Effect of Mineral Salt Blocks Containing Sodium Bicarbonate or Selenium on Ruminal pH, Rumen Fermentation and Milk Production and Composition in Crossbred Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Welfare Statement
2.2. Animals, Diets, Experimental Design and Treatments
2.3. Data Record, Sampling Procedures and Analysis Methods
2.4. Statistical Analysis
3. Results
3.1. Feed Intake and Nutrient Digestibility
3.2. Milk Yield and Composition
3.3. Ruminal Fermentation Parameters and Blood Urea Nitrogen
4. Discussion
4.1. Feed Intake and Nutrient Digestibility
4.2. Milk Yield and Composition
4.3. Ruminal Fermentation Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kariuna, A. Minerals in Feeds, Water and Plasma of Dairy Cows in Lopburi and Saraburi Provinces. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 1994. [Google Scholar]
- Davidov, I.; Radinović, M.; Erdeljan, M.; Jurakić, Ž.; Kovačević, Z. Zinc Effect on milk somatic cell count in dairy cows. Acta Sci. Vet. 2014, 42, 1226. [Google Scholar]
- Garg, M.R.; Bhanderi, B.M.; Sherasia, P.L. Trace minerals status of feeds and fodders in Junagadh district of Gujarat. Indian J. Dairy Sci. 2002, 55, 154–158. [Google Scholar]
- Bhanderi, B.M.; Garg, M.R.; Sherasia, P.L. Assessment of minerals status of dairy animals in South-West zone of Punjab. J. Buffalo Sci. 2015, 4, 33–39. [Google Scholar] [CrossRef]
- Santra, A.; Chaturvedi, O.H.; Tripathi, M.K.; Kumar, R.; Karim, S.A. Effect of dietary sodium bicarbonate supplementation on fermentation characteristics and ciliate protozoal population in rumen of lambs. Small Ruminant Res. 2003, 47, 203–212. [Google Scholar] [CrossRef]
- Cooper, R.; Klopfenstein, T. Effect of Rumensin and Feed Intake Variation on Ruminal pH; University of Nebraska: Lincoln, NE, USA, 1996. [Google Scholar]
- Cooper, S.B.D.; Kyriazakis, I.; Nolan, J.V. Diet selection in sheep: The role of the rumen environment in the selection of a diet from two feeds that differ in their energy density. British J. Nutri. 1995, 74, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Murphy, M.R. Statistical evaluation of early- and mid-lactation dairy cow responses to dietary sodium bicarbonate addition. Anim. Feed Sci. Technol. 2005, 119, 43–54. [Google Scholar] [CrossRef]
- Raucha, R.E.; Robinson, P.H.; Erasmus, L.J. Effects of sodium bicarbonate and calcium magnesium carbonate supplementation on performance of high producing dairy cows. Anim Feed Sci. Technol. 2012, 177, 180–193. [Google Scholar] [CrossRef]
- Ichijo, T.; Nagahama, K.; Ohkubo, A.; Ikuta, K.; Okada, K.; Sato, S. Effects of a salt lick containing sodium bicarbonate on ruminal pH and volatile fatty acid concentration in cattle. J. Japan Vet. Med. Assoc. 2014, 67, 844–849. [Google Scholar] [CrossRef][Green Version]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270–281. [Google Scholar] [CrossRef]
- Iraguha, B.; Hamudikuwanda, H.; Mushonga, B.; Kandiwa, E.; Mpatswenumugabo, J.P. Comparison of cow-side diagnostic tests for subclinical mastitis of dairy cows in Musanze district, Rwanda. J. S. Afr. Vet. Assoc. 2017, 88, a1464. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Fraker, P.J. The role of metals in the production of toxic oxygen metabolites by mononuclear phagocytes. In Nutrition Modulation of the Immune Responses; Cunningham-Rundles, S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1993; p. 127. [Google Scholar]
- Craven, N.; Williams, M.R. Defenses of the bovine mammary gland against infection and prospects for their enhancement. Vet. Immunol. Immunopathol. 1985, 10, 71–127. [Google Scholar] [CrossRef]
- Cope, C.M.; Mackenzie, A.M.; Wilde, D.; Sinclair, L.A. Effects of level and form of dietary zinc on dairy cow performance and health. J. Dairy Sci. 2009, 92, 2128–2135. [Google Scholar] [CrossRef]
- Ali, M.; Mehboob, H.A.; Mirza, M.A.; Raza, H.; Osredkar, M. Effect of hydrolysable tannin supplementation on production performance of dairy crossbred cows. J. Anim. Plant. Sci. 2017, 27, 1088–1093. [Google Scholar]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Abd Ellah, M.R.; Rushdi, M.; Keiji, O.; Jun, Y. Oxidative stress and bovine liver diseases: Role of glutathione peroxidase and glucose6-phosphate dehydrogenase. Jpn. J. Vet. Res. 2007, 54, 163–173. [Google Scholar] [PubMed]
- Smith, K.L.; Harrison, J.H.; Hancock, D.D.; Todhunter, D.A.; Conrad, H.R. Effect of Vitamin E and Selenium Supplementation on Incidence of Clinical Mastitis and Duration of Clinical Symptoms. J. Dairy Sci. 1984, 67, 1293–1300. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis: Animal Feeds, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber and non-Starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Keulen, J.; Young, B.A. Evaluation of acid-Insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 1997, 44, 282–287. [Google Scholar] [CrossRef]
- Schneider, B.H.; Flatt, W.P. The Evaluation of Feed through Digestibility Experiment; The University of Georgia Press: Athens, GA, USA, 1975. [Google Scholar]
- Cai, Y. Analysis method for silage. In Field and Laboratory Methods for Grassland Science; Tosho Printing Co. Ltd.: Tokyo, Japan, 2004. [Google Scholar]
- SAS. User’s Guide: Statistic, Version 6, 12th ed.; SAS Inst. Inc.: Cary, NC, USA, 1998. [Google Scholar]
- Kearl, L.C. Nutrient Requirements of Ruminants in Developing Countries; International Feed Stuffs Institute, Utah Agriculture Experimental Station, Utah State University: Logon, UT, USA, 1982. [Google Scholar]
- Broderick, G.A.; Stevenson, M.J.; Patton, R.A. Effect of dietary protein concentration and degradability on response to rumen-protected methionine in lactating dairy cows. J. Dairy Sci. 2009, 92, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.K.A.; Shook, G.E. An optimum transformation for somatic cell concentration in milk. J. Dairy Sci. 1980, 63, 487–490. [Google Scholar] [CrossRef]
- Wachirapakorn, C.; Parmaluk, P.; Wanapat, M.; Pakdee, P.; Cherdthong, A. Effects of levels of crude protein and ground corn cobs in total mixed ration on intake, rumen fermentation and milk production in crossbred Holstein Friesian lactating dairy cows. J. Appl. Anim. Res. 2014, 42, 263–268. [Google Scholar] [CrossRef]
- Wittayakun, S.; Innaree, S.; Chainetr, W.; Innaree, W. Influence of dietary fiber and sodium bicarbonate on digestibility, rumen fermentation, blood metabolites and performance of dairy cows fed pineapple peel-concentrate mixed diets. Thammasat Int. J. Sci. Technol. 2015, 20, 8–18. [Google Scholar]
- Zhaoa, X.G.; Wanga, M.; Tan, Z.L.; Tang, S.X.; Sun, Z.H.; Zhou, C.S.; Han, X.F. Effects of rice straw particle size on chewing activity, feed intake, rumen fermentation and digestion in goats. Asian-Aust. J. Anim. Sci. 2009, 22, 1256–1266. [Google Scholar] [CrossRef]
- Chládek, G.; Zapletal, D. A free-choice intake of mineral blocks in beef cows during the grazing season and in winter. Livest. Sci. 2007, 106, 41–46. [Google Scholar] [CrossRef]
- Thiangtum, W.; Yawongsa, A.; Schonewille, J.T.; Rukkwamsuk, T.; Yuangklang, C.; Wa Verstegen, M.; Hendriks, W.H. An attempt to define the sodium requirement of lactating dairy cows in a tropical environment. J. Sci. Food Agric. 2011, 91, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Pechová, A.; Pavlata, I.; Lokajová, E. Zinc supplementation and somatic cell count in milk of dairy cows. Acta Vet. Brno. 2006, 75, 355–361. [Google Scholar] [CrossRef][Green Version]
- Bakhshizadeh, S.; Mirzaei Aghjehgheshlagh, F.; Taghizadeh, A.; Seifdavati, J.; Navidshad, B. Effect of zinc sources on milk yield, milk composition and plasma concentration of metabolites in dairy cows. S. Afr. J. Anim. Sci. 2019, 49, 884–891. [Google Scholar] [CrossRef]
- Dibley, M.J. Zinc in Present Knowledge in Nutrition, 8th ed.; Bowman, B.A., Russell, R.M., Eds.; Int. Life Sei. Inst. Press: Washington, DC, USA, 2001; p. 329. [Google Scholar]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CABI Publishing: Wallingford, UK, 2010. [Google Scholar]
- Machado, V.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.S.; Kacar, C.; Foditsch, C.; Felippe, M.J.; Gilbert, R.O.; Bicalho, R.C. The effect of injectable trace minerals (selenium, copper, zinc, and manganese) on peripheral blood leukocyte activity and serum superoxide dismutase activity of lactating Holstein cows. Vet. J. 2014, 200, 299–304. [Google Scholar] [CrossRef]
- Warken, A.C.; Lopes, L.S.; Bottari, N.B.; Glombowsky, P.; Galli, G.M.; Morsch, V.M.; Schetinger, M.R.S.; Da Silva, A.S. Mineral supplementation stimulates the immune system and antioxidant responses of dairy cows and reduces somatic cell counts in milk. An. Acad. Bras. Cienc. 2018, 90, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Colakoglu, H.E.; Kuplulu, O.; Vural, M.R.; Kuplulu, S.; Yazlik, M.O.; Polat, I.M.; Oz, B.; Kaya, U.; Bayramoglu, R. Evaluation of the relationship between milk glutathione peroxidase activity, milk composition and various parameters of subclinical mastitis under seasonal variations. Vet. Arhiv. 2017, 87, 557–570. [Google Scholar] [CrossRef]
- Gong, J.; Xiao, M. Effect of organic selenium supplementation on selenium status, oxidative stress, and antioxidant status in selenium-adequate dairy cows during the periparturient period. Biol. Trace Elem. Res. 2018, 186, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Beauchemin, K.A. Altering physically effective fiber intake through forage proportion and particle length: Chewing and ruminal pH. J. Dairy Sci. 2007, 90, 2826–2838. [Google Scholar] [CrossRef]
- Wanapat, M.; Gunun, P.; Anantasook, N.; Kang, S. Changes of ruminal pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. J. Agric. Sci. 2014, 152, 675–685. [Google Scholar] [CrossRef]
- Abe, T. Mineral-salt block-efficient and unique supplemental method of trace mineral for cattle in Japan. J. Integr. Field. Sci. 2004, 1, 65–66. [Google Scholar]
- Krause, K.M.; Dhuyvetter, D.V.; Oetzel, G.R. Effect of a low-Moisture buffer block on ruminal pH in lactating dairy cattle induced with subacute ruminal acidosis. J. Dairy Sci. 2009, 92, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Cottee, G.; Kyriazakis, I.; Widowski, T.M.; Lindinger, M.I.; Cant, J.P.; Duffield, T.F.; Osborne, V.R.; McBride, B.W. The effects of subacute ruminal acidosis on sodium bicarbonate-supplemented water intake for lactating dairy cows. J. Dairy Sci. 2004, 87, 2248–2253. [Google Scholar] [CrossRef]
- Mao, S.; Huo, W.; Liu, J.; Zhang, R.; Zhu, W. In vitro effects of sodium bicarbonate buffer on rumen fermentation, levels of lipopolysaccharide and biogenic amine, and composition of rumen microbiota. J. Sci. Food Agric. 2017, 97, 1276–1285. [Google Scholar] [CrossRef]
- Ramos, S.C.; Jeong, C.-D.; Mamuad, L.L.; Kim, S.-H.; Son, A.-R.; Miguel, M.A.; Islam, M.; Cho, Y.-I.; Lee, S.-S. Enhanced ruminal fermentation parameters and altered rumen bacterial community composition by formulated rumen buffer agents fed to dairy cows with a high-concentrate diet. Agriculture 2021, 11, 554. [Google Scholar] [CrossRef]
| Composition | Unit | Mineral Salt Block (per 1 kg) | ||
|---|---|---|---|---|
| MSB-Se | MSB-Na | MSB-Com | ||
| Copper, Cu | mg | 150 | 150 | 450 |
| Cobalt, Co | mg | 25 | 25 | 60 |
| Ferrous, Fe | mg | - | - | 2100 |
| Iodine, I | mg | 50 | - | 150 |
| Manganese, Mn | mg | 500 | 630 | 420 |
| Selenium, Se | mg | 15 | - | 10 |
| Zinc, Zn | mg | 500 | 620 | 280 |
| Phosphorus, P | g | - | - | 100 |
| Sodium, Na | g | 382 | - | 210 |
| Calcium, Ca | g | - | - | 80 |
| Magnesium, Mg | g | - | 10 | 2.5 |
| Salt | g | - | 390 | - |
| Molasses | g | 18 | - | |
| Sodium bicarbonate, NaHCO3 | g | - | 500 | - |
| Item | Concentrate | Rice Straw |
|---|---|---|
| Concentrate ingredients | ||
| Cassava chip | 47.0 | |
| Corn meal | 7.0 | |
| Soybean meal | 20.0 | |
| Fined rice bran | 5.0 | |
| Palm kernel meal | 9.5 | |
| Bean pods meal | 4.0 | |
| Sugar | 3.0 | |
| Urea | 2.5 | |
| Salt | 0.5 | |
| Dicalcium phosphate | 1.0 | |
| Premix † | 0.5 | |
| Total | 100.0 | |
| Chemical composition, %DM | ||
| DM | 90.08 | 91.97 |
| Crude protein | 17.62 | 2.84 |
| Ether extract | 3.48 | 1.77 |
| NDF | 16.11 | 90.52 |
| ADF | 10.24 | 49.63 |
| Ash | 4.42 | 14.05 |
| ME, Mcal/kg DM | 2.79 | 1.54 |
| Item | Mineral Salt Block | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | MSB-Se | MSB-Na | MSB-Com | C vs. MSB | T | ||
| Average BW, kg | 464.94 | 467.46 | 465.61 | 469.21 | 3.762 | 0.72 | 0.87 |
| Intake, kg/d | |||||||
| Concentrate | 9.41 | 9.03 | 9.30 | 9.11 | 0.697 | 0.76 | 0.98 |
| Rice straw | 5.25 | 5.72 | 5.46 | 5.27 | 0.150 | 0.21 | 0.19 |
| Total | 14.66 | 14.75 | 14.76 | 14.38 | 0.780 | 0.98 | 0.98 |
| %BW | 3.15 | 3.06 | 3.18 | 3.15 | 0.166 | 0.95 | 0.97 |
| g/kgBW0.75 | 146.23 | 146.72 | 147.39 | 142.61 | 7.712 | 0.96 | 0.97 |
| R:C ratio | 35.96 | 39.02 | 37.16 | 36.85 | 1.471 | 0.45 | 0.55 |
| Mineral block lick, g/d | 0.00 a | 21.43 b | 14.29 bc | 11.90 c | 1.943 | ** | ** |
| Nutrient intake, kg/d | |||||||
| OM | 13.50 | 13.56 | 13.59 | 13.24 | 0.737 | 0.97 | 0.98 |
| CP | 1.81 | 1.75 | 1.79 | 1.76 | 0.126 | 0.79 | 0.98 |
| EE | 0.42 | 0.41 | 0.42 | 0.41 | 0.026 | 0.85 | 0.94 |
| NDF | 6.27 | 6.64 | 6.44 | 6.26 | 0.214 | 0.51 | 0.59 |
| ADF | 3.58 | 3.76 | 3.66 | 3.55 | 0.122 | 0.59 | 0.65 |
| Nutrient digestibility, % | |||||||
| DM | 60.58 | 58.56 | 60.42 | 59.57 | 1.336 | 0.39 | 0.63 |
| OM | 64.00 | 61.34 | 63.25 | 62.51 | 1.411 | 0.35 | 0.61 |
| CP | 71.78 | 67.50 | 70.00 | 69.22 | 1.311 | 0.09 | 0.32 |
| EE | 86.57 | 83.89 | 84.28 | 83.48 | 1.150 | 0.11 | 0.24 |
| NDF | 47.22 | 49.23 | 48.56 | 49.00 | 0.897 | 0.15 | 0.45 |
| ADF | 43.57 | 43.39 | 41.40 | 43.82 | 2.965 | 0.84 | 0.93 |
| Energy intake | |||||||
| Mcal ME/d | 32.93 | 31.64 | 32.61 | 31.69 | 2.388 | 0.74 | 0.97 |
| Microbial crude protein | |||||||
| kg/d | 1.25 | 1.08 | 1.12 | 1.09 | 0.081 | 0.75 | 0.97 |
| Item | Mineral Salt Block | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | MSB-Se | MSB-Na | MSB-Com | C vs. MSB | T | ||
| Milk production | |||||||
| Milk yield, kg/d | 12.95 | 13.06 | 12.46 | 12.21 | 0.292 | 0.31 | 0.23 |
| 4%FCM | 12.72 | 13.49 | 12.47 | 12.56 | 0.619 | 0.87 | 0.66 |
| ECM †, kg | 12.97 | 13.48 | 12.70 | 12.64 | 0.537 | 0.96 | 0.69 |
| Milk composition | |||||||
| Fat, % | 3.84 | 4.19 | 4.04 | 4.15 | 0.236 | 0.34 | 0.74 |
| Protein, % | 3.48 | 3.40 | 3.56 | 3.40 | 0.104 | 0.81 | 0.66 |
| Lactose, % | 5.14 | 5.09 | 5.12 | 5.11 | 0.024 | 0.28 | 0.60 |
| Solid-not-fat, % | 9.32 | 9.19 | 9.37 | 9.21 | 0.118 | 0.68 | 0.66 |
| Total solids, % | 13.16 | 13.37 | 13.41 | 13.36 | 0.278 | 0.51 | 0.91 |
| Fat/protein ratio | 1.11 | 1.22 | 1.14 | 1.23 | 0.073 | 0.32 | 0.58 |
| Milk efficiency, kg/kg DM | 0.87 | 0.89 | 0.84 | 0.86 | 0.051 | 0.88 | 0.87 |
| 4%FCM/kg DM | 0.86 | 0.93 | 0.84 | 0.89 | 0.071 | 0.77 | 0.83 |
| NUE ‡ | 24.05 | 24.86 | 23.76 | 23.74 | 2.195 | 0.98 | 0.98 |
| SCC, ×103 cell/ml | 319.50 | 132.25 | 114.75 | 139.00 | 48.82 | * | 0.07 |
| SCS § | 4.54 a | 3.24 b | 3.09 b | 3.18 b | 0.265 | ** | 0.02 |
| Item | Mineral Salt Block | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | MSB-Se | MSB-Na | MSB-Com | C vs. MSB | T | ||
| Rumen end-products | |||||||
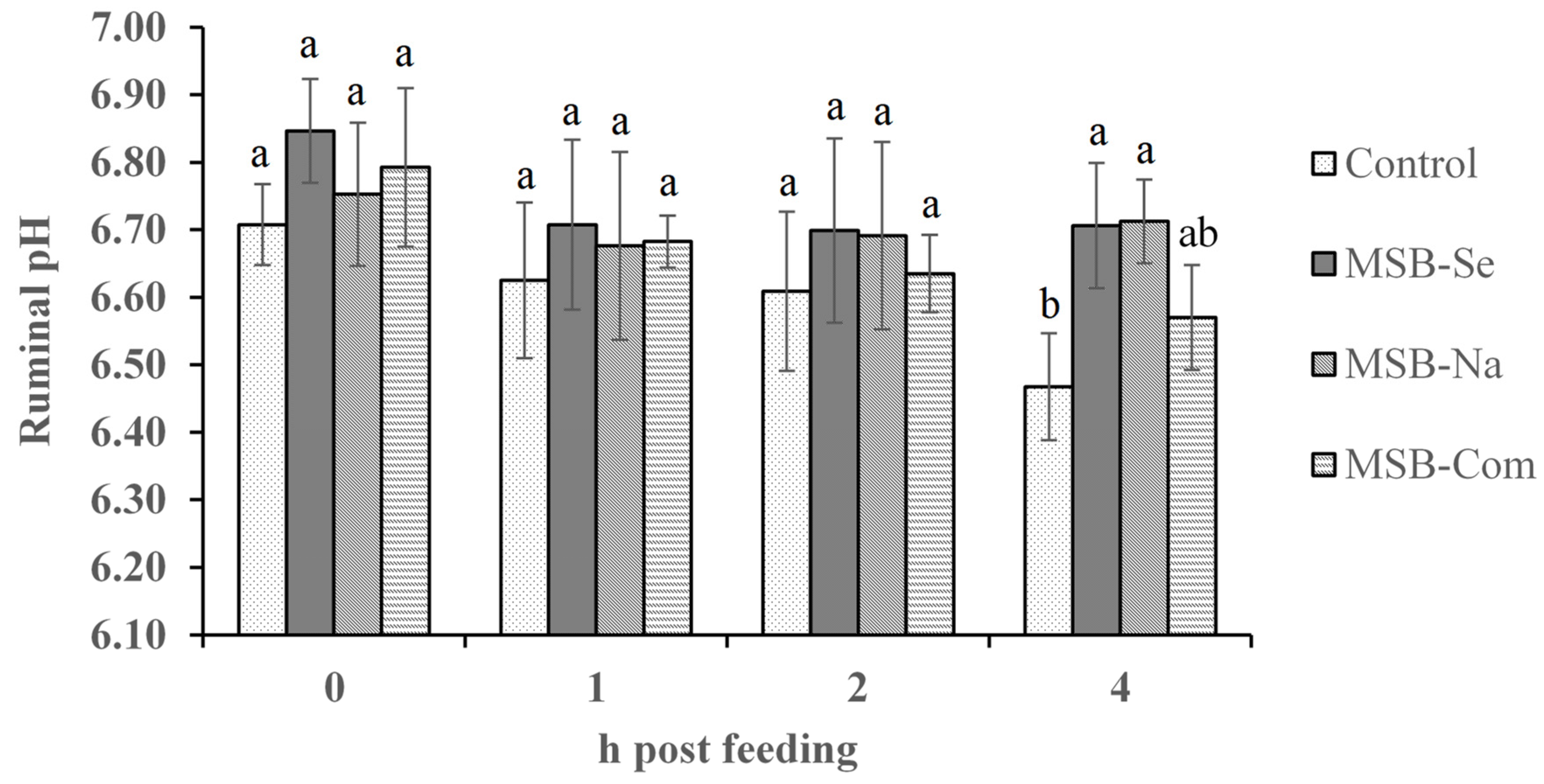
| pH | 6.60 | 6.74 | 6.71 | 6.67 | 0.048 | 0.11 | 0.30 |
| NH3-N, mg/dL | 17.20 | 19.50 | 18.16 | 18.81 | 0.689 | 0.09 | 0.21 |
| TVFA, mM | 97.44 | 99.34 | 97.58 | 98.40 | 0.831 | 0.34 | 0.42 |
| C2, % | 67.80 | 67.66 | 67.79 | 67.51 | 0.030 | 0.69 | 0.87 |
| C3, % | 21.13 | 21.18 | 21.06 | 21.31 | 0.274 | 0.87 | 0.93 |
| C4, % | 11.08 | 11.16 | 11.15 | 11.18 | 0.090 | 0.43 | 0.86 |
| C2/C3 | 3.39 | 3.40 | 3.38 | 3.31 | 0.062 | 0.70 | 0.73 |
| Blood metabolites | |||||||
| BUN, mg/dL | 19.56 | 18.75 | 19.69 | 19.81 | 1.045 | 0.91 | 0.89 |
| Glucose, mg/dL | 58.25 | 55.63 | 55.88 | 57.69 | 1.371 | 0.29 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insoongnern, H.; Srakaew, W.; Prapaiwong, T.; Suphrap, N.; Potirahong, S.; Wachirapakorn, C. Effect of Mineral Salt Blocks Containing Sodium Bicarbonate or Selenium on Ruminal pH, Rumen Fermentation and Milk Production and Composition in Crossbred Dairy Cows. Vet. Sci. 2021, 8, 322. https://doi.org/10.3390/vetsci8120322
Insoongnern H, Srakaew W, Prapaiwong T, Suphrap N, Potirahong S, Wachirapakorn C. Effect of Mineral Salt Blocks Containing Sodium Bicarbonate or Selenium on Ruminal pH, Rumen Fermentation and Milk Production and Composition in Crossbred Dairy Cows. Veterinary Sciences. 2021; 8(12):322. https://doi.org/10.3390/vetsci8120322
Chicago/Turabian StyleInsoongnern, Hathaichanok, Wuttikorn Srakaew, Tipwadee Prapaiwong, Napongphot Suphrap, Saisamorn Potirahong, and Chalong Wachirapakorn. 2021. "Effect of Mineral Salt Blocks Containing Sodium Bicarbonate or Selenium on Ruminal pH, Rumen Fermentation and Milk Production and Composition in Crossbred Dairy Cows" Veterinary Sciences 8, no. 12: 322. https://doi.org/10.3390/vetsci8120322
APA StyleInsoongnern, H., Srakaew, W., Prapaiwong, T., Suphrap, N., Potirahong, S., & Wachirapakorn, C. (2021). Effect of Mineral Salt Blocks Containing Sodium Bicarbonate or Selenium on Ruminal pH, Rumen Fermentation and Milk Production and Composition in Crossbred Dairy Cows. Veterinary Sciences, 8(12), 322. https://doi.org/10.3390/vetsci8120322







