 | 22.63 | 24.14 | 0.886 | 0.111 |
| SD | 13.67 | 0.58 | 0.07 | 1.07 | 4.84 | 5.59 | 0.210 | 0.08 |
| Minimum | 6.90 | 0.78 | 0.007 | 0.004 | 11.10 | 10.30 | 0.307 | 0.023 |
| Maximum | 80.10 | 9.92 | 0.56 | 13.86 | 41.80 | 39.70 | 1.723 | 1.25 |
| Sum | 12057.70 | 529.96 | 10.48 | 106.11 | 9662.8 | 10307.5 | 378.39 | 47.34 |
| Range | 73.20 | 9.13 | 0.56 | 13.85 | 30.70 | 29.40 | 1.42 | 1.22 |
| Some authors a | 23.2–44.7 | 0.01–0.06 | 3.32–4.24 | 0.04–0.102 | 12.5–25.9 | 12.2–46.1 | 0.55–1.4 | 0.99 ± 0.03 |
| Radostits et al., 2006 [4] | 10–29 | — | 0.17–0.51 | — | 15.7 | 21.35 | — | 0.52 |
| Kaneko et al., 2008 [2] | 10.2–29.0 | — | — | — | 5.16–5.54 | — | — | — |
| Underwood and Suttle, 2002 [36] | 17.4 ±5.2 | — | — | — | 3–9 | — | — | — |
| Araujo, 2008 [37] | 23.2–44.7 | — | — | — | — | 12.2–38.2 | 2.6–15.2 | — |
| Herdt and Hoff, 2011 [38] | 19.7–44.7 | — | 0.029–0.34 | — | 9.42–17.27 | 9.18–290.7 | 0.823–1.772 | 0.02–0.37 |
| Rollin and Guyot, 2013 [39] | — | — | — | — | 13–18 | 14–21 | 1.02–1.78 | — |
| García-Belenger et al., 1991 [40] b | — | — | — | — | — | — | 0.13–0.48 | — |
| Bartolomé et al., 2005 [30] b | 17.71 | — | — | — | — | — | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalera-Valente, F.; Alonso, M.E.; Lomillos, J.M.; Gaudioso, V.R.; Alonso, Á.J.; González-Montaña, J.R. Effect of Intense Exercise on Plasma Macrominerals and Trace Elements in Lidia Bulls. Vet. Sci. 2021, 8, 97. https://doi.org/10.3390/vetsci8060097
Escalera-Valente F, Alonso ME, Lomillos JM, Gaudioso VR, Alonso ÁJ, González-Montaña JR. Effect of Intense Exercise on Plasma Macrominerals and Trace Elements in Lidia Bulls. Veterinary Sciences. 2021; 8(6):97. https://doi.org/10.3390/vetsci8060097
Chicago/Turabian StyleEscalera-Valente, Francisco, Marta E. Alonso, Juan M. Lomillos, Vicente R. Gaudioso, Ángel J. Alonso, and José Ramiro González-Montaña. 2021. "Effect of Intense Exercise on Plasma Macrominerals and Trace Elements in Lidia Bulls" Veterinary Sciences 8, no. 6: 97. https://doi.org/10.3390/vetsci8060097
APA StyleEscalera-Valente, F., Alonso, M. E., Lomillos, J. M., Gaudioso, V. R., Alonso, Á. J., & González-Montaña, J. R. (2021). Effect of Intense Exercise on Plasma Macrominerals and Trace Elements in Lidia Bulls. Veterinary Sciences, 8(6), 97. https://doi.org/10.3390/vetsci8060097







