Ischemia-Induced Cognitive Impairment Is Improved via Remyelination and Restoration of Synaptic Density in the Hippocampus after Treatment with COG-Up® in a Gerbil Model of Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Groups
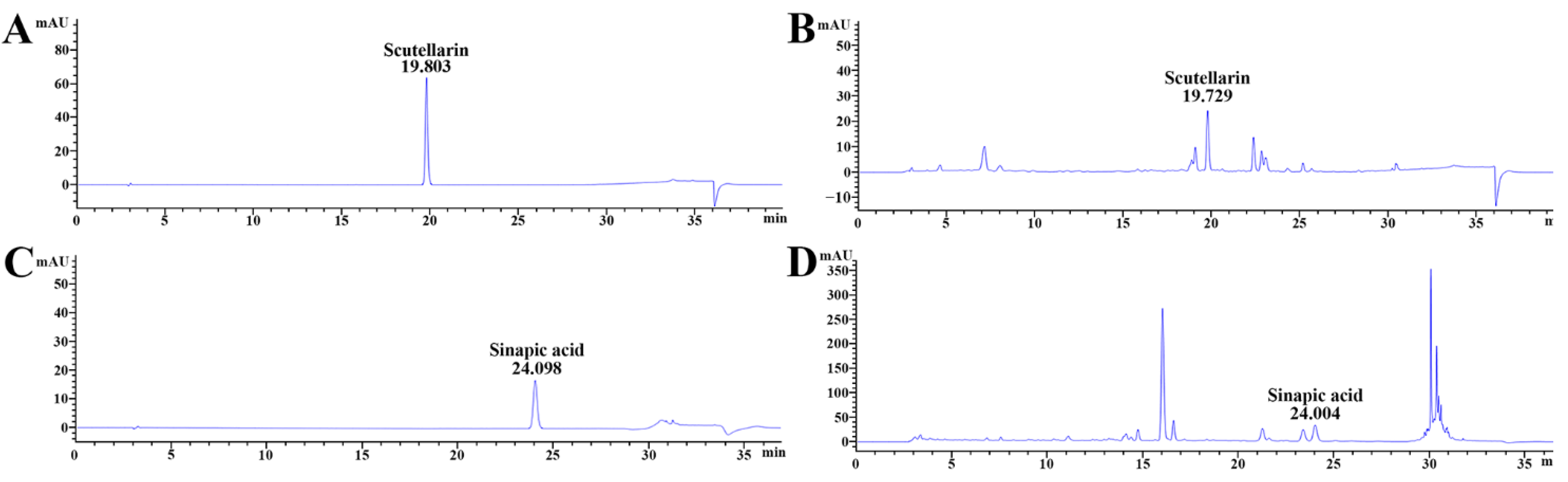
2.3. Qualitative Analysis of COG-Up®
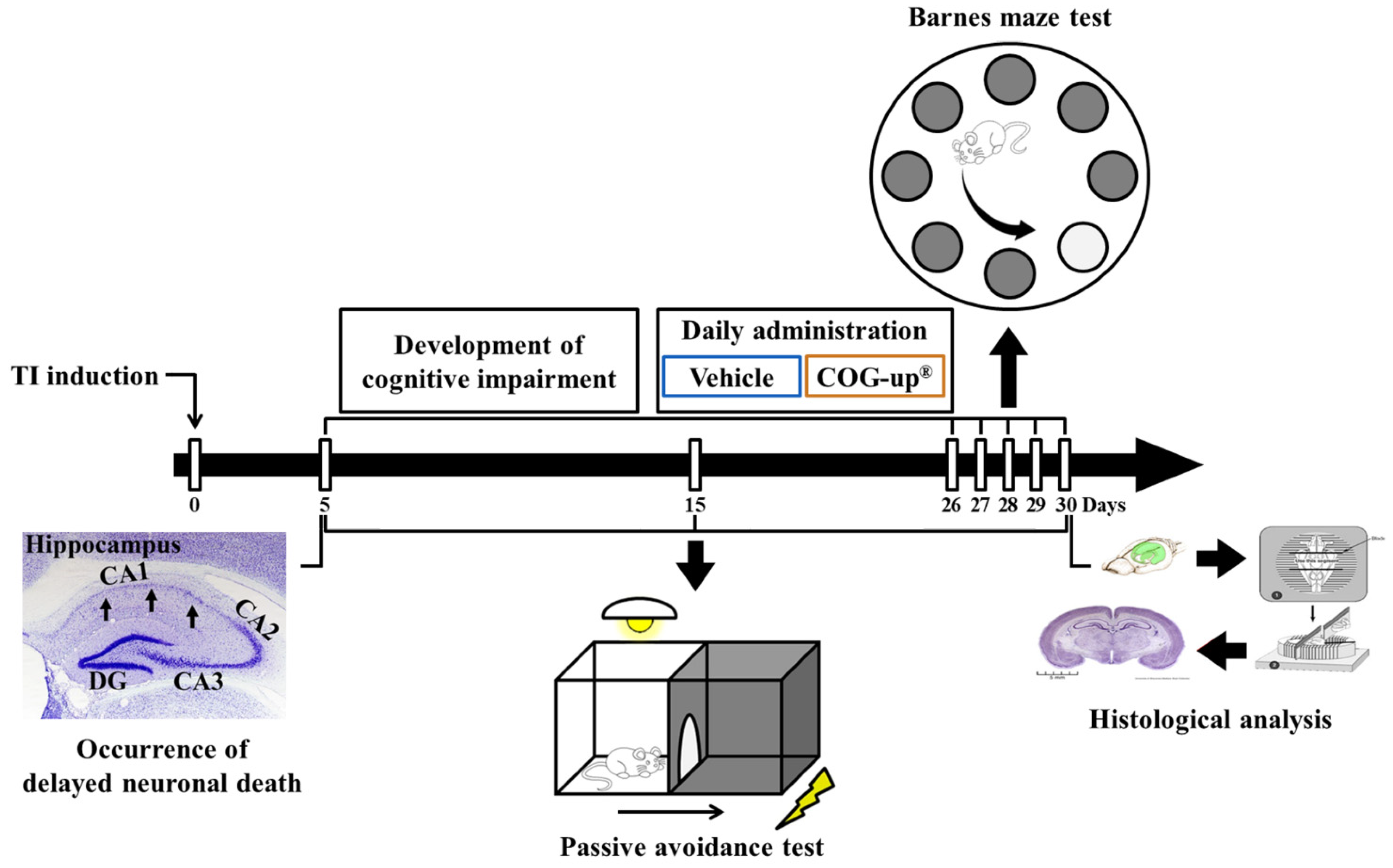
2.4. Induction of TI and Treatment with COG-Up®
2.5. Barnes Maze Test (BMT)
2.6. Passive Avoidance Test (PAT)
2.7. Preparation of Brain Sections
2.8. Cresyl Violet (CV) Staining
2.9. Fluoro-Jade B (FJB) Histofluorescence
2.10. Immunohistochemistry
2.11. Double Immunofluorescence
2.12. Statistical Analysis
3. Results
3.1. Major Ingredients of COG-Up®
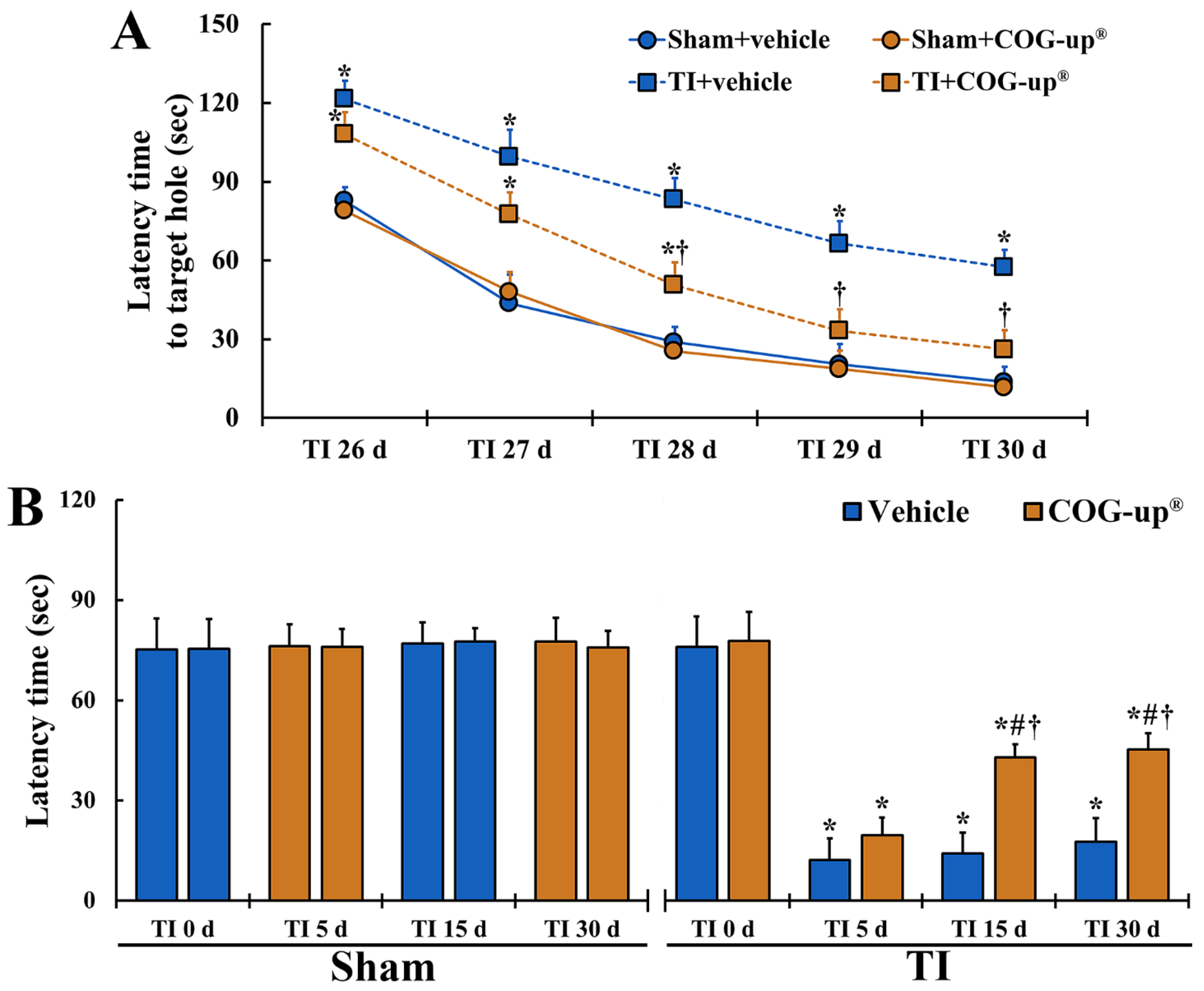
3.2. Cognitive Function
3.2.1. Spatial Memory by BMT
3.2.2. Learning Memory by PAT
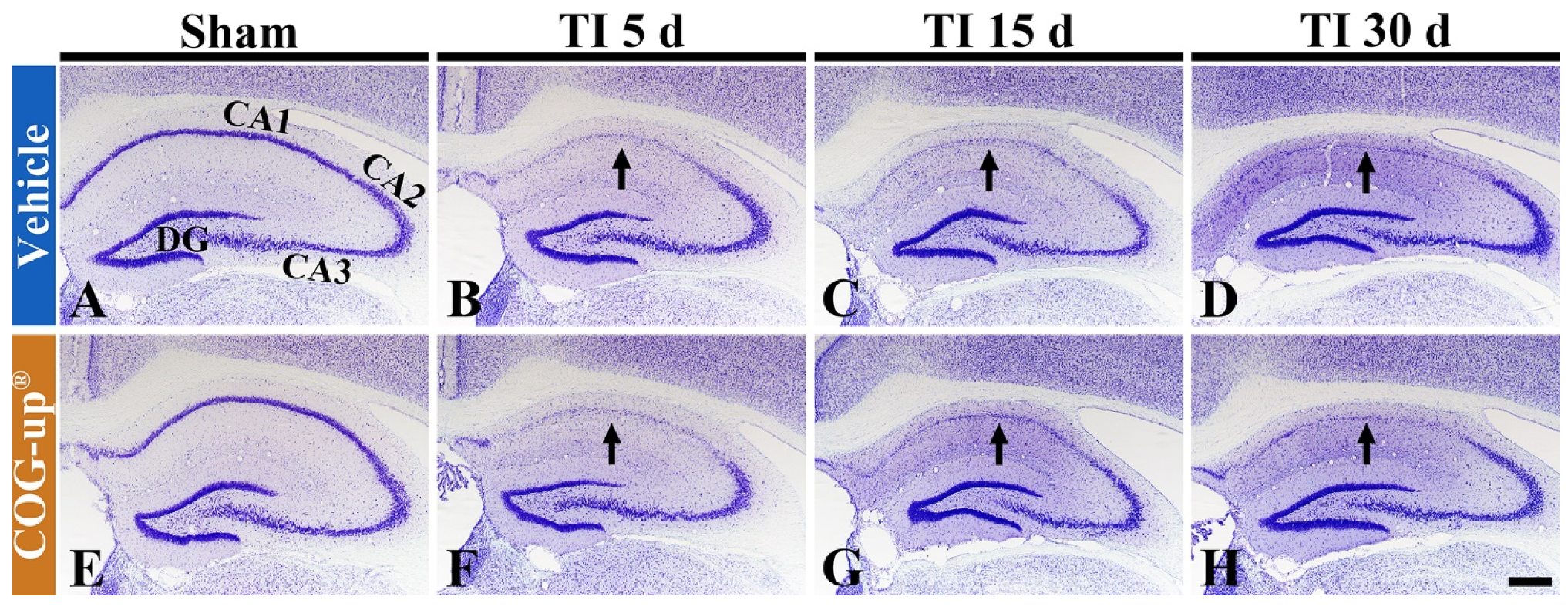
3.3. Cellular Change in the Hippocampus
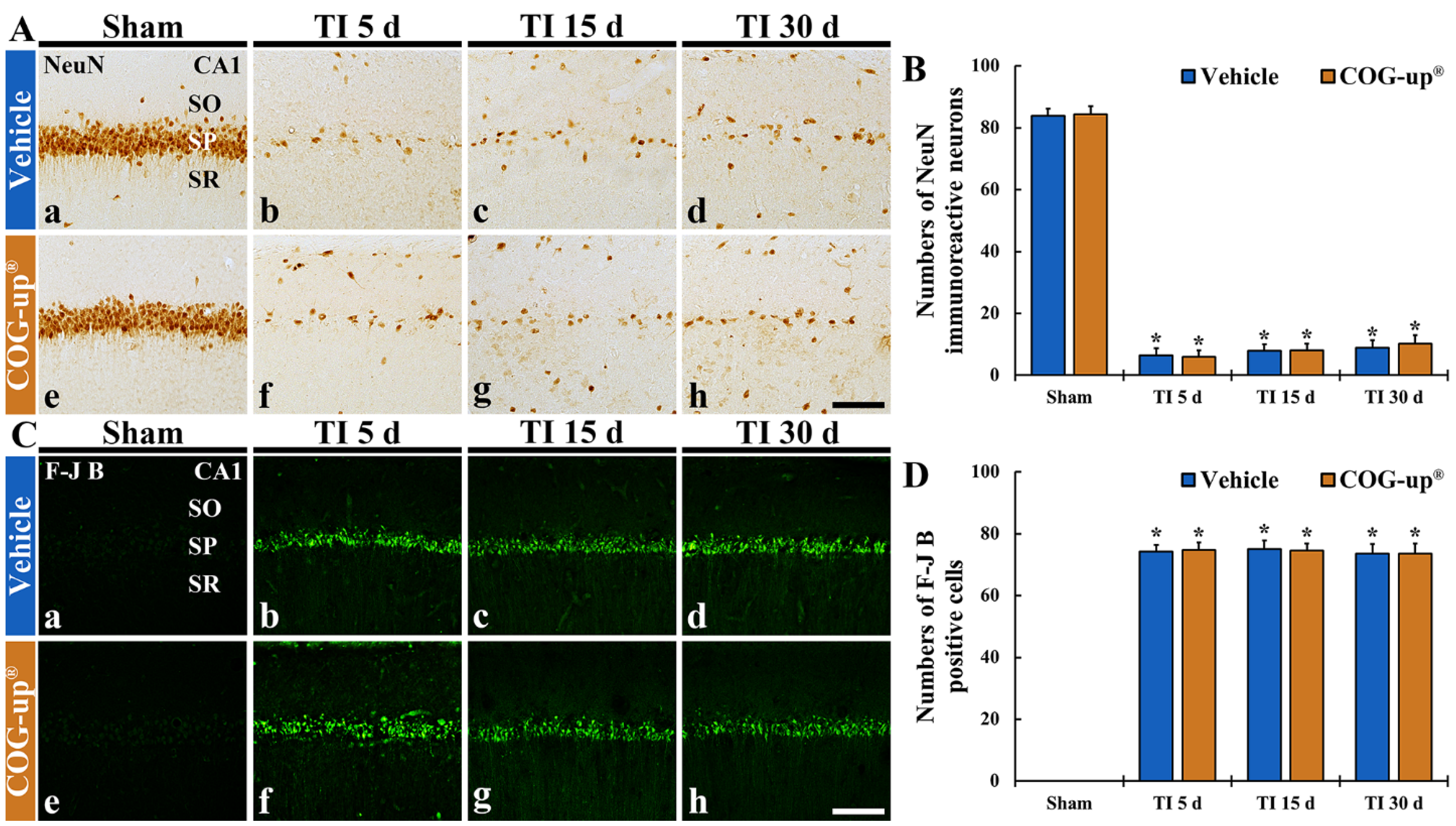
3.4. TI-Induced Neuronal Death (Loss) in the CA1 Region
3.4.1. Findings by NeuN Immunohistochemistry
3.4.2. Findings by FJB Histofluorescence
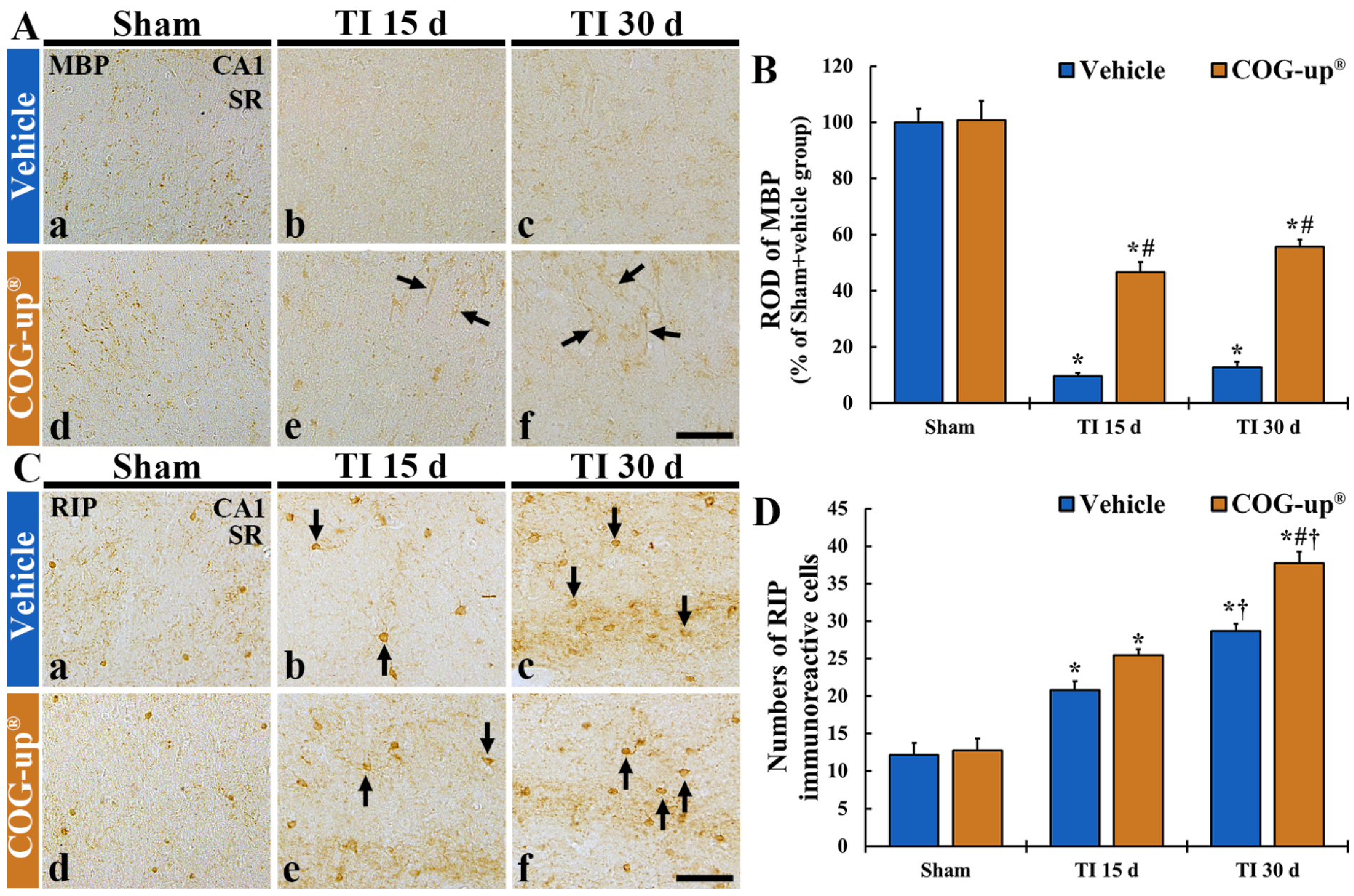
3.5. Myelin Using MBP Immunohistochemistry
3.6. Oligodendrocytes Using RIP Immunohistochemistry
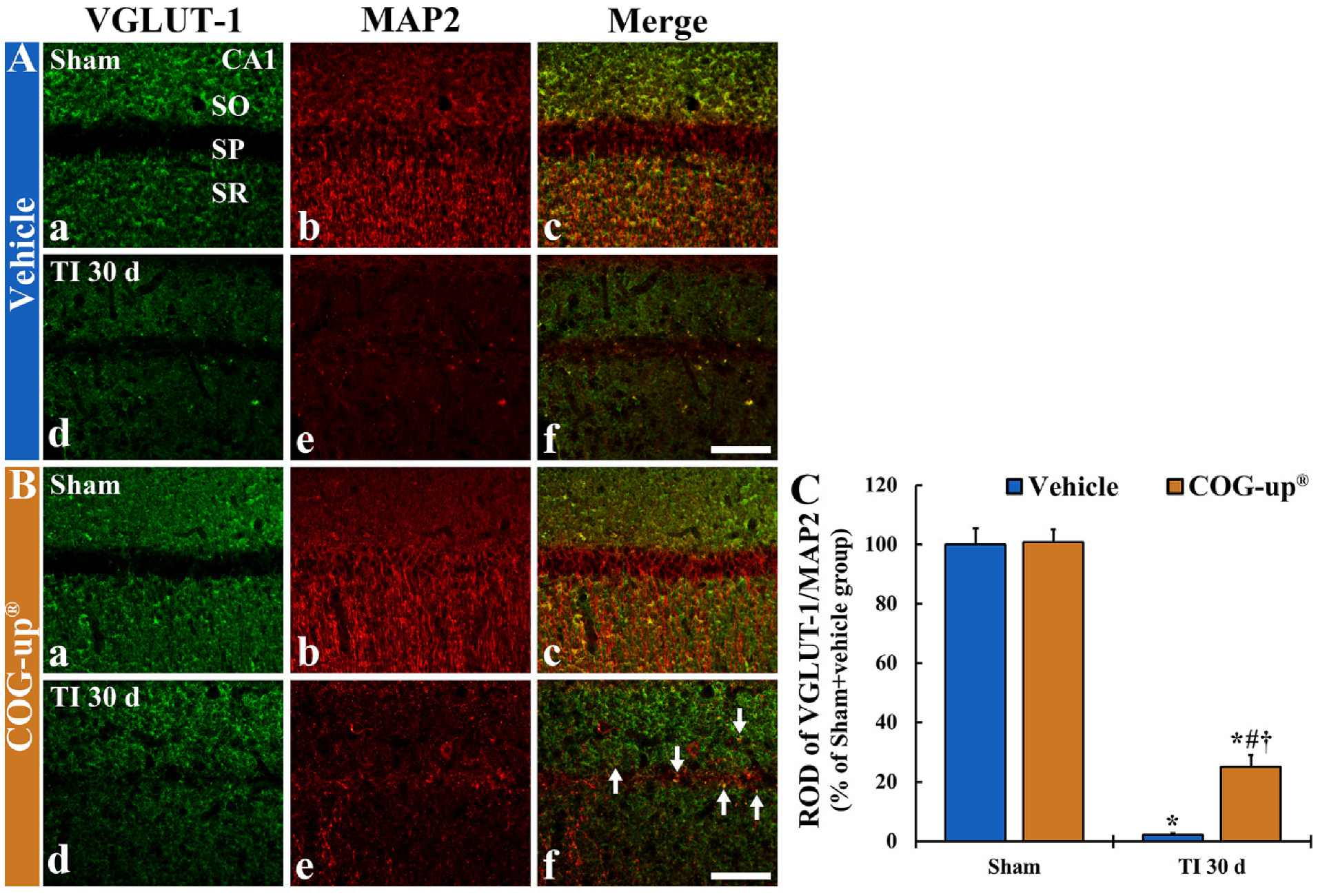
3.7. Synaptic Density Using Double Immunofluorescence for VGLUT-1/MAP2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lin, L.; Wang, X.; Yu, Z. Ischemia-reperfusion injury in the brain: Mechanisms and potential therapeutic strategies. Biochem. Pharmacol. Open Access 2016, 5, 213. [Google Scholar]
- Xu, M.S.; Yin, L.M.; Cheng, A.F.; Zhang, Y.J.; Zhang, D.; Tao, M.M.; Deng, Y.Y.; Ge, L.B.; Shan, C.L. Cerebral ischemia-reperfusion is associated with upregulation of cofilin-1 in the motor cortex. Front. Cell Dev. Biol. 2021, 9, 634347. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Park, J.H.; Lee, Y.L.; Kang, I.J.; Kim, D.W.; Hwang, I.K.; Lee, C.H.; Yan, B.C.; Kim, Y.M.; Lee, T.K.; et al. Melatonin improves vascular cognitive impairment induced by ischemic stroke by remyelination via activation of ERK1/2 signaling and restoration of glutamatergic synapses in the gerbil hippocampus. Biomed. Pharmacother. 2018, 108, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Della-Morte, D.; Rundek, T. Dizziness and vertigo. Front. Neurol. Neurosci. 2012, 30, 22–25. [Google Scholar] [PubMed] [Green Version]
- Liao, L.Y.; Lau, B.W.; Sanchez-Vidana, D.I.; Gao, Q. Exogenous neural stem cell transplantation for cerebral ischemia. Neural Regen. Res. 2019, 14, 1129–1137. [Google Scholar] [PubMed]
- Sweatt, J.D. Hippocampal function in cognition. Psychopharmacology 2004, 174, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Ahn, J.H.; Chen, B.H.; Shin, B.N.; Cho, J.H.; Kim, I.H.; Park, J.H.; Lee, J.C.; Tae, H.J.; Lee, Y.L.; Lee, J.; et al. Intravenously infused F3.Olig2 improves memory deficits via restoring myelination in the aged hippocampus following experimental ischemic stroke. Cell Transplant. 2016, 25, 2129–2144. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Park, J.H.; Yan, B.C.; Kim, I.H.; Cho, G.S.; Jeoung, D.; Kwon, Y.G.; Kim, Y.M.; Lee, Y.L.; Shin, H.C.; et al. Effects of transient cerebral ischemia on the expression of DNA methyltransferase 1 in the gerbil hippocampal ca1 region. Neurochem. Res. 2013, 38, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Park, J.H.; Ahn, J.H.; Lee, J.C.; Won, M.H.; Kang, I.J. Postsynaptic density protein (PSD)-95 expression is markedly decreased in the hippocampal ca1 region after experimental ischemia-reperfusion injury. J. Neurol. Sci. 2013, 330, 111–116. [Google Scholar] [CrossRef]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Park, Y.E.; Noh, Y.; Kim, D.W.; Lee, T.K.; Ahn, J.H.; Kim, B.; Lee, J.C.; Park, C.W.; Park, J.H.; Kim, J.D.; et al. Experimental pretreatment with YES-10®, a plant extract rich in scutellarin and chlorogenic acid, protects hippocampal neurons from ischemia/reperfusion injury via antioxidant role. Exp. Ther. Med. 2021, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, J.; Wang, J.; Li, Y.; Liu, W.; Xia, J. Lycopene supplementation protects vascular dementia gerbils against the impairment of learning and memory. Folia Neuropathol. 2021, 59, 161–173. [Google Scholar] [CrossRef]
- Qu, X.; Qi, D.; Dong, F.; Wang, B.; Guo, R.; Luo, M.; Yao, R. Quercetin improves hypoxia-ischemia induced cognitive deficits via promoting remyelination in neonatal rat. Brain Res. 2014, 1553, 31–40. [Google Scholar] [CrossRef]
- Nave, K.A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [PubMed]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef] [Green Version]
- Kugaya, A.; Sanacora, G. Beyond monoamines: Glutamatergic function in mood disorders. CNS Spectr. 2005, 10, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W.; Murphy, E.R. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol. Sci. 2006, 27, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, M.J.; Lee, J.R.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Roots of erigeron annuus attenuate acute inflammation as mediated with the inhibition of NF-kappa B-associated nitric oxide and prostaglandin E2 production. Evid.-Based Complement. Altern. Med. 2013, 2013, 297427. [Google Scholar] [CrossRef]
- Park, S.K.; Ha, J.S.; Kim, J.M.; Kang, J.Y.; Lee du, S.; Guo, T.J.; Lee, U.; Kim, D.O.; Heo, H.J. Antiamnesic effect of broccoli (Brassica oleracea var. Italica) leaves on amyloid beta (Aβ)1-42-induced learning and memory impairment. J. Agric. Food Chem. 2016, 64, 3353–3361. [Google Scholar] [CrossRef]
- Carpenter, J.W.; Marion, C. Exotic Animal Formulary-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Mahar, I.; Albuquerque, M.S.; Mondragon-Rodriguez, S.; Cavanagh, C.; Davoli, M.A.; Chabot, J.G.; Williams, S.; Mechawar, N.; Quirion, R.; Krantic, S. Phenotypic alterations in hippocampal NPY- and PV-expressing interneurons in a presymptomatic transgenic mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2016, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain atlas of the Mongolian gerbil (meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct. Funct. 2016, 221 (Suppl. 1), 1–272. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, F.; Zou, X.; Torbey, M. Comparison of unbiased estimation of neuronal number in the rat hippocampus with different staining methods. J. Neurosci. Methods 2015, 254, 73–79. [Google Scholar] [CrossRef]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.J.; Miller, K.M.; Fugaccia, I.; Scheff, S.W. Regional distribution of Fluoro-Jade B staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005, 193, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dhar, A.; Kaundal, R.K. Fetpps protects against global cerebral ischemic-reperfusion injury in gerbils. Pharmacol. Res. 2007, 55, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Meurer, R.T.; Martins, D.T.; Hilbig, A.; Ribeiro Mde, C.; Roehe, A.V.; Barbosa-Coutinho, L.M.; Fernandes Mda, C. Immunohistochemical expression of markers Ki-67, NeuN, synaptophysin, p53 and HER2 in medulloblastoma and its correlation with clinicopathological parameters. Arq. De Neuro-Psiquiatr. 2008, 66, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paizs, M.; Engelhardt, J.I.; Siklos, L. Quantitative assessment of relative changes of immunohistochemical staining by light microscopy in specified anatomical regions. J. Microsc. 2009, 234, 103–112. [Google Scholar] [CrossRef]
- Cho, J.H.; Kwon, J.E.; Cho, Y.; Kim, I.; Kang, S.C. Anti-inflammatory effect of Angelica gigas via heme oxygenase (HO)-1 expression. Nutrients 2015, 7, 4862–4874. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Park, J.H.; Ahn, J.H.; Kim, J.D.; Cho, J.H.; Lee, T.K.; Won, M.H. Stronger antioxidant enzyme immunoreactivity of Populus tomentiglandulosa extract than ascorbic acid in rat liver and kidney. Iran. J. Basic Med. Sci. 2019, 22, 963–967. [Google Scholar] [PubMed]
- Lee, T.K.; Kang, I.J.; Sim, H.; Lee, J.C.; Ahn, J.H.; Kim, D.W.; Park, J.H.; Lee, C.H.; Kim, J.D.; Won, M.H.; et al. Therapeutic effects of decursin and Angelica gigas nakai root extract in gerbil brain after transient ischemia via protecting BBB leakage and astrocyte endfeet damage. Molecules 2021, 26, 2161. [Google Scholar] [CrossRef]
- Shin, J.W.; Kweon, K.J.; Kim, D.K.; Kim, P.; Jeon, T.D.; Maeng, S.; Sohn, N.W. Scutellarin ameliorates learning and memory deficit via suppressing beta-amyloid formation and microglial activation in rats with chronic cerebral hypoperfusion. Am. J. Chin. Med. 2018, 46, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Lee, S.W.; Oh, M.S.; Lee, H.J. Effects of sinapic acid of 4 vessel occlusion model-induced ischemia and cognitive impairments in the rat. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2011, 9, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Liu, M.; Liu, J.; Zhang, T.; Zhang, L.; Li, H.; Luo, Z. Shenmayizhi decoction improves the mitochondrial structure in the brain and ameliorates cognitive impairment in VCI rats via the AMPK/UCP2 signaling pathway. Neuropsychiatr. Dis. Treat. 2021, 17, 1937–1951. [Google Scholar] [CrossRef]
- Baaklini, C.S.; Rawji, K.S.; Duncan, G.J.; Ho, M.F.S.; Plemel, J.R. Central nervous system remyelination: Roles of glia and innate immune cells. Front. Mol. Neurosci. 2019, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Kamen, Y.; Pivonkova, H.; Karadottir, R.T. Neuronal activity-dependent myelin repair after stroke. Neurosci. Lett. 2019, 703, 139–144. [Google Scholar] [CrossRef]
- Kirvell, S.L.; Elliott, M.S.; Kalaria, R.N.; Hortobagyi, T.; Ballard, C.G.; Francis, P.T. Vesicular glutamate transporter and cognition in stroke: A case-control autopsy study. Neurology 2010, 75, 1803–1809. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Li, M.; Yang, X.; Qi, Z.; Xu, B.; Liu, W.; Xu, Z.; Deng, Y. Research progress on the role of type i vesicular glutamate transporter (VGLUT1) in nervous system diseases. Cell Biosci. 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Balschun, D.; Moechars, D.; Callaerts-Vegh, Z.; Vermaercke, B.; Van Acker, N.; Andries, L.; D’Hooge, R. Vesicular glutamate transporter vglut1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb. Cortex 2010, 20, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Gou, Z.; Du, Y.; Fan, Y.; Liang, L.; Yan, Y.; Lin, P.; Jin, M.; Du, Y. Glutamatergic and central cholinergic dysfunction in the CA1, CA2 and CA3 fields on spatial learning and memory in chronic cerebral ischemia-induced vascular dementia of rats. Neurosci. Lett. 2016, 620, 169–176. [Google Scholar] [CrossRef] [PubMed]
| Primary Antibodies | Dilution | Suppliers |
| Mouse anti-neuronal nuclei (NeuN) | 1:1000 | Chemicon, Temecula, CA, USA |
| Rabbit anti-myelin basic protein (MBP) | 1:200 | Abcam, Cambridge, UK |
| Mouse anti-receptor interacting protein (RIP) | 1:200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| Secondary Antibodies | Dilution | Suppliers |
| Biotinylated horse anti-mouse IgG | 1:250 | Vector Laboratories Inc., Burlingame, CA, USA |
| Biotinylated goat anti-rabbit IgG | 1:250 | Vector Laboratories Inc., Burlingame, CA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-K.; Hong, J.; Lee, J.-W.; Kim, S.-S.; Sim, H.; Lee, J.-C.; Kim, D.W.; Lim, S.S.; Kang, I.J.; Won, M.-H. Ischemia-Induced Cognitive Impairment Is Improved via Remyelination and Restoration of Synaptic Density in the Hippocampus after Treatment with COG-Up® in a Gerbil Model of Ischemic Stroke. Vet. Sci. 2021, 8, 321. https://doi.org/10.3390/vetsci8120321
Lee T-K, Hong J, Lee J-W, Kim S-S, Sim H, Lee J-C, Kim DW, Lim SS, Kang IJ, Won M-H. Ischemia-Induced Cognitive Impairment Is Improved via Remyelination and Restoration of Synaptic Density in the Hippocampus after Treatment with COG-Up® in a Gerbil Model of Ischemic Stroke. Veterinary Sciences. 2021; 8(12):321. https://doi.org/10.3390/vetsci8120321
Chicago/Turabian StyleLee, Tae-Kyeong, Junkee Hong, Ji-Won Lee, Sung-Su Kim, Hyejin Sim, Jae-Chul Lee, Dae Won Kim, Soon Sung Lim, Il Jun Kang, and Moo-Ho Won. 2021. "Ischemia-Induced Cognitive Impairment Is Improved via Remyelination and Restoration of Synaptic Density in the Hippocampus after Treatment with COG-Up® in a Gerbil Model of Ischemic Stroke" Veterinary Sciences 8, no. 12: 321. https://doi.org/10.3390/vetsci8120321
APA StyleLee, T.-K., Hong, J., Lee, J.-W., Kim, S.-S., Sim, H., Lee, J.-C., Kim, D. W., Lim, S. S., Kang, I. J., & Won, M.-H. (2021). Ischemia-Induced Cognitive Impairment Is Improved via Remyelination and Restoration of Synaptic Density in the Hippocampus after Treatment with COG-Up® in a Gerbil Model of Ischemic Stroke. Veterinary Sciences, 8(12), 321. https://doi.org/10.3390/vetsci8120321







