Abstract
Copaifera reticulata Ducke is a popularly known species known as copaíba that is widely spread throughout the Amazon region. The tree yields an oleoresin which is extensively used in local traditional medicine mainly as an anti-inflammatory and antinociceptive agent. The aim of the present study was to assess the anti-inflammatory potential of this oleoresin obtained from a national forest in the central Amazon which presented an unusual chemical composition. The chemical composition of volatile compounds of oleoresin was analyzed by gas chromatography-mass spectrometry. The acute toxicity assay was performed with a single dose of 2000 mg/kg. The anti-inflammatory potential was evaluated by carrageenan-induced paw edema and air pouch assays using four different C. reticulata oleoresin concentrations (10, 100, and 400 mg/kg). The exudate was evaluated for nitrite concentration through the colorimetric method and for TNF-α, IL-1β, and PGE2 by ELISA. C. reticulata oleoresin collected in the Amazonian summer contained six major sesquiterpene compounds (β-bisabolene, cis-eudesma-6,11-diene, trans-α-bergamotene, β-selinene, α-selinene, and β-elemene) and was nontoxic at a dose of 2000 mg/kg, showing low acute toxicity. Different from oleoresin obtained from other sites of the Brazilian Amazon, the major volatile compound found was β-Bisabolene with 25.15%. This β-Bisabolene-rich oleoresin reduced the formation of paw edema induced by carrageenan and reduced the global number of cells in the air pouch assay, as well as exudate volume and nitrite, TNF-α, IL-1β, and prostaglandin E2 levels (p < 0.05). C. reticulata oleoresin with a high β-Bisabolene concentration showed anti-inflammatory activity, reducing vascular permeability and consequently edema formation, and thus reducing cell migration and the production of inflammatory cytokine, confirming its traditional use by local Amazonian communities.
1. Introduction
Plants are a major primary source for molecules with a wide range of biological activity, from classical activities such as anti-inflammatory, and antimicrobial [1,2] to additional biological properties such as anticancer activity [3,4] and microbiome regulation [5], with recent studies showing that Amazon can be a source of plant with a broad array of biological activities [6,7].
Inflammation is a response of the immune system to tissue damage caused by biological, mechanical, or chemical stimuli [8] and is mediated by many signaling molecules released by local cells in the presence of an injury. These substances are responsible for the formation of the main signs in most cases, but in others, when some inflammatory mediators are dysregulated (for example, nitric oxide (NO), eicosanoids, and cytokines), extremely severe pathological disorders can emerge as a consequence, such as septic shock, autoimmune diseases and chronic inflammatory diseases [9].
In communities in the Amazon region, people use natural products in traditional medicine to treat some health problems, such as inflammation and pain [10,11]. Copaifera reticulata Ducke (Fabaceae) is a common Amazonian species known for its ethnopharmacological application. It is a tree popularly known as copaíba or pau d’óleo that grows to a height of up to 40 m [10] with a trunk rich in an oleoresin that is extracted by perforation of the wood. Oleoresin was first used in folk medicine as an anti-inflammatory and analgesic agent after native Indians observed the behavior of wounded animals, which rubbed their bodies against the trunk of copaíba trees in an attempt to heal their wounds. Indians also used to apply the oleoresin to the navels of newborns and to warriors who returned from their battles with exposed wounds [12].
The anti-inflammatory activity of Copaifera sp. oleoresin samples has been studied during the last decade after extraction from C. cearensis [13], C. duckei [14], and C. multijuga [13,15,16,17,18,19]. C. reticulata oleoresin has also been previously investigated for its anti-inflammatory activity, but only in the zymosan-induced pleurisy model in Swiss mice [6], in the tongue injury model in male Wistar rats [20], and in the arthritis model in male Holtzman rats [21].
Previous research in vitro using cell culture revealed anti-inflammatory activity of Copaifera species. Dias et al. [22] observed in Copaifera-treated cells a reduction in the production of tumor necrosis factor, interferon-gamma, interleukin (IL)-17, nitric oxide, and hydrogen peroxide. Destryana et al. [23] found that copaiba was able to reduce the levels of nitric oxide, IL-6, IL-8, and IL-1β produced by macrophages.
C. reticulata oleoresin contains sesquiterpenes in the volatile fraction and diterpenes in the resinous fraction. The major compounds reported are sesquiterpenes: the most common is β-caryophyllene, which has anti-inflammatory, antitumor, and antimicrobial activity, followed by β-bisabolene, which has anti-inflammatory and analgesic properties [24,25]. Diterpenes are minor compounds, but they have some important activities such as cytotoxicity against tumor cells (kolavenol and hardwickiic acid) and anti-inflammatory activities (copalic acid) [10].
Considering that the chemical components of oleoresin of Copaifera reticulata have significant seasonal influence on their constitution and that the anti-inflammatory pharmacological activity of oleoresin extracted from the copaibeiras in the western region of Pará, in the Tapajós National Forest (Belterra Para), has not yet been reported in in vivo or in vitro studies, the objective of the present study was to evaluate the anti-inflammatory effects of C. reticulata oleoresin using male Wistar rats and carrageenan-induced inflammation as an experimental model.
2. Material and Methods
2.1. Plant Material
C. reticulata oleoresin was collected in Floresta Nacional do Tapajós (FLONA) during the dry period (Amazonian summer) of 2011 at Belterra, Pará State, Brazil. The species was identified by the taxonomist Regina Célia Viana Martins da Silva and a voucher specimen was deposited in the Herbarium of Embrapa Oriental under registration NID: 69/2011. Oil extraction for scientific purposes was authorized and approved by the Brazilian regulatory agency (protocol number: 44380-1). The trees were mechanically perforated with a traditional auger (2 cm in diameter and 45 cm in length) producing two holes at 1.00 and 1.50 m from the soil, respectively. Oleoresin samples were collected and stored in plastic containers protected from light and then transferred to glass vials (10 mL) for later analysis. After the full flow of oleoresin, the tree holes were sealed with a PVC-type pipe 0.75 cm in diameter and 10 cm in length) covered with a plastic cap in order to facilitate the next collections and to avoid wood waste [26].
The oleoresin tested in this study was endotoxin free, as determined according to the instructions of the Lonza Kinetic-QCL Chromogenic Limulus Lysate (LAL) Endotoxin Assay Kit (Walkersville, MD, USA), with a sensitivity range of 0.005–50.0 EU/mL.
2.2. Chemical Characterization of Volatile Compounds from C. reticulata Oleoresin
The chemical composition of the volatile compounds from oleoresin was analyzed by gas chromatography-mass spectrometry (GC-MS) using an Agilent gas chromatographer model HP-6890, a selective mass detector Agilent model HP-5975, a capillary column HP-5 MS (30 m × 0.25 mm × 0.25 µm), injector temperature = 250 °C, column temperature = 80 °C, heating rate = 5 °C/min up to 280 °C (20 min) and detector temperature = 300 °C; carrier gas = helium (flow of 1 mL/min); selective mass detector operating at 70 eV, m/z = 30 to 500 a.m.u. Oleoresin was dissolved in ethyl acetate at 20 mg/mL. Major volatile compounds were identified by comparison between the retention indices (RI) of the investigated substances and the RI available in the NIST library.
2.3. Animals
Wistar rats (140–220 g) 8 to 12 weeks of age were used in the experiments. Animals were kept in individual plastic cages (supplied with wood sawdust changed every three days) at 23 ± 0.5 °C on a dark-light cycle of 12 h/12 h and had access to water and food ad libitum prior to and during the experiments. The experiments were approved by the Ethics Committee for Animal Use, Federal University of Western Pará-UFOPA (protocol number: 07004/2013) and were conducted according to best practices of animal welfare.
We used a total of 62 rats, 12 of which were female and used for the acute toxicity study. In the study of paw edema, 25 male rats were used and in the air pouch test another 25 male rats were used.
2.4. Drugs and Solutions
The following drugs and solutions were used to perform the experiments: ethanol (Synth PA-ACS, Diadema, Brazil), carrageenan (Sigma Chemical Co., St. Louis, MO, USA), sodium pentobarbital (Hypnol® 3% Fontoveter, Itapira, Brazil), Tween 80 (Sigma Chemical Co.), dexamethasone disodium phosphate (Sigma Chemical Co.), and Griess reagent (Sigma Chemical Co.).
Oleoresin was diluted in 15% ethanol P.A. and 0.312% Tween 80. The vehicle consisted of 0.312% Tween 80, 15% ethanol, and 0.9% saline solution.
2.5. Acute Toxicity Assay
The acute toxicity assay followed the guidelines of the Organization for Economic Co-operation and Development (OCDE)-423/2001 [27]. This protocol states that, when previous studies have assessed the acute toxicity of a known sample, the initial dose used can be the maximum dose without side effects. As previously reported by Sachetti et al. [25], C. reticulata oleoresin was tested in a single dose of 2000 mg/kg. We used twelve 9-week-old female rats weighing 150 to 180 g. Six rats received the oleoresin and six were kept as the control group, receiving the same dose (2000 mg/kg) of water. The acute toxicity assay was performed with three rats per group (control and treated) and repeated after 14 days with another three rats per group, totaling twelve rats. Several parameters such as general activity, irritability, contortion, ataxia, tremors, convulsions, piloerection, hypothermia, breathing, cyanosis, hyperemia, and death were analyzed.
2.6. Experimental Design
The animals were treated with three different doses of C. reticulata oleoresin (low, medium, and high), which were defined according to the acute toxicity assay, corresponding to 1/200, 1/20, and 1/5 of the maximal dose with biological safety (2000 mg). The standard anti-inflammatory drug was dexamethasone (0.6 mg/kg, orally).
A total of 50 rats were submitted to two different anti-inflammatory activity tests: carrageenan-induced paw edema assay (n = 25) and carrageenan-induced air pouch assay (n = 25). The first group was treated only with vehicle and the pro-inflammatory stimulus (Carrageenan). In the second group, the animals were treated with the standard drug and pro-inflammatory stimulus. The third, fourth, and fifth groups were treated with oleoresin at 10, 100, and 400 mg/kg, respectively, and the pro-inflammatory stimulus.
2.7. Anti-Inflammatory Activity
2.7.1. Carrageenan-Induced Paw Edema Assay
Paw edema was measured as described by Koo et al. [28]. Animals (n = 5 per group) were treated orally with vehicle, dexamethasone (0.6 mg/kg), and oleoresin (10, 100, and 400 mg/kg,); 0.2 mL of 1% carrageenan was injected into the intraplantar region of the right hind paw and an equal volume of saline solution was injected into the left hind paw. The volume (mL) of the paw was measured with a digital plethysmometer immediately after carrageenan administration (0) and at later intervals (1, 2, 3, and 4 h) and the difference in volume between the two paws was calculated.
2.7.2. Carrageenan-Induced Air Pouch Assay
Air pouches were produced in the animals by subcutaneous injection with 20 mL of sterile air in the intrascapular region of Wistar rats as described by Tao et al. [29]. Pouches were reinflated with 10 mL air after the 3rd and 6th days. Oral treatment (n = 5 per group) with vehicle, dexamethasone (0.6 mg/kg), and oleoresin (10, 100, and 400 mg/kg) was performed on the 9th day. One hour after the beginning of treatment, 2 mL of 1% carrageenan was administered directly into the pouch. On the 10th day, the animals were sacrificed with a sodium pentobarbital overdose 16 h after the stimulus with carrageenan. After incision in the wall of the air pouch, the content was collected with a sterile Pasteur pipette and the exudate volume was measured. Cells were counted (cells/mm3) in a Neubauer chamber and the cytokines listed below were determined.
Euthanasia was performed by intraperitoneal injection of a sodium pentobarbital overdose, corresponding to two to three times the dose considered anesthetic (100 mg/kg), as recommended by Guidelines for the Practice of Euthanasia of the National Council for the Control of Animal Experimentation (CONCEA) [30].
2.7.3. Determination of Nitrite, Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL-1β) and Prostaglandin E2 (PGE2) in the Exudate
Nitrite levels were measured in the exudate samples by the addition of 500 µL Griess reagent to 500 µL of exudate in a spectrophotometer at 540 nm. Nitrite concentration was determined by comparison with the calibration curve with serial dilutions of sodium nitrite as described by Koo et al. [19]. The concentrations of TNF-α, IL-1β, and PGE2 in the exudate were determined with ELISA kits for rats (eBioscience, San Diego, CA, USA) and the results are expressed as pg/mL
2.8. Statistical Analysis
Data were analyzed statistically using GraphPad Prism (6.0) software and submitted to analysis of variance (one-way or two-way ANOVA). The Newman–Keuls or Bonferroni multiple comparison test was used for comparison between groups. Results were considered significant for values of * p < 0.05; ** p < 0.01; *** p < 0.001.
3. Results
3.1. Chemical Composition of Volatile Compounds of C. reticulata Oleoresin
According to Table 1, the characterization of oleoresin volatile compounds showed the presence of six major sesquiterpenes corresponding to 74.8% of the total compounds: β-bisabolene (25.15%), cis-eudesma-6,11-diene (14.20%), trans-α-bergamotene (12.76%), β-selinene (8.70%), α-selinene (7.03%), and β-elemene (6.96%). It is important to note that the percentages of the compounds listed in Table 1 are related to the total number of compounds identified in the GC-MS analysis; they are not the percentage of the compounds in the extract and that the GC-MS analysis identified only volatile compounds.

Table 1.
Qualitative and quantitative composition of volatile compounds from Copaifera reticulata oleoresin by CG-MS.
Table 2 presents a comparative result from the chemical composition of volatile compounds from C. reticulata obtained from different areas of the Brazilian Amazon. Our samples were obtained from FLONA Tapajós, located in the central Amazon, and had different chemical compositions from samples from Belém within the same state (Pará) but located in the eastern Amazon and from samples obtained from Acre State located in the western Amazon. While the same plant from other sites from the Amazon had limited amounts of sesquiterpenes, the sample used in the present study yielded as much as 25% of one single compound (β-Bisabolene).

Table 2.
Bibliographic references compared to our results.
3.2. Acute Toxicity
The animals treated with a 2000 mg/kg dose of oleoresin did not show any signs of toxicity and no deaths occurred. For this reason, it was established that the acute oral toxicity of the oleoresin was higher than 2000 mg/kg and the product was classified as category 5 with a high level of biological safety.
3.3. Paw edema
The oleoresin was able to reduce edema in the first hour of evaluation by 14.56% (0.61 ± 0.08 mL), 36.69% (0.45 ± 0.02 mL) and 40.89% (0.42 ± 0.03 mL) at 10, 100, and 400 mg/kg, respectively, compared to the control (0.71 ± 0.01). In the second hour, only the groups treated with 100 and 400 mg/kg oleoresin showed a significant antiedema effect, with a reduction in paw edema of 20.66% (0.77 ± 0.02 mL) and 17.97% (0.79 ± 0.04 mL), respectively, compared to the vehicle-treated group (0.97 ± 0.05 mL). After the third (T3) and fourth (T4) hours, only the group treated with 100 mg/kg oleoresin showed a significant antiedema activity, with a reduction in edema of 17.94% (0.62 ± 0.04 mL) and 12.72% (0.58 ± 0.04 mL), respectively, compared to the vehicle-treated group (T3 = 0.75 ± 0.02 and T4 = 0.66 ± 0.03 mL). Dexamethasone, 0.6 mg/kg, prevented edema formation along the 4 h evaluation (Figure 1).
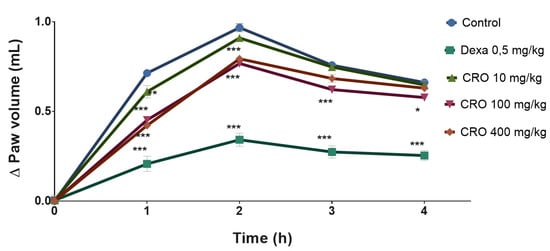
Figure 1.
Effect of C. reticulata oleoresin (10, 100, and 400 mg/kg) and dexamethasone (0.6 mg/kg) administered orally to Wistar rats on the edematogenic stimulus induced by 1% carrageenan (200 µL, intraplantar). Each point represents the mean ± SE of a group of 5 animals. * p < 0.05; ** p < 0.01; *** p < 0.001 compared to control; two-way ANOVA, Bonferroni test.
3.4. Volume of Exudate from the Air Pouch
Oleoresin showed a dose-dependent effect on the production of exudate in the air pouch, reducing it by 52.22% (1.49 ± 0.12 mL), 60.43% (1.23 ± 0.04 mL), and 64.08% (1.12 ± 0.01 mL) at 10, 100, and 400 mg/kg, respectively, compared to the total volume produced by the control group (3.12 ± 0.08 mL). Dexamethasone reduced the exudate volume by 90.25% (0.30 ± 0.02) (Figure 2).
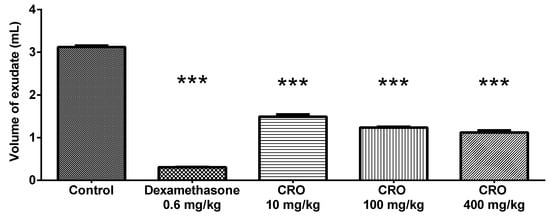
Figure 2.
Volume of exudate produced in the air pouch. Effect of C. reticulata oleoresin—CRO (10, 100, and 400 mg/kg, orally) and dexamethasone (0.6 mg/kg, orally) on Wistar rats using the air pouch model. Each point represents the mean ± SE of a group of 5 animals. *** p < 0.001 compared to control (vehicle); one-way ANOVA, Newman–Keuls multiple comparison test.
3.5. Cell Count in the Air Pouch Exudate
Oleoresin reduced the number of cells in the air pouch. Dexamethasone inhibited cell recruitment by 85.40% (11.98 ± 4.03 cells/mm3) compared to the control (81.92 ± 1.29 cells/mm3). The groups treated with 10, 100, and 400 mg/kg oleoresin showed a reduction in cell numbers of 26.80% (59.97 ± 14.34 cells/mm3), 51.60% (39.66 ± 5.84 cells/mm3), and 54.30% (37.45 ± 8.50 cells/mm3), respectively (Figure 3).
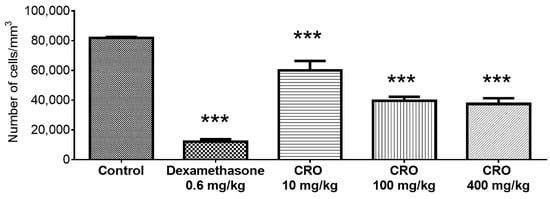
Figure 3.
Count of the total number of cells of the exudate in the air pouch. Effect of C. reticulata oleoresin—CRO (10, 100, and 400 mg/kg, orally) and dexamethasone (0.6 mg/kg, orally) on Wistar rats using the air pouch model. Each point represents the mean ± SE of a group of 5 animals. *** p < 0.001 compared to control (vehicle); one-way ANOVA, Newman–Keuls multiple comparison test.
3.6. Determination of Nitrite Levels
Oleoresin (10, 100, and 400 mg/kg) significantly reduced nitrite levels in the air pouch by 44.00% (39.45 ± 2.78 µM), 56.90% (30.40 ± 1.20 µM), and 64.00% (25.35 ± 0.75 µM), respectively. Dexamethasone reduced these levels by 65.80% (24.19 ± 0.70 µM) (Figure 4A).
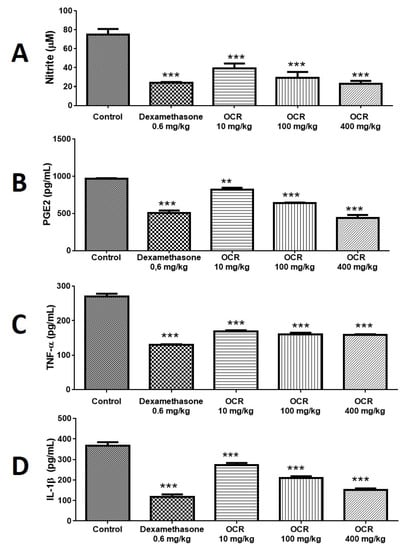
Figure 4.
Effect of C. reticulata oleoresin—CRO (10, 100, and 400 mg/kg) and dexamethasone (Dexa) (0.6 mg/kg) administered orally to Wistar rats on inflammation mediators using the air pouch model: determination of nitrite (A), PGE2 (B), TNF-α (C), and IL-1β (D) in the air pouch exudate. Each point represents the mean ± SE of a group of 5 animals. ** p < 0.01; *** p < 0.001, compared to control (vehicle); one-way ANOVA, Newman–Keuls multiple comparison test.
3.7. Determination of PGE2 Levels
Oleoresin promoted a dose-dependent response of PGE2 levels at concentrations of 10, 100, and 400 mg/kg, reducing the production of this prostaglandin by 15.11% (817.60 ± 27.54 pg/mL), 33.64% (639.20 ± 8.37 pg/mL), and 54.73% (436.00 ± 43.63 pg/mL), respectively, compared to the control (963.20 ± 10.28 pg/mL). Dexamethasone reduced the concentration of PGE2 by 47.78% (503.00 ± 35.83 pg/mL) (Figure 4B).
3.8. Determination of TNF-α Levels
Oleoresin, 10, 100, and 400 mg/kg, had a significant effect, reducing the TNF-α concentration by 37.26% (169.40 ± 2.71 pg/mL), 40.45% (160.80 ± 4.49 pg/mL), and 41.41% (158.20 ± 2.44 pg/mL), respectively, compared to the control (270.00 ± 7.84 pg/mL). Dexamethasone reduced TNF-α levels by 48.00% (129.60 ± 1.86 pg/mL) (Figure 4C).
3.9. Determination of IL-1β Levels
Oleoresin, 10, 100, and 400 mg/kg, significantly reduced IL-1β levels by 25.93% (272.00 ± 11.58), 42.86% (209.80 ± 8.44), and 58.93% (150.80 ± 8.07), respectively, compared to control (367.20 ± 17.49). Dexamethasone reduced IL-1β levels by 67.75% (118.40 ± 11.18 pg/mL) (Figure 4D).
4. Discussion
The chemical characterization of volatile compounds from C. reticulata oleoresin showed the presence of sesquiterpenes, including β-bisabolene (25.15%), eudesma cis-6,11-diene (14.20%), and trans-α-bergamotene (12.76%). These results are in agreement with Bardají et al. [31], who reported the presence of β-bisabolene (24.91%) and trans-α-bergamotene (21.99%) as the main oleoresin volatile compounds of C. reticulata, collected from Brasil Novo, in the state of Pará, Brazil. However, according to a previous report in Brazil [10], there is wide variability in the chemical composition of oleoresin of the genus Copaifera, depending on the species, location, seasonal variations, light intensity, soil nutrients, and attack by pathogens and herbivores. The chemical composition of volatile compounds of C. reticulata was found with a wide variability of the sesquiterpene fraction [32]. One limitation of the present study was the chemical analytical method, since GC-MS only identified volatile compounds and was based only on comparison of RI values to those provided by the NIST library. In addition, studies carried out by Veiga [13] and Gomes et al. [16] demonstrated the different chemical compositions and concentrations of compounds in the same species of C. reticulata. However, all samples of oleoresin of that species contained β-bisabolene. In a comparative analysis of the chemical composition of oleoresin from Copaifera reticulata obtained in the present study to previous reports [13,16], the presence of β-bisabolene was observed in the three oleoresins, even at different concentrations. However, the major compounds in the three different studies did not match, thus demonstrating variability in the chemical composition of the species. Therefore, although the anti-inflammatory activity of C. reticulata has already been reported, our study corroborates this result since the chemical composition of volatile compounds of the tested oleoresin, as well as the representativeness of the compounds, are also different from the oleoresin described in the literature.
Oleoresin was nontoxic at a dose of 2000 mg/kg, showing low acute toxicity. It significantly reduced the paw edema in the first hour in a dose-dependent manner during the phase of release of mediators such as histamine, serotonins, and/or kinins. By the second hour (second phase of edema formation), the group treated with 100 mg/kg showed a reduction in edema volume at all tested times. It is known that the second phase is characterized by a high production of prostaglandins that promote edema formation [8]. Our results showed that oleoresin interfered with the production of mediators such as PGE2. C. multijuga oleoresin and its hexane and chloroform fractions administered at 150 mg/kg were able to reverse the edematogenic effect of carrageenan by at least 50% [19].
In the air pouch model, a thin layer of fibroblasts and macrophages is formed in the intrascapular region closely similar to the synovial region and the administration of carrageenan simulates an inflammatory response in the human joints. Carrageenan activates a Toll-like receptor (Toll 4 type) with the consequent activation of the nuclear transcription factor NF-κB. When activated, this factor results in the production and release of pro-inflammatory cytokines that recruit cells to the site of inflammation, and also results in the production of NO and other inflammatory mediators [33]. Administration of carrageenan induces a response that favors an ideal environment for the collection of many cells and extracellular fluids rich in pro-inflammatory cytokines. These products can be qualified, quantified, and used as evaluative parameters of inflammation [33,34,35]. On this basis, our results showed that C. reticulata oleoresin reduced the fluid overflow into the cavity of the air pouch. This effect is probably related to the reduction in vascular permeability through the production and release of inflammatory mediators. The reduction in exudate volume agrees with the reduction in paw edema. It is known that the chemotactic and exudative process is directly related to macrophage activity regarding the production of mediators and the increase in vascular permeability [36]. Therefore, oleoresin may perhaps interfere with macrophage activity by reducing the production and release of inflammatory mediators such as PGE2, TNF-α, IL-1β, and NO.
Inducer agents such as carrageenan and lipopolysaccharides (LPS) and pro-inflammatory cytokines such as TNF-α stimulate the expression of induced nitric oxide synthase (iNOS), which regulates the production of NO by macrophages and other cells activated by cytokines [37]. NO has the ability to destroy pathogens by inducing oxidative stress in macrophages or polymorphonuclear cells, also acting on the modulation of cell development and cytokine production, and can act on the endothelium to promote vasodilation, to increase permeability, and produce an exudate [33]. In the present study, oleoresin reduced nitrite levels in the air pouch and consequently vascular permeability, since the volume of exudate was significantly reduced. These results agree with those obtained from the paw edema assay because oleoresin also inhibited the formation of edema in the rat paws. Veiga et al. [13] evaluated oleoresins from some Copaifera species and observed the inhibition of nitrergic activity in peritoneal macrophages of LPS-stimulated mice when NO production was inhibited.
Considering the importance of cytokines in the inflammatory process, we investigated the effect of C. reticulata oleoresin on TNF-α and IL-1β levels in the exudate formed inside the air pouch. Oleoresin significantly reduced the levels of these cytokines as well as NO levels. Gomes et al. [38] determined the levels of TNF-α in the ascitic fluid extracted from animals with Ehrlich ascites tumor cells and observed a significant reduction after treatment with oleoresin. The present results also agree with Gelmini et al. [39], who determined the production of TNF-α and IL-1β by LPS-stimulated human monocytes pre-treated with a purified fraction of C. langsdorffii (0.1–100 µM) containing sesquiterpenes (31.74%), diterpenic acids (44.73%), and diterpenes (5.58%).
Macrophages have an important function in the inflammatory response through the production and release of many mediators. When macrophages release TNF-α, arachidonic acid from cell membrane phospholipids is metabolized by cyclooxygenases, yielding prostanoids and PGE2 in particular. PGE2 acts on vascular permeability and is an important mediator that acts on the development of several inflammatory diseases, on pain induction, and leukocyte recruitment [8]. C. reticulata oleoresin reduced the synthesis of PGE2 in the air pouch, in agreement with its anti-edematogenic action, inhibiting edema formation in the second phase. The second phase of the paw edema assay is characterized by a high production of prostaglandins which contribute to the maintenance of edema [8]. Thus, a reduction in PGE2 seems to be directly related to the decrease in paw edema and exudate in the air pouch.
Veiga et al. [13] evaluated the anti-inflammatory activity of C. reticulata (100, 200, and 400 mg/kg) using a zymosan-induced inflammation model in male Swiss mice. Oleoresin inhibited total leukocyte and neutrophil accumulation only at 400 mg/kg but did not inhibit the protein extravasation induced by zymosan. Ghizoni et al. [21] evaluated the action of C. reticulata oleoresin on the systemic inflammation of arthritic male Holtzman rats. Severe arthritis in the paw edema model was induced by Freund’s adjuvant (heat-inactivated Mycobacterium tuberculosis derived from the H37 Rv human strain) and rats were treated with oleoresin at 580 and 1150 mg/kg. Treatment was only partially effective since it was able to decrease the contralateral paw edema, but not the secondary lesions due to arthritis in the tail and ears. Teixeira et al. [20] investigated the anti-inflammatory activity of C. reticulata oleoresin using a model of injury to rat tongues. Oleoresin therapy (200 mg/kg) modulated the inflammatory response by decreasing the chronic inflammatory infiltrate, edema, and specifically the number of macrophages in the injured area.
The present results showed that oleoresin rich in β-Bisabolene extracted from FLONA had a significant anti-inflammatory activity confirmed by the carrageenan-induced inflammation model, reducing vascular permeability and consequently edema formation, and thus reducing cell migration and the production of NO, PGE2, IL-1β, and TNF-α. This study supports the anti-inflammatory potential of C. reticulata oleoresin, confirming the traditional medicinal use of this natural product by local people in the Amazon region. Further studies are required to evaluate the specific compounds associated with different degrees of anti-inflammatory activity, but the results from this study combined with the recent literature showing a similar activity with different chemical compositions based on volatile compounds may indicate that the Amazon tree Copaiba can be a source for multiple molecules with anti-inflammatory properties [13,16].
Author Contributions
Conceptualization, T.M.P.M., E.O., L.E.S.B., A.H.H.M. and W.P.M.; Data curation, T.M.P.M., E.C.P.d.O., A.H.H.M. and W.P.M.; Formal analysis, J.S.d.A.J., É.B.S.d.S., T.M.P.M., A.A.M.K., L.C.B., E.C.P.d.O. and L.E.S.B.; Funding acquisition, E.O. and A.H.H.M.; Investigation, J.S.d.A.J., A.A.M.K., L.C.B., L.E.S.B. and W.P.M.; Methodology, J.S.d.A.J., É.B.S.d.S., A.A.M.K., A.S., L.C.B., E.C.P.d.O., L.E.S.B. and W.P.M.; Project administration, A.S., E.O., A.H.H.M. and W.P.M.; Resources, É.B.S.d.S., A.A.M.K. and W.P.M.; Supervision, T.M.P.M., A.S. and W.P.M.; Validation, J.S.d.A.J., É.B.S.d.S., A.S., L.C.B. and E.C.P.d.O.; Visualization, E.C.P.d.O.; Writing—original draft, J.S.d.A.J.; Writing—review & editing, L.C.B., E.O., L.E.S.B., A.H.H.M. and W.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external funding.
Institutional Review Board Statement
The experiments were approved by Ethics Committee for Animal Use, Federal University of Western Pará-UFOPA (protocol number: 07004/2013) and were conducted according to best practices of animal welfare.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the raw data collected in this research is fully and completely available upon request to the corresponding author.
Acknowledgments
We wish to thank Regina C. V. M. da Silva for the taxonomic identification of C. reticulata. A.H.H.M. is grateful to CNPq for his research productivity fellowship.
Conflicts of Interest
The authors declare no competing interests.
References
- Mutuku, A.; Mwamburi, L.; Keter, L.; Ondicho, J.; Korir, R.; Kuria, J.; Chemweno, T.; Mwitari, P. Evaluation of the antimicrobial activity and safety of Rhus vulgaris (Anacardiaceae) extracts. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Adegbaju, O.D.; Otunola, G.A.; Afolayan, A.J. Anti-inflammatory and cytotoxic evaluation of extracts from the flowering stage of Celosia argentea. BMC Complement. Med. Ther. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Nelson, V.K.; Sahoo, N.K.; Sahu, M.; Sudhan, H.H.; Pullaiah, C.P.; Muralikrishna, K.S. In vitro anticancer activity of Eclipta alba whole plant extract on colon cancer cell HCT-116. BMC Complement. Med. Ther. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Silva, B.S.; Barata, L.E.S.; Arévalo, M.R.; Vieira, L.Q.; Castro, W.; Ruiz, A.L.T.G.; Della Torre, A.; Castro, K.C.F.; Sartoratto, A.; Baratto, L.C.; et al. Chemical Composition and Antiproliferative Activity of the Ethanolic Extract of Cyperus articulatus L. (Cyperaceae). Plants 2021, 10, 2084. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-Inflammatory Properties and Gut Microbiota Modulation of Porphyra tenera Extracts in Dextran Sodium Sulfate-Induced Colitis in Mice. Antioxidants 2020, 9, 988. [Google Scholar] [CrossRef]
- Assis, F.; Silva, N.; Moraes, W.; Barata, L.; Minervino, A. Chemical Composition and In Vitro Antiplasmodial Activity of the Ethanolic Extract of Cyperus articulatus var. nodosus Residue. Pathogens 2020, 9, 889. [Google Scholar] [CrossRef]
- Américo, V.L.D.S.; Nunes, K.M.; De Assis, F.F.V.; Dias, S.R.; Passos, C.T.S.; Morini, A.C.; De Araújo, J.A.; Castro, K.C.F.; Castro, K.C.F.; Da Silva, S.K.R.; et al. Efficacy of Phytopharmaceuticals From the Amazonian Plant Libidibia ferrea for Wound Healing in Dogs. Front. Veter.-Sci. 2020, 7, 244. [Google Scholar] [CrossRef]
- Riedel, R.; Marrassini, C.; Anesini, C.; Gorzalczany, S. Anti-Inflammatory and Antinociceptive Activity of Urera aurantiaca. Phytotherapy Res. 2014, 29, 59–66. [Google Scholar] [CrossRef]
- Vargas, F.D.S.; De Almeida, P.D.O.; Aranha, E.; Boleti, A.P.D.A.; Newton, P.; De Vasconcellos, M.C.; Junior, V.F.V.; Lima, E.S. Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins. Molecules 2015, 20, 6194–6210. [Google Scholar] [CrossRef] [Green Version]
- Veiga Junior, V.F.; Pinto, A.C. O gênero copaifera L. Quím. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Calderon, L.D.A.; Zuliani, J.P.; Da Silva, L.H.P.; Ii, I.; Silva-Jardim, I.; Silva, A.; Iii, I.; Ciancaglini, P.; Stábeli, R.G. Amazonian biodiversity: A view of drug development for Leishmaniasis and malaria. J. Braz. Chem. Soc. 2009, 20, 1011–1023. [Google Scholar] [CrossRef]
- De Albuquerque, K.C.O.; Da Veiga, A.D.S.S.; Silva, J.V.D.S.E.; Brigido, H.P.C.; Ferreira, E.P.D.R.; Costa, E.V.S.; Marinho, A.M.D.R.; Percário, S.; Dolabela, M.F. Brazilian Amazon Traditional Medicine and the Treatment of Difficult to Heal Leishmaniasis Wounds with Copaifera. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Veiga, V.; Rosas, E.; Carvalho, M.; Henriques, M.D.G.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Cascon, V.; Possebon, L.S.; Morimoto, M.S.S.; Cardoso, L.G.; Kaplan, M.A.C.; Gilbert, B. Topical antiinflammatory and analgesic activities of Copaifera duckei dwyer. Phytotherapy Res. 2005, 19, 946–950. [Google Scholar] [CrossRef]
- Veiga, V.F.; Zunino, L.; Patitucci, M.L.; Pinto, A.C.; Calixto, J.B. The inhibition of paw oedema formation caused by the oil of Copaifera multijuga Hayne and its fractions. J. Pharm. Pharmacol. 2006, 58, 1405–1410. [Google Scholar] [CrossRef]
- Gomes, N.M.; Rezende, C.M.; Fontes, S.P.; Matheus, M.E.; Fernandes, P.D. Antinociceptive activity of Amazonian Copaiba oils. J. Ethnopharmacol. 2007, 109, 486–492. [Google Scholar] [CrossRef]
- Kobayashi, C.; Fontanive, T.O.; Enzweiler, B.G.; De Bona, L.R.; Massoni, T.; Apel, M.A.; Henriques, A.T.; Richter, M.F.; Ardenghi, P.; Suyenaga, E.S. Pharmacological evaluation of Copaifera multijuga oil in rats. Pharm. Biol. 2010, 49, 306–313. [Google Scholar] [CrossRef]
- Lucca, L.G.; De Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga, V.F.; De Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory Effect from a Hydrogel Containing Nanoemulsified Copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech 2017, 19, 522–530. [Google Scholar] [CrossRef]
- Gomes, N.D.M.; de Rezende, C.M.; Fontes, S.P.; Matheus, M.E.; Pinto, A.D.C.; Fernandes, P.D. Characterization of the antinociceptive and anti-inflammatory activities of fractions obtained from Copaifera multijuga Hayne. J. Ethnopharmacol. 2010, 128, 177–183. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Silva, R.D.B.; Lameira, O.A.; Webber, L.P.; Couto, R.S.D.; Martins, M.D.; Lima, R.R. Copaiba oil-resin (Copaifera reticulata Ducke) modulates the inflammation in a model of injury to rats tongues. BMC Complement. Altern. Med. 2017, 17, 313. [Google Scholar] [CrossRef] [Green Version]
- Ghizoni, C.V.C.; Ames, A.P.A.; Lameira, O.A.; Amado, C.A.B.; Nakanishi, A.B.S.; Bracht, L.; Natali, M.R.M.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-Inflammatory and Antioxidant Actions of Copaiba Oil Are Related to Liver Cell Modifications in Arthritic Rats. J. Cell. Biochem. 2017, 118, 3409–3423. [Google Scholar] [CrossRef]
- Dias, D.S.; Fontes, L.B.A.; Crotti, A.; Aarestrup, B.J.V.; Aarestrup, F.M.; Filho, A.A.D.S.; Corrêa, J.O.A. Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE). Molecules 2014, 19, 12814–12826. [Google Scholar] [CrossRef] [Green Version]
- Destryana, R.A.; Young, D.G.; Woolley, C.L.; Huang, T.-C.; Wu, H.-Y.; Shih, W.-L. Antioxidant and Anti-inflammation Activities of Ocotea, Copaiba and Blue Cypress Essential Oils in Vitro and in Vivo. J. Am. Oil Chem. Soc. 2014, 91, 1531–1542. [Google Scholar] [CrossRef]
- Pieri, F.; Mussi, M.; Moreira, M. Óleo de copaíba (Copaifera sp.): Histórico, extração, aplicações industriais e propriedades medicinais. Rev. Bras. de Plantas Med. 2009, 11, 465–472. [Google Scholar] [CrossRef]
- Sachetti, C.G.; Fascineli, M.L.; Sampaio, J.A.; Lameira, O.A.; Caldas, E.D. Avaliação da toxicidade aguda e potencial neurotóxico do óleo-resina de copaíba (Copaifera reticulata Ducke, Fabaceae). Rev. Bras. Farm. 2009, 19, 937–941. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.C.; Lameira, O.A.; Zoghbi, M.G.B. Identificação da época de coleta do óleo-resina de copaíba (Copaifera spp.) no município de Moju, PA. Rev. Bras. Plantas Med. 2006, 8, 14–23. [Google Scholar]
- Organization for Economic Cooperation and Development OECD Test Guidelines for the Chemicals. Guideline 423, Acute Oral Toxicity-Acute Toxic Class Method; Organization for Economic Co-operation and Development: Paris, France, 2001. [Google Scholar]
- Koo, H.-J.; Lim, K.-H.; Jung, H.-J.; Park, E.-H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef]
- Tao, X.; Ma, L.; Mao, Y.; Lipsky, P.E. Suppression of carrageenan-induced inflammation in vivo by an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F. Inflamm. Res. 1999, 48, 139–148. [Google Scholar] [CrossRef]
- Concea Redolução Normativa No 13, de 20 de Setembro de 2013. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/31061978/do1-2013-09-26-resolucao-normativa-n-13-de-20-de-setembro-de-2013-31061974 (accessed on 19 May 2021).
- Bardaji, D.K.R.; da Silva, J.J.M.; Bianchi, T.C.; Eugênio, D.D.S.; Oliveira, P.; Leandro, L.F.; Rogez, H.; Veneziani, R.C.S.; Ambrosio, S.R.; Tavares, D.C.; et al. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 2016, 40, 18–27. [Google Scholar] [CrossRef]
- Herrero-Jáuregui, C.; Casado, M.A.; Zoghbi, M.D.G.B.; Martins-Da-Silva, R.C. Chemical Variability of Copaifera reticulataDucke Oleoresin. Chem. Biodivers. 2011, 8, 674–685. [Google Scholar] [CrossRef]
- Mattei, R.A.; Dalmarco, E.M.; Fröde, T.S. Etanercept administration prevents the inflammatory response induced by carrageenan in the murine air pouch model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 1247–1257. [Google Scholar] [CrossRef]
- Romano, M.; Faggioni, R.; Sironi, M.; Sacco, S.; Echtenacher, B.; Di Santo, E.; Salmona, M.; Ghezzi, P. Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediat. Inflamm. 1997, 6, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, E.B.; Sakai, Y.I.; De Gaspari, E. A mouse air pouch model for evaluating the immune response to Taenia crassiceps infection. Exp. Parasitol. 2014, 137, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Bastos, G.; Silveira, A.; Salgado, C.; Picanço-Diniz, D.; Nascimento, J.D. Physalis angulata extract exerts anti-inflammatory effects in rats by inhibiting different pathways. J. Ethnopharmacol. 2008, 118, 246–251. [Google Scholar] [CrossRef]
- Dusse, L.; Vieira, L.M.; Carvalho, M.D.G. Revisão sobre óxido nítrico. J. Bras. Patol. Med. Lab. 2003, 39, 343–350. [Google Scholar] [CrossRef]
- Gomes, N.D.M.; Rezende, C.D.M.; Fontes, S.P.; Hovell, A.M.C.; Landgraf, R.G.; Matheus, M.E.; Pinto, A.D.C.; Fernandes, P.D. Antineoplasic activity of Copaifera multijuga oil and fractions against ascitic and solid Ehrlich tumor. J. Ethnopharmacol. 2008, 119, 179–184. [Google Scholar] [CrossRef]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magni, P.; Ruscica, M.; Cavalchini, A.; Facino, R.M. GC–MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).