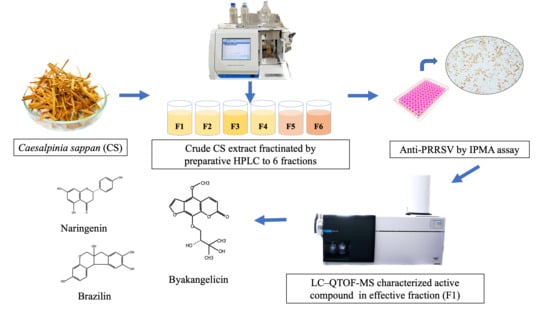
Effect of Ethanolic Caesalpinia sappan Fraction on In Vitro Antiviral Activity against Porcine Reproductive and Respiratory Syndrome Virus
Abstract
1. Introduction
2. Materials and Methods
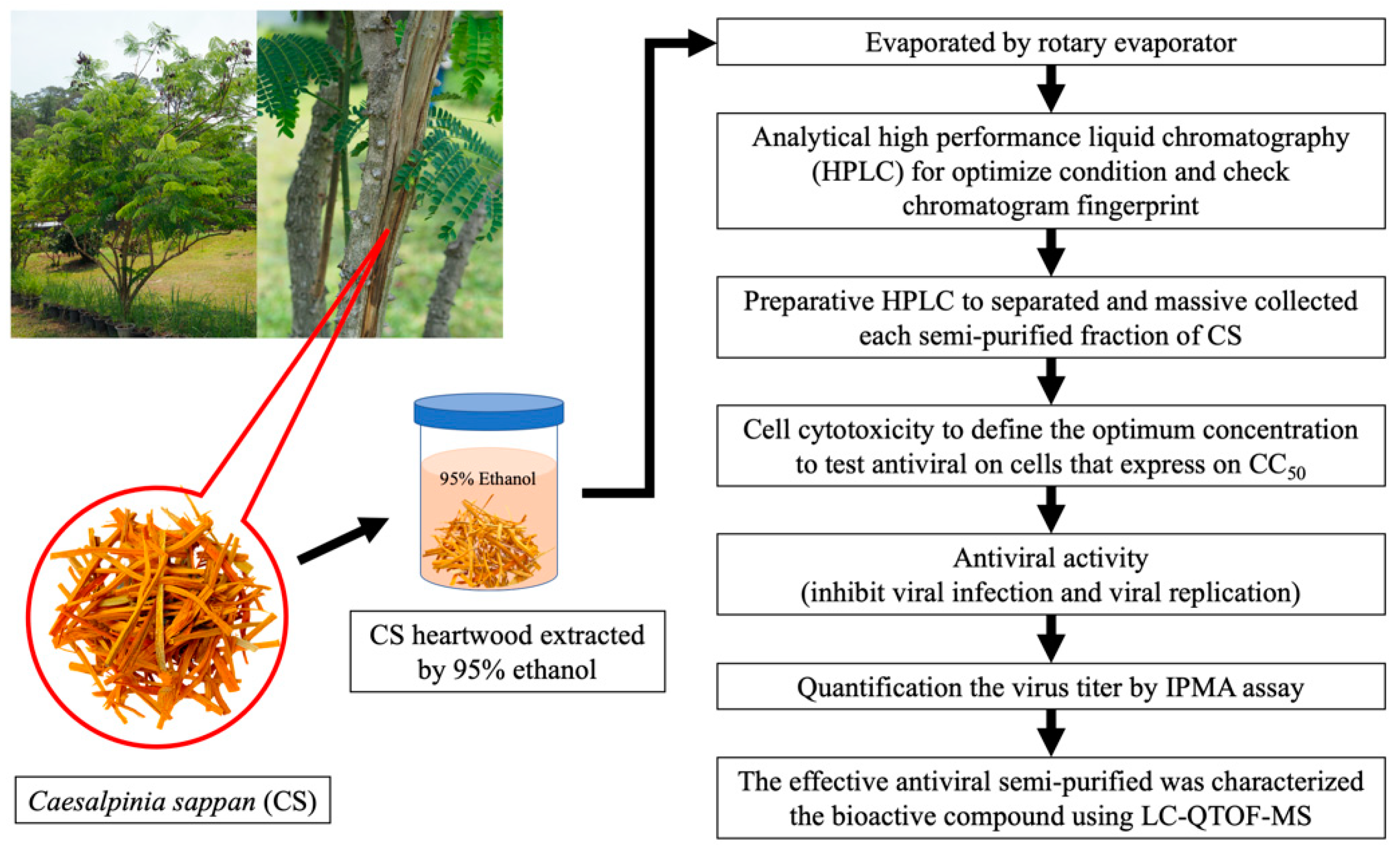
2.1. Experimental Plan
2.2. Sample Collection and Extraction
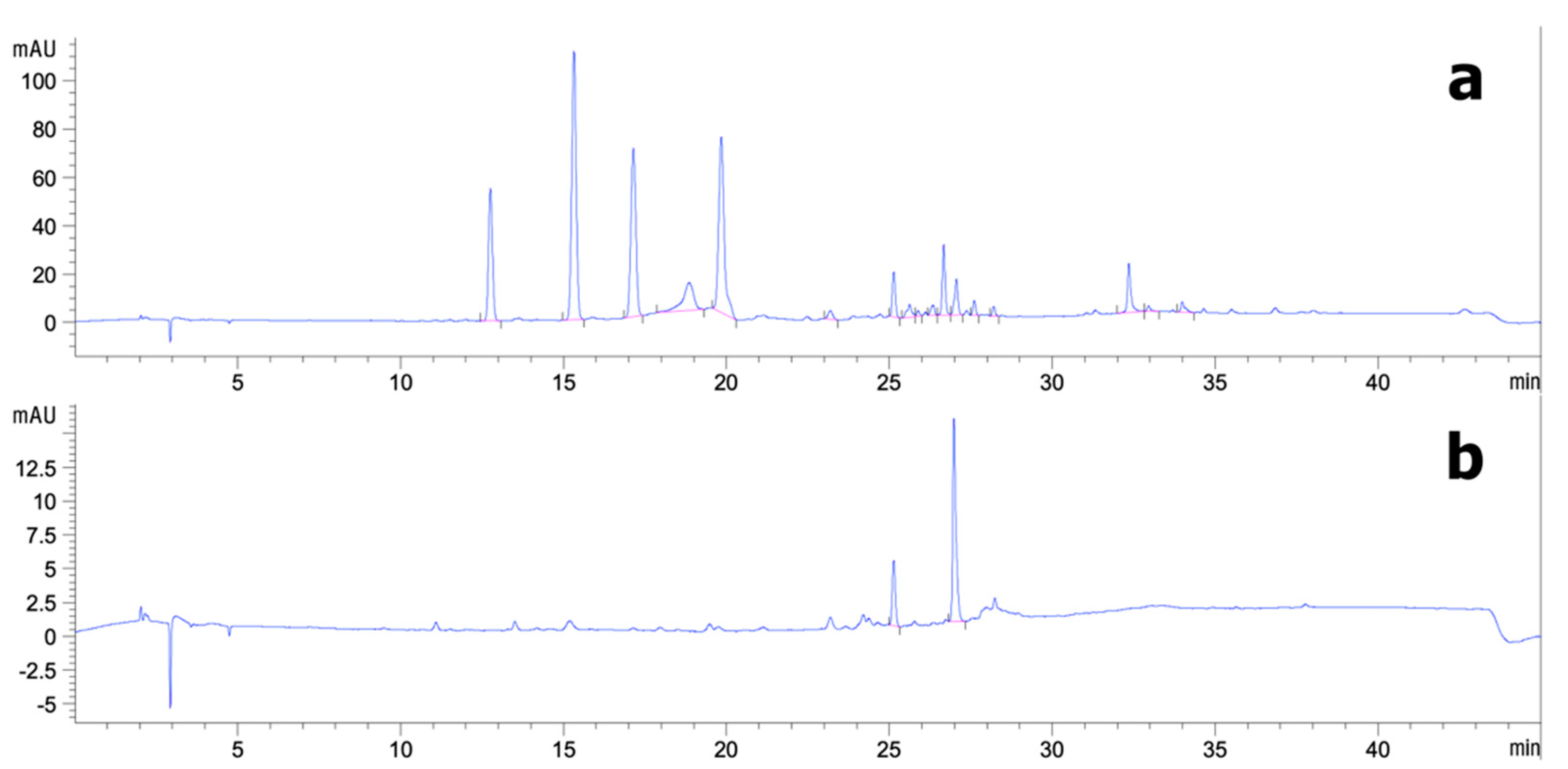
2.3. Analytical High-Performance Liquid Chromatography (HPLC)
2.4. Semi-Purified Fractionation of CS Extract by Preparative HPLC
2.5. Cells and Virus
2.6. Cell Cytotoxicity
2.7. Inhibition of Viral Infection Assay
2.8. Inhibition of Viral Replication Assay
2.9. Virus Titer
2.10. Characterization of Semi-Purified Fraction by LC-QTOF-MS
2.11. Statistical Analysis
3. Results
3.1. Extraction Yield of Ethanolic CS Heartwood Crude Extract
3.2. Analytical HPLC and Preparative HPLC Semi-Purification of CS Extract
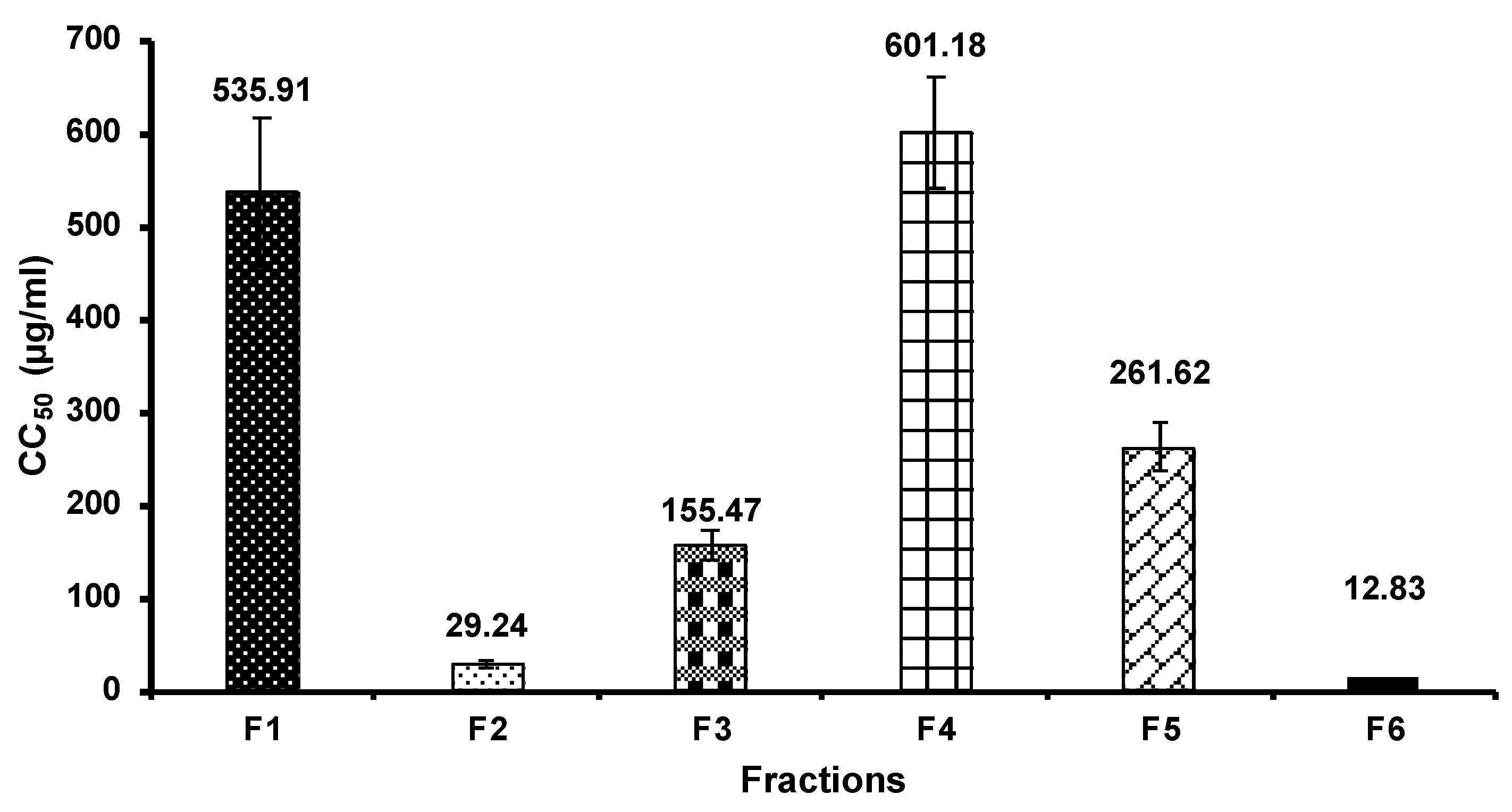
3.3. Cell Cytotoxicity
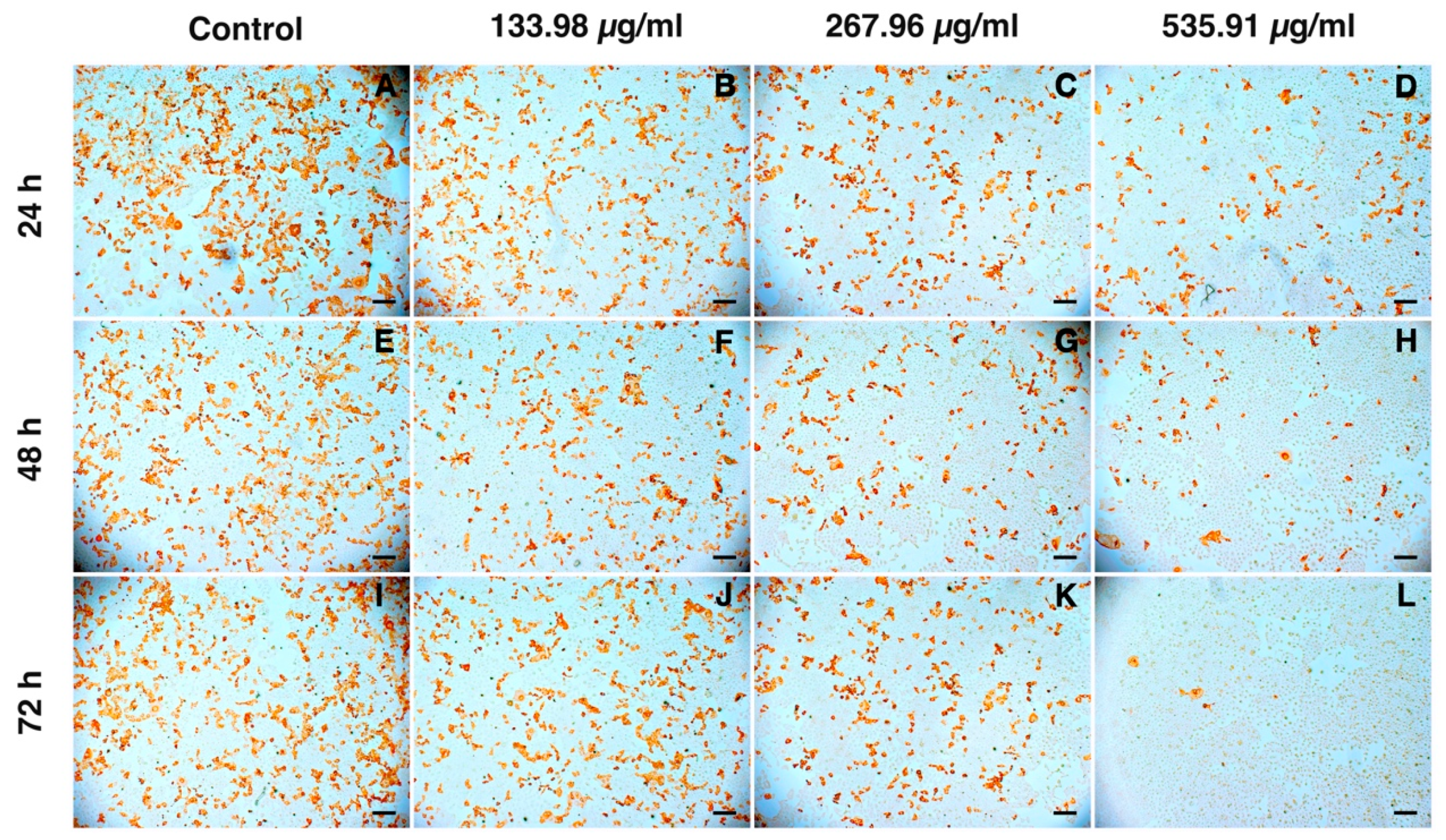
3.4. Inhibition of PRRSV Infection
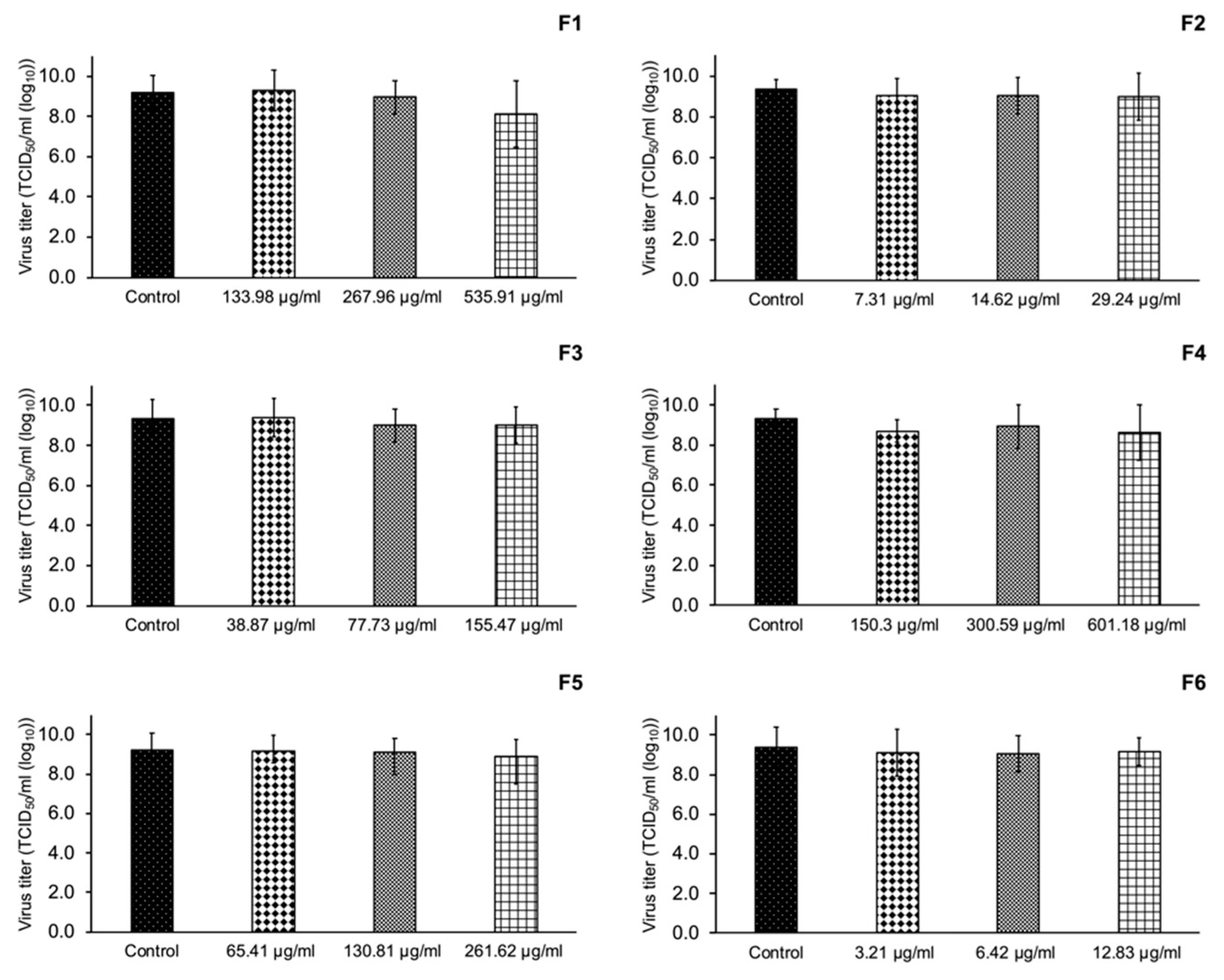
3.5. Inhibition of PRRSV Replication
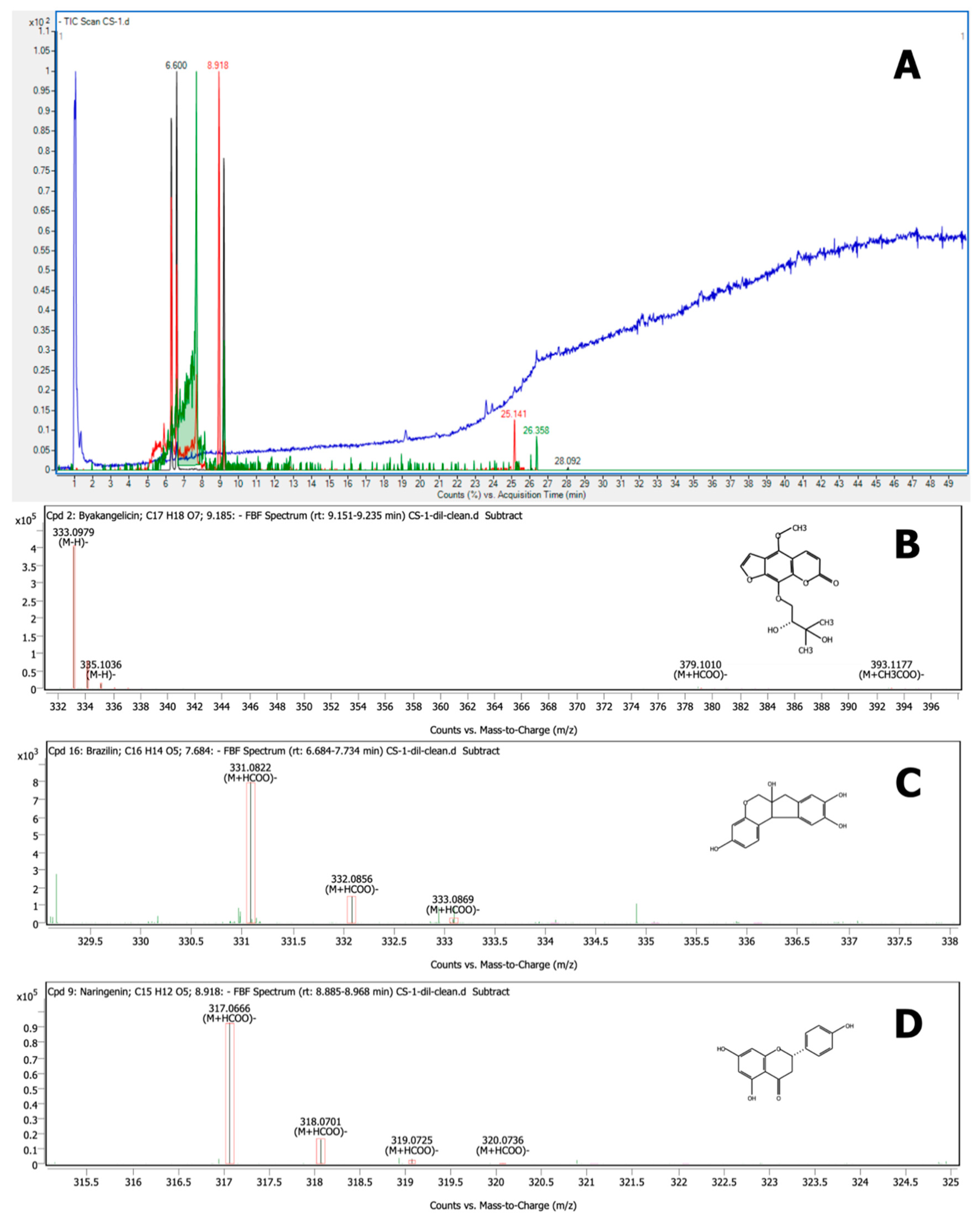
3.6. Characterization by LC–QTOF-MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Y.; Snijder, E.J. The PRRSV replicase: Exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010, 154, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Bai, X.; Cui, T.; Zhou, H.; Chen, Y.; Xie, J.; Shi, Q.; Wang, H.; Zhang, G. In Vitro Antiviral Activity of Germacrone Against Porcine Reproductive and Respiratory Syndrome Virus. Curr. Microbiol. 2016, 73, 317–323. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Xu, X.; Leng, X.; Li, S.; Wen, Y.; Wang, F.; Xia, M.; Cheng, S.; Wu, H. Pathological and immunological characteristics of piglets infected experimentally with a HP-PRRSV TJ strain. BMC Vet. Res. 2016, 12, 230. [Google Scholar] [CrossRef]
- Benfield, D.A.; Nelson, E.; Collins, J.E.; Harris, L.; Goyal, S.M.; Robison, D.; Christianson, W.T.; Morrison, R.B.; Gorcyca, D.; Chladek, D. Characterization of Swine Infertility and Respiratory Syndrome (SIRS) Virus (Isolate ATCC VR-2332). J. Vet. Diagn. Investig. 1992, 4, 127–133. [Google Scholar] [CrossRef]
- Pu, X.; Liang, J.; Shang, R.; Wang, X.; Wang, Z.; Hua, L.; Liu, Y. Influence of Hypericum perforatum Extract on Piglet Infected with Porcine Respiratory and Reproductive Syndrome Virus. Agric. Sci. China 2009, 8, 730–739. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Sun, Y.; Yang, Q.; Ren, J.; Liu, J.; Wang, H.; Feng, W. Cryptoporus volvatus Extract Inhibits Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) In Vitro and In Vivo. PLoS ONE 2013, 8, e63767. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.P.; Genzow, M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine 2011, 29, 8192–8204. [Google Scholar] [CrossRef]
- De Lamache, D.D.; Moges, R.; Siddiq, A.; Allain, T.; Feener, T.D.; Muench, G.P.; McKenna, N.; Yates, R.M.; Buret, A.G. Immuno-modulating properties of Tulathromycin in porcine monocyte-derived macrophages infected with porcine reproductive and respiratory syndrome virus. PLoS ONE 2019, 14, e0221560. [Google Scholar] [CrossRef]
- Xiong, W.; Sun, Y.; Zeng, Z. Antimicrobial use and antimicrobial resistance in food animals. Environ. Sci. Pollut. Res. 2018, 25, 18377–18384. [Google Scholar] [CrossRef]
- Liu, Y.; Che, T.M.; Song, M.; Lee, J.J.; Almeida, J.A.S.; Bravo, D.; Van Alstine, W.G.; Pettigrew, J.E. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2013, 91, 5668–5679. [Google Scholar] [CrossRef]
- Pringproa, K.; Khonghiran, O.; Kunanoppadol, S. In Vitro Virucidal and Virustatic Properties of the Crude Extract of Cynodon dactylon against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Med. Int. 2014, 2014, 947589. [Google Scholar] [CrossRef] [PubMed]
- Arjin, C.; Pringproa, K.; Hongsibsong, S.; Ruksiriwanich, W.; Seel-audom, M.; Mekchay, S.; Sringarm, K. In vitro screening antiviral activity of Thai medicinal plants against porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 2020, 16, 102. [Google Scholar] [CrossRef]
- Saenjum, C.; Chaiyasut, C.; Chansakaow, S. Antioxidant activity and protective effects on DNA damage of Caesalpinia sappan L. extract. J. Med. Plants Res. 2010, 4, 1594–1608. [Google Scholar] [CrossRef]
- Liang, C.-H.; Chan, L.-P.; Chou, T.-H.; Chiang, F.-Y.; Yen, C.-M.; Chen, P.-J.; Ding, H.-Y.; Lin, R.-J. Brazilein from Caesalpinia sappan L. Antioxidant Inhibits Adipocyte Differentiation and Induces Apoptosis through Caspase-3 Activity and Anthelmintic Activities against Hymenolepis nana and Anisakis simplex. Evid. Based Complement. Altern. Med. 2013, 2013, 864892. [Google Scholar] [CrossRef]
- Puttipan, R.; Chansakaow, S.; Khongkhunthian, S.; Okonogi, S. Caesalpinia sappan: A promising natural source of antimicrobial agent for inhibition of cariogenic bacteria. Drug Discov. Ther. 2018, 12, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Settharaksa, S.; Monton, C.; Charoenchai, L. Optimization of Caesalpinia sappan L. heartwood extraction procedure to obtain the highest content of brazilin and greatest antibacterial activity. J. Integr. Med. 2019, 17, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Hosokawa, T.; Nagai, M.; Nagumo, S. In Vitro Study for Inhibition of NO Production about Constituents of Sappan Lignum. Biol. Pharm. Bull. 2007, 30, 193–196. [Google Scholar] [CrossRef]
- Ruamrungsri, N.; Siengdee, P.; Sringarm, K.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. In vitro cytotoxic screening of 31 crude extracts of Thai herbs on a chondrosarcoma cell line and primary chondrocytes and apoptotic effects of selected extracts. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 434–444. [Google Scholar] [CrossRef]
- Srinivasan, R.; Selvam, G.G.; Karthik, S.; Mathivanan, K.; Baskaran, R.; Karthikeyan, M.; Gopi, M.; Govindasamy, C. In vitro antimicrobial activity of Caesalpinia sappan L. Asian Pac. J. Trop. Biomed. 2012, 2, S136–S139. [Google Scholar] [CrossRef]
- Hu, C.M.; Liu, Y.H.; Cheah, K.P.; Li, J.S.; Lam, C.S.K.; Yu, W.Y.; Choy, C.S. Heme oxygenase-1 mediates the inhibitory actions of brazilin in RAW264.7 macrophages stimulated with lipopolysaccharide. J. Ethnopharmacol. 2009, 121, 79–85. [Google Scholar] [CrossRef] [PubMed]
- You, E.J.; Khil, L.Y.; Kwak, W.J.; Won, H.S.; Chae, S.H.; Lee, B.H.; Moon, C.K. Effects of brazilin on the production of fructose-2,6-bisphosphate in rat hepatocytes. J. Ethnopharmacol. 2005, 102, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, E.P.; Saputri, F.C.; Mun’im, A. The Antidiabetic Effectivity of Indonesian Plants Extracts via DPP-IV Inhibitory Mechanism. J. Young Pharm. 2019, 11, 161–164. [Google Scholar] [CrossRef]
- Moon, C.-K.; Park, K.-S.; Kim, S.-G.; Won, H.-S.; Chung, J.-H. Brazilin protects cultured rat hepatocytes from BrCCl3-induced toxicity. Drug Chem. Toxicol. 1992, 15, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Shu, S.; Qin, H.; Ming, S.; Lee, Y.; Wang, Y.; Du, G. In vitro Anti-Influenza Viral Activities of Constituents from Caesalpinia sappan. Planta Med. 2009, 75, 337–339. [Google Scholar] [CrossRef]
- Bunluepuech, K.; Tewtrakul, S. Anti-HIV-1 integrase activity of Thai medicinal plants in longevity preparations. Songklanakarin J. Sci. Technol. 2011, 33, 693–697. [Google Scholar]
- Zhu, L.; Li, B.; Liu, X.; Huang, G.; Meng, X. Purification of six lignans from the stems of Schisandra chinensis by using high-speed counter-current chromatography combined with preparative high-performance liquid chromatography. Food Chem. 2015, 186, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints 12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Barros, A.V.; Araújo, L.M.; De Oliveira, F.F.; Da Conceição, A.O.; Simoni, I.C.; Fernandes, M.J.B.; Arns, C.W. In Vitro Evaluation of the Antiviral Potential of Guettarda angelica Against Animal Herpesviruses. Acta Sci. Vet. 2012, 40, 1–7. [Google Scholar]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID50 for Quantitation of Virus Infectivity. Virol. Sin. 2020, 36, 141–144. [Google Scholar] [CrossRef]
- Chellappan, D.R.; Purushothaman, A.K.; Brindha, P. Gastroprotective potential of hydro-alcoholic extract of Pattanga (Caesalpinia sappan Linn.). J. Ethnopharmacol. 2017, 197, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections-A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Huang, H.S.; Ma, C.G.; Chen, Z.W. Advances in the research on pharmacological actions of flavone compounds. Zhongguo Zhong Yao Za Zhi 2000, 25, 589–592. [Google Scholar] [PubMed]
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research Progress of the Antiviral Bioactivities of Natural Flavonoids. Nat. Prod. Bioprospect. 2020, 10, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Kaewprom, K.; Chen, Y.; Lin, C.; Chiou, M.-T.; Lin, C.-N. Antiviral activity of Thymus vulgaris and Nepeta cataria hydrosols against porcine reproductive and respiratory syndrome virus. Thai J. Vet. Med. 2017, 47, 25–33. [Google Scholar]
- Anantikulchai, P.; Emprom, P.; Pringproa, K.; Yamsakul, P. In vitro Cytotoxicity Test and Antiviral Activity of Curcuminoids from Turmeric Extract Against PRRS Virus. Vet. Integr. Sci. 2017, 15, 199–205. [Google Scholar] [CrossRef]
- Adnan, A.; Allaudin, Z.N.; Hani, H.; Loh, H.; Khoo, T. Virucidal activity of Garcinia parvifolia leaf extracts in animal cell culture. MC Complementary Altern. Med. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Kiyonga, A.N.; Hong, G.; Kim, H.S.; Suh, Y.-G.; Jung, K. Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim]Tf2N Ionic Liquid. Molecules 2021, 26, 830. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, X.M.; Wang, R.R.; Liu, Q.M.; Zheng, Y.T.; Zhou, J.; Chen, J.J. Anti-HIV Active Constituents from Angelica apaensis. Nat. Prod. Res. Dev. 2008, 239–244. [Google Scholar]
- Nirmal, N.P.; Rajput, M.S.; Prasad, R.G.S.V.; Ahmad, M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: A review. Asian Pac. J. Trop. Med. 2015, 8, 421–430. [Google Scholar] [CrossRef]
- Bae, I.-K.; Min, H.-Y.; Han, A.-R.; Seo, E.-K.; Lee, S.K. Suppression of lipopolysaccharide-induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2005, 513, 237–242. [Google Scholar] [CrossRef]
- Choi, B.M.; Lee, J.A.; Gao, S.S.; Eun, S.Y.; Kim, Y.S.; Ryu, S.Y.; Choi, Y.H.; Park, R.; Kwon, D.Y.; Kim, B.R. Brazilin and the extract from Caesalpinia sappan L. protect oxidative injury through the expression of heme oxygenase-1. BioFactors 2007, 30, 149–157. [Google Scholar] [CrossRef]
- Batubara, I.; Mitsunaga, T.; Ohashi, H. Brazilin from Caesalpinia sappan wood as an antiacne agent. J. Wood Sci. 2010, 56, 77–81. [Google Scholar] [CrossRef]
- Xu, H.X.; Lee, S.F. The antibacterial principle of Caesalpina sappan. Phyther. Res. 2004, 18, 647–651. [Google Scholar] [CrossRef]
- Linda Laksmiani, N.P.; Febryana Larasanty, L.P.; Jaya Santika, A.A.G.; Andika Prayoga, P.A.; Kharisma Dewi, A.A.I.; Kristiara Dewi, N.P.A. Active compounds activity from the medicinal plants against SARS-CoV-2 using in silico assay. Biomed. Pharmacol. J. 2020, 13, 873–881. [Google Scholar] [CrossRef]
- Hartogh, D.J.D.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phyther. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Dos Santos, C.N.D.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S. Potential Inhibitor of COVID-19 Main Protease (M pro) from Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020, 1–14. [Google Scholar] [CrossRef]

| Sample | Weight (g) | Yield (g) | % Yield |
|---|---|---|---|
| CS heartwood | 500.323 | 35.124 | 7.020 |
| Fractions | Retention Time (min) | Yield (mg/g Extract) |
|---|---|---|
| F1 | 12.76 | 39.54 ± 5.85 |
| F2 | 15.32 | 51.94 ± 5.12 |
| F3 | 17.14 | 42.32 ± 5.46 |
| F4 | 18.85 | 33.75 ± 4.60 |
| F5 | 19.84 | 45.75 ± 3.22 |
| F6 | 26.99 | 37.43 ± 4.46 |
| No. | Compound Name | Structure | Rt | Matching Score (%) | m/z * | (M − H)− | Mass | Mass Diff (Tgt/ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Catechin | C15 H14 O6 | 4.466 | 98.52 | 289.0658 | 289.0718 | 290.079 | −0.10 |
| 2 | Isorhamnetin | C16 H12 O7 | 5.750 | 95.57 | 315.0509 | 315.0510 | 316.0583 | −0.16 |
| 3 | Kaempferol | C15 H10 O6 | 5.750 | 93.02 | 285.0402 | 285.0425 | 286.0476 | −0.36 |
| 4 | (+)–Epicatechin | C15 H14 O6 | 6.517 | 97.80 | 349.0928 | 289.0718 | 290.079 | −0.13 |
| 5 | Brazilein | C16 H12 O5 | 6.600 | 99.64 | 283.0613 | 283.0612 | 284.0685 | 0.16 |
| 6 | Kaempferide | C16 H12 O6 | 7.051 | 98.79 | 299.056 | 299.0561 | 300.0633 | −0.16 |
| 7 | Brazilin | C16 H14 O5 | 7.684 | 99.83 | 331.0822 | 285.0768 | 286.084 | −0.41 |
| 8 | Naringenin | C15 H12 O5 | 8.918 | 99.80 | 317.0666 | 271.0612 | 272.0684 | −0.34 |
| 9 | Byakangelicin | C17 H18 O7 | 9.185 | 99.97 | 333.0979 | 333.0980 | 334.1052 | −0.27 |
| 10 | Tricin | C17 H14 O7 | 9.935 | 99.10 | 329.0665 | 329.0667 | 330.0738 | −0.44 |
| 11 | Wogonin | C16 H12 O5 | 9.935 | 99.10 | 329.0665 | 283.0612 | 284.0683 | −0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arjin, C.; Hongsibsong, S.; Pringproa, K.; Seel-audom, M.; Ruksiriwanich, W.; Sutan, K.; Sommano, S.R.; Sringarm, K. Effect of Ethanolic Caesalpinia sappan Fraction on In Vitro Antiviral Activity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Sci. 2021, 8, 106. https://doi.org/10.3390/vetsci8060106
Arjin C, Hongsibsong S, Pringproa K, Seel-audom M, Ruksiriwanich W, Sutan K, Sommano SR, Sringarm K. Effect of Ethanolic Caesalpinia sappan Fraction on In Vitro Antiviral Activity against Porcine Reproductive and Respiratory Syndrome Virus. Veterinary Sciences. 2021; 8(6):106. https://doi.org/10.3390/vetsci8060106
Chicago/Turabian StyleArjin, Chaiwat, Surat Hongsibsong, Kidsadagon Pringproa, Mintra Seel-audom, Warintorn Ruksiriwanich, Kunrunya Sutan, Sarana Rose Sommano, and Korawan Sringarm. 2021. "Effect of Ethanolic Caesalpinia sappan Fraction on In Vitro Antiviral Activity against Porcine Reproductive and Respiratory Syndrome Virus" Veterinary Sciences 8, no. 6: 106. https://doi.org/10.3390/vetsci8060106
APA StyleArjin, C., Hongsibsong, S., Pringproa, K., Seel-audom, M., Ruksiriwanich, W., Sutan, K., Sommano, S. R., & Sringarm, K. (2021). Effect of Ethanolic Caesalpinia sappan Fraction on In Vitro Antiviral Activity against Porcine Reproductive and Respiratory Syndrome Virus. Veterinary Sciences, 8(6), 106. https://doi.org/10.3390/vetsci8060106