Prevalence of Echinococcus granulosus sensu lato in Owned Dogs in Lagos State, Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
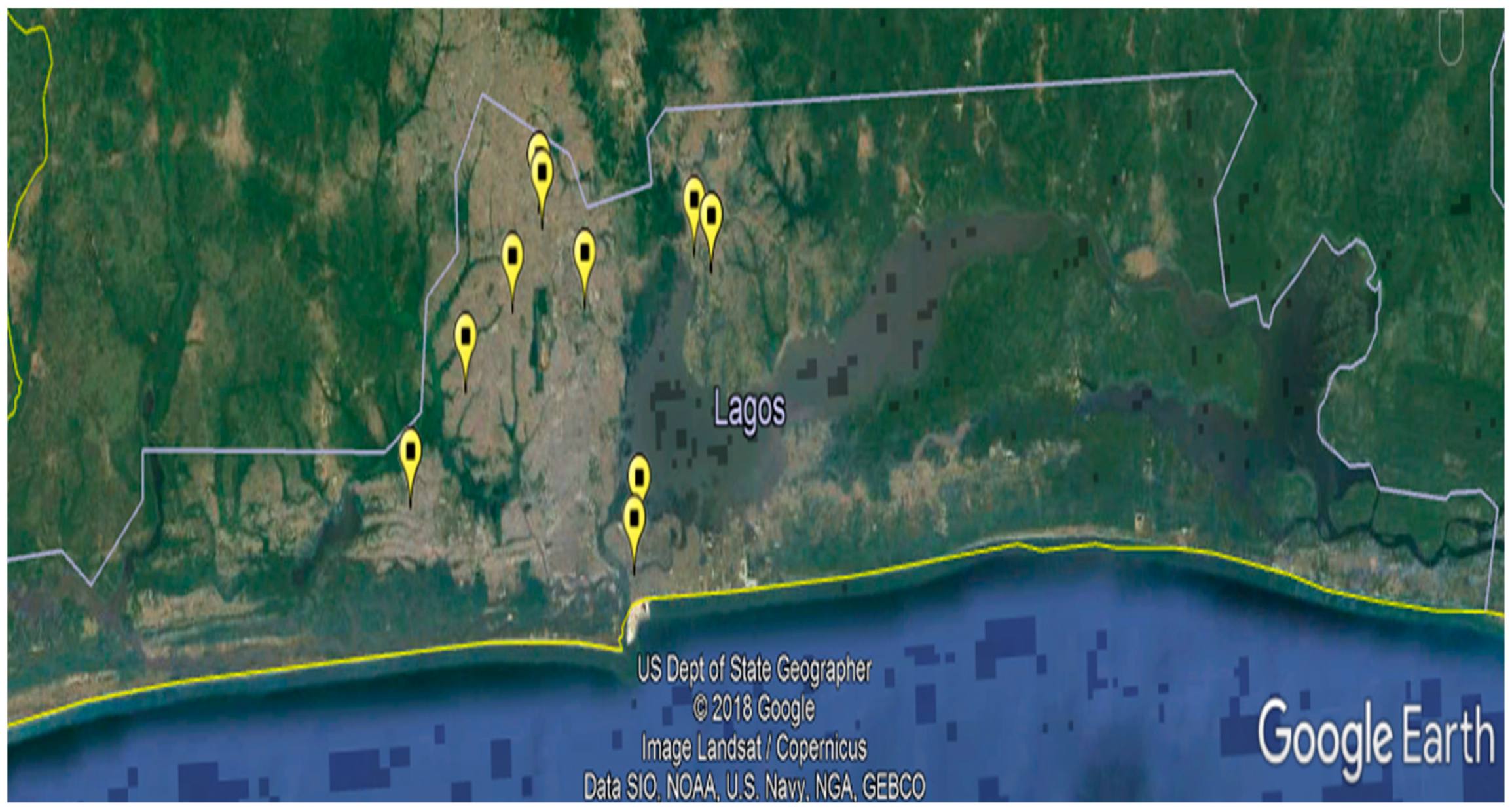
2.2. Sample Size and Sampling
2.3. Fecal Samples Collection
2.4. Data Collection
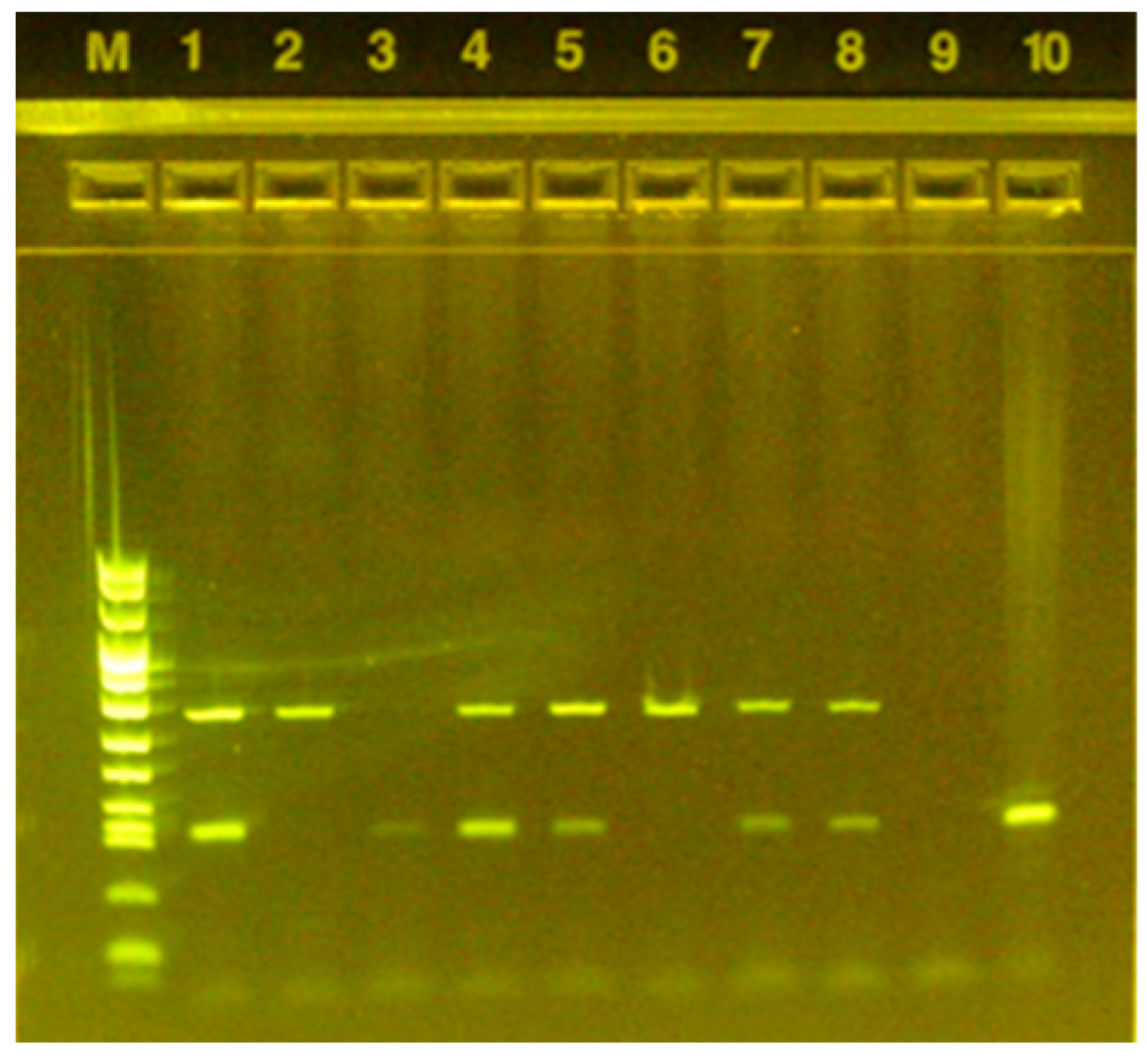
2.5. Copromicroscopic and Molecular Analyses
2.6. Statistical Analysis
3. Results
3.1. Socio-Demographic Variables of Dog Owners and Dogs Characteristc
3.2. E. granulosus s.l. Infection and Associated Factors in Dogs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romig, T.; Ebi, D.; Wassermann, M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet. Parasitol. 2015, 213, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hüttner, M.; Nakao, M.; Wassermann, T.; Siefert, L.; Boomker, J.D.F.; Dinkel, A.; Sako, Y.; Mackenstedt, U.; Romig, T.; Ito, A. Genetic characterization and phylogenetic position of Echinococcus felidis Ortlepp, 1937 (Cestoda: Taeniidae) from the African lion. Int. J. Parasitol. 2008, 38, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Rojas, C.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Echinococcosis. Available online: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (accessed on 16 April 2021).
- Eckert, J.; Gemmell, M.A.; Meslin, F.-X.; Pawłowski, Z.S. World Health Organization World Organisation for Animal Health WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern 2001. Available online: https://www.who.int/echinococcosis/resources/929044522X/en/ (accessed on 16 April 2021).
- Amarir, F.E.; Saadi, A.; Marcotty, T.; Rhalem, A.; Oukessou, M.; Sahibi, H.; Obtel, M.; Bouslikhane, M.; Sadak, A.; Kirschvink, N. Cystic echinococcosis in three locations in the Middle Atlas, Morocco: Estimation of the infection rate in the dog reservoir. Vector Borne Zoonotic Dis. 2020, 20, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Oba, P.; Ejobi, F.; Omadang, L.; Chamai, M.; Okwi, A.L.; Othieno, E.; Inangolet, F.O.; Ocaido, M. Prevalence and risk factors of Echinococcus granulosus infection in dogs in Moroto and Bukedea districts in Uganda. Trop. Anim. Health Prod. 2016, 48, 249–254. [Google Scholar] [CrossRef]
- Lahmar, S.; Lahmar, S.; Boufana, B.; Bradshaw, H.; Craig, P.S. Screening for Echinococcus granulosus in dogs: Comparison between arecoline purgation, coproELISA and coproPCR with necropsy in pre-patent infections. Vet. Parasitol. 2007, 144, 287–292. [Google Scholar] [CrossRef]
- Buishi, I.E.; Njoroge, E.M.; Bouamra, O.; Craig, P.S. Canine echinococcosis in northwest Libya: Assessment of coproantigen ELISA, and a survey of infection with analysis of risk-factors. Vet. Parasitol. 2005, 130, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Magambo, J.; Njoroge, E.; Zeyhle, E. Epidemiology and control of echinococcosis in sub-Saharan Africa. Parasitol. Int. 2006, 55, S193–S195. [Google Scholar] [CrossRef]
- Romig, T.; Omer, R.A.; Zeyhle, E.; Hüttner, M.; Dinkel, A.; Siefert, L.; Elmahdi, I.E.; Magambo, J.; Ocaido, M.; Menezes, C.N.; et al. Echinococcosis in sub-Saharan Africa: Emerging complexity. Vet. Parasitol. 2011, 181, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.N.; Xu, Y.Y.; Cadavid-Restrepo, A.M.; Lou, Z.Z.; Yan, H.B; Li, L.; Fu, B.Q.; Gray, D.J.; Clements, A.A.; Barnes, T.S.; et al. Estimating the prevalence of Echinococcus in domestic dogs in highly endemic for echinococcosis. Infect. Dis. Poverty 2018, 7, 77. [Google Scholar] [CrossRef]
- Bitrus, D.; Weka, R.; Yakubu, R.; Ogo, I.N.; Kamani, J.; Ikeh, E. Seroprevalence and associated risk factors of human cystic echinococcosis in some parts of Plateau State, Nigeria. Niger. J. Parasitol. 2020, 41, 30–34. [Google Scholar] [CrossRef]
- Adediran, O.A.; Kolapo, T.U.; Uwalaka, E.C. Echinococcus granulosus prevalence in dogs in southwest Nigeria. J. Parasitol. Res. 2014, 2014, 124358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ola-Fadunsin, S.D.; Uwabujo, P.I.; Halleed, I.N.; Richards, B. Prevalence and financial loss estimation of parasitic diseases detected in slaughtered cattle in Kwara State, North-central Nigeria. J. Parasit. Dis. 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Igwenagu, E.; Onyiche, E.T.; Saidu, A.M.; Chahari, A.M.; Waziri, A.; Kayeri, B.K. Prevalence of hydatidosis and fertility of hydatid cyst in slaughtered camels in Maiduguri, Nigeria. Ife J. Sci. 2018, 20, 299. [Google Scholar] [CrossRef]
- Ohiolei, J.A.; Yan, H.B; Li, L.; Magaji, A.A.; Luka, J.; Zhu, G.Q.; Isaac, C.; Odoya, M.E.; Wu, Y.T.; Alvi, M.A.; et al. Cystic echinococcosis in Nigeria: First insight into the genotypes of Echinococcus granulosus in animals. Parasites Vectors 2019, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Ayanwale, F.O.; Dipeolu, O.O.; Esuruoso, G.O. The incidence of echinococcus infection in dogs, sheep and goats slaughtered in Ibadan, Nigeria. Int. J. Zoonoses 1982, 9, 65–67. [Google Scholar] [PubMed]
- Ukwueze, K.O.; Ishola, O.O.; Dairo, M.D.; Awosanya, E.J.; Cadmus, S.I. Seroprevalence of brucellosis and associated factors among livestock slaughtered in oko-oba abattoir, lagos state, southwestern Nigeria. Pan Afr. Med. J. 2020, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dauda, G.B.; Cheryl, M.; Folorunso, O.F.; Ighodalo, I. Abattoir characteristics and seroprevalence of bovine brucellosis in cattle slaughtered at Bodija Municipal Abattoir, Ibadan, Nigeria. J. Vet. Med. Anim. Health 2015, 7, 164–168. [Google Scholar] [CrossRef][Green Version]
- Statistics on Population Density by Local Government Area Year 2006. Available online: http://mepb.lagosstate.gov.ng/storage/sites/29/2020/08/Digest-of-Statistics-2018.pdf (accessed on 17 April 2021).
- Hambolu, S.E.; Dzikwi, A.A.; Kwaga, J.K.P.; Kazeem, H.M.; Umoh, J.U.; Hambolu, D.A. Dog ecology and population studies in Lagos State, Nigeria. Glob. J. Health Sci. 2014, 6, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2007. [Google Scholar]
- Guo, Z.; Li, W.; Peng, M.; Duo, H.; Shen, X.; Fu, Y.; Irie, T.; Gan, T.; Kirino, Y.; Nasu, T.; et al. Epidemiological study and control trial of taeniid cestode infection in farm dogs in qinghai province, China. J. Vet. Med. Sci. 2014, 76, 395–400. [Google Scholar] [CrossRef][Green Version]
- Ritchie, L.S.; Pan, C.; Hunter, G.W. A comparison of the zinc sulfate and the formalin-ether (406th mgl) technic. Med. Bull. US Army Far East 1953, 1, 111–113. [Google Scholar]
- Rojekittikhun, W.; Mahittikorn, A.; Prummongkol, S.; Puangsa-Art, S.; Chaisiri, K.; Kusolsuk, T. Evaluation of Sugar Flotation and Formalin-Ether Concentration Techniques in the Examination of GI Parasites of Refuge Dogs and Cats in Kanchanaburi Province, Thailand. J. Trop. Med. Parasitol. 2015, 38, 17–24. [Google Scholar]
- Young, K.H.; Bullock, S.L.; Melvin, D.M.; Spruill, C.L. Ethyl Acetate as a Substitute for Diethyl Ether in the Formalin-Ether Sedimentation Technique. J. Clin. Microbiol. 1979, 10, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, D.; Deplazes, P.; Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007, 134, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Alishani, M.; Sherifi, K.; Rexhepi, A.; Hamidi, A.; Armua-Fernandez, M.T.; Grimm, F.; Hegglin, D.; Deplazes, P. The impact of socio-cultural factors on transmission of Taenia spp. and Echinococcus granulosus in Kosovo. Parasitology 2017, 144, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.I.; Wafa, M.I.; Kawther, M.I.; Badereddin, B.A. Incidence and the history of Echinococcus granulosus infection in dogs within the past few decades in Libya: A review. J. Vet. Med. Anim. Health 2017, 9, 1–10. [Google Scholar] [CrossRef][Green Version]
- Öge, H.; Öge, S.; Gönenç, B.; Sarımehmetoğlu, O.; Özbakış, G. Coprodiagnosis of Echinococcus granulosus infection in dogs from Ankara, Turkey. Vet. Parasitol. 2017, 242, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, V.; Pantchev, N.; Gawlowska, S.; Vrhovec, M.G.; Bauer, C. Echinococcus multilocularis infections in domestic dogs and cats from Germany and other European countries. Vet. Parasitol. 2008, 157, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ohiolei, J.A.; Li, L.; Ebhodaghe, F.; Yan, H.B; Isaac, C.; Bo, X.W.; Fu, B.Q.; Jia, W.Z. Prevalence and distribution of Echinococcus spp. in wild and domestic animals across Africa: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2020, 67, 2345–2364. [Google Scholar] [CrossRef] [PubMed]
- Otero-Abad, B.; Torgerson, P.R. A Systematic Review of the Epidemiology of Echinococcosis in Domestic and Wild Animals. PLoS Negl. Trop. Dis. 2013, 7, e2249. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.; Carmena, D.; Joseph, L.; Martínez, J.; Guisantes, J.A. Dog echinococcosis in northern Spain: Comparison of coproantigen and serum antibody assays with coprological exam. Vet. Parasitol. 2006, 142, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moein, K.A.; Hamza, D.A. Norway rat (Rattus norvegicus) as a potential reservoir for Echinococcus granulosus: A public health implication. Acta Parasitol. 2016, 61, 815–819. [Google Scholar] [CrossRef] [PubMed]
| Variables | Characteristics | Frequency | Percentage (95% CI) |
|---|---|---|---|
| Age (Years) | 17–35 | 59 | 44.4 (35.9–52.8) |
| >35 | 74 | 55.6 (47.2–64.1) | |
| Gender | Male | 97 | 72.9 (65.4–80.5) |
| Female | 36 | 27.1 (19.5–34.6) | |
| Ethnic origin | Yoruba | 73 | 54.9 (46.4–63.3) |
| Igbo | 33 | 24.8 (17.5–32.2) | |
| Hausa | 2 | 1.5 (0.0–3.6) | |
| Others 1 | 25 | 18.8 (12.2–25.4) | |
| Highest educational level | No formal education | 1 | 0.8 (0.0–2.2) |
| Primary | 0 | 0 | |
| Secondary | 24 | 18.1 (11.5–24.6) | |
| Tertiary | 108 | 81.2 (74.6–87.8) | |
| Marital status | Single | 40 | 30.1 (22.3–37.9) |
| Married | 93 | 69.9 (62.1–77.7) | |
| Occupational status | Unemployed | 17 | 12.8 (7.1–18.5) |
| Employed | 54 | 40.6 (32.2–49.0) | |
| Self employed | 62 | 46.6 (38.1–55.1) |
| Variables | Characteristics | Frequency | Percentage (95%) CI |
|---|---|---|---|
| Age (months) | 2–12 | 83 | 38.3 (31.8–44.7) |
| >12 | 134 | 61.7 (55.3–68.2) | |
| Sex | Male | 117 | 53.9 (47.3–60.6) |
| Female | 100 | 46.1 (39.5–52.7) | |
| Breed | Alsatian | 48 | 22.1 (16.6–27.6) |
| Lhasa Apso | 28 | 12.9 (8.4–17.4) | |
| Boerboel | 25 | 11.5 (7.3–15.8) | |
| Caucasian | 25 | 11.5 (7.3–15.8) | |
| Rottweiler | 19 | 8.8 (5.0–12.5) | |
| Terrier | 10 | 4.6 (1.8–7.4) | |
| Pit bull | 9 | 4.2 (1.5–6.8) | |
| Others 1 | 45 | 20.7 (15.3–26.1) | |
| Local | 8 | 3.7 (1.2–6.2) | |
| Location | Ikeja | 102 | 47.0 (40.4–53.6) |
| Lagos Island | 51 | 23.5 (17.9–29.1) | |
| Badagry | 40 | 18.4 (13.3–23.6) | |
| Ikorodu | 24 | 11.1 (6.9–15.2) |
| Variables | Characteristics | E.g. s.l. Positive n = 12 (%) | E.g. s.l. Negative n = 205 (%) | Odds Ratio OR, (95% CI) | p Value |
|---|---|---|---|---|---|
| Age (months) | 2–12 | 4 (33.3) | 79 (38.5) | 0.80 (0.17, 3.10) | 1.00 |
| >12 | 8 (66.7) | 126 (61.5) | |||
| Sex | Male | 5 (41.7) | 112 (54.6) | 0.59 (0.14, 2.26) | 0.55 |
| Female | 7 (58.3) | 93 (45.4) | |||
| Breed | Local | 1 (8.3) | 7 (3.4) | 2.56 (0.05, 23.08) | 0.37 |
| Exotic | 11 (91.7) | 198 (96.6) | |||
| Location | Badagry | 9 (75) | 31 (15.1) | Ref. | |
| Ikorodu | 1 (8.3) | 23 (11.2) | 0.15 (0.003, 1.24) | 0.08 | |
| Lagos Island | 1 (8.3) | 50 (24.4) | 0.07 (0.002, 0.55) | 0.004 * | |
| Ikeja | 1 (8.3) | 101 (49.3) | 0.04 (0.001, 0.27) | <0.001 * |
| Variables | Characteristics | Owners of E.g. s.l. Positive Dogs n = 11 (%) | Owners of E.g. s.l. Negative Dogs n = 122 (%) | Odds Ratio OR, (95% CI) | p Value |
|---|---|---|---|---|---|
| Dog Owners’ Characteristics | |||||
| Age (Years) | 14–35 | 5 (45.5) | 54 (44.3) | 1.05 (0.24, 4.38) | 1.00 |
| >35 | 6 | 68 | |||
| Gender | Male | 10 (90.9) | 87 (71.3) | 3.99 (0.53, 179.50) | 0.29 |
| Female | 1 | 35 | |||
| Ethnic origin | Yoruba | 3 (27.3) | 70 (57.4) | 0.28 (0.05, 1.24) | 0.07 |
| # Others | 8 | 52 | |||
| Highest educational level | Secondary and below | 3 (27.3) | 22 (18.0) | 1.70 (0.27, 7.83) | 0.43 |
| Tertiary | 8 | 100 | |||
| Marital status | Single | 4 (36.4) | 36 (29.5) | 1.36 (0.28, 5.76) | 0.73 |
| Married | 7 | 86 | |||
| Occupational status | Unemployed | 3 (27.3) | 14 (11.5) | 2.86 (0.44, 13.87) | 0.15 |
| Employed | 8 | 108 | |||
| Management and Environmental Factors | |||||
| Number of dogs in household | 1 | 8 (72.7) | 71 (58.2) | 1.91 (0.43, 11.70) | 0.52 |
| >1 | 3 | 51 | |||
| Allow dog to roam | Yes | 1 (9.1) | 40 (32.8) | 0.21 (0.01, 1.54) | 0.17 |
| No | 10 | 82 | |||
| Purpose for keeping dog | Security | 8 | 34 | Ref. | |
| Companion | 2 (18.2) | 47 (38.5) | 0.18 (0.02, 1.00) | 0.05 * | |
| Breeding and others | 1 (9.1) | 41 (33.6) | 0.11 (0.00, 0.86) | 0.03 * | |
| Feed raw meat to dog | Yes | 2 (18.2) | 13 (10.7) | 1.85 (0.18, 10.48) | 0.36 |
| No | 9 | 109 | |||
| Feed offal to dog | Yes | 2 (18.2) | 29 (23.8) | 0.71 (0.07, 3.7) | 1.00 |
| No | 9 | 93 | |||
| Slaughter slab in the vicinity | Yes | 1 (9.1) | 16 (13.1) | 0.66 (0.01, 5.28) | 1.00 |
| No | 10 | 106 | |||
| Dewormed dog | Yes | 11 (100.0) | 122 (100.) | ∞ (∞, ∞) | ∞ |
| No | 0 | 0 | |||
| Frequency of deworming | Once a month | 2 | 31 | Ref | |
| 4 times a year | 6 (54.6) | 37 (30.3) | 2.49 (0.41, 26.89) | 0.47 | |
| When necessary | 3 (27.3) | 54 (44.3) | 0.86 (0.09, 10.85) | 1.00 | |
| Use of anthelmintic | Praziquantel | 1 | 48 | Ref. | |
| Other drugs | 5 (45.5) | 32 (26.2) | 7.34 (0.77, 361.90) | 0.10 | |
| Unknown | 5 (45.5) | 42 (34.4) | 5.63 (0.60, 275.80) | 0.19 | |
| Source of water | Borehole | 10 | 77 | Ref. | |
| Tap | 1 (9.1) | 33 (27.1) | 0.24 (0.01, 1.78) | 0.26 | |
| Deep well | 0 (0.0) | 12 (9.8) | 0.00 (0.1, 3.33) | 0.51 | |
| Type of food | Home-made only | 4 | 27 | Ref. | |
| Canned food only | 1 (9.1) | 17 (13.9) | 0.40 (0.01, 4.55) | 0.77 | |
| Home-made and canned | 5 (45.5) | 61 (50.0) | 0.56 (0.11, 3.04) | 0.62 | |
| Others | 1 (9.1) | 17 (13.9) | 0.40 (0.01, 4.55) | 0.77 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awosanya, E.J.; Ligali, Z.; Duedu, K.O.; Peruzzu, A.; Masala, G.; Bonelli, P. Prevalence of Echinococcus granulosus sensu lato in Owned Dogs in Lagos State, Nigeria. Vet. Sci. 2021, 8, 101. https://doi.org/10.3390/vetsci8060101
Awosanya EJ, Ligali Z, Duedu KO, Peruzzu A, Masala G, Bonelli P. Prevalence of Echinococcus granulosus sensu lato in Owned Dogs in Lagos State, Nigeria. Veterinary Sciences. 2021; 8(6):101. https://doi.org/10.3390/vetsci8060101
Chicago/Turabian StyleAwosanya, Emmanuel Jolaoluwa, Zaynab Ligali, Kwabena Obeng Duedu, Angela Peruzzu, Giovanna Masala, and Piero Bonelli. 2021. "Prevalence of Echinococcus granulosus sensu lato in Owned Dogs in Lagos State, Nigeria" Veterinary Sciences 8, no. 6: 101. https://doi.org/10.3390/vetsci8060101
APA StyleAwosanya, E. J., Ligali, Z., Duedu, K. O., Peruzzu, A., Masala, G., & Bonelli, P. (2021). Prevalence of Echinococcus granulosus sensu lato in Owned Dogs in Lagos State, Nigeria. Veterinary Sciences, 8(6), 101. https://doi.org/10.3390/vetsci8060101






