Milk Somatic Cell Count and Polymorphonuclear Cells in Healthy Quarters of Cows That Underwent Blanket and Selective Dry Therapy: An Italian Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Groups and Inclusion Criteria
- Quarter milk collection and analysis 24 h before drying off;
- Administration of the assigned DCT to each cow;
- Quarter milk collection and analysis after the dry period.
2.2. Statistical Analysis
- SCS = 3 + log2(SCC/100);
- NS = log2(N);
- MS = log2(M);
- LS = log2(L);
- PMNS = 3 + log2(PMN/100).
3. Results
3.1. Data Overview
3.2. Analysis of Variance
4. Discussion
4.1. Descriptive Statistics and Correlations
4.2. Fixed Effects
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, P.; Barkema, H.W.; Brito, L.F.; Narayana, S.G.; Miglior, F. Symposium review: Novel strategies to genetically improve mastitis resistance in dairy cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Barkema, H.W.; Jacques, M.; Lavallée-Bourget, E.-M.; Malouin, F.; Saini, V.; Stryhn, H.; Dufour, S. Invited review: Incidence, risk factors, and effects of clinical mastitis recurrence in dairy cows. J. Dairy Sci. 2018, 101, 4729–4746. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Egger-Danner, C.; Mészáros, G.; Fuerst, C.; Penasa, M.; Sölkner, J.; Fuerst-Waltl, B. Genetic associations of lactose and its ratios to other milk solids with health traits in Austrian Fleckvieh cows. J. Dairy Sci. 2019, 102, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; van Hoeij, R.J.; Kemp, B.; van Knegsel, A.T.M. Evaluation of customized dry-period strategies in dairy cows. J. Dairy Sci. 2021, 104, 1887–1899. [Google Scholar] [CrossRef]
- Winder, C.B.; Sargeant, J.M.; Kelton, D.F.; Leblanc, S.J.; Duffield, T.F.; Glanville, J.; Wood, H.; Churchill, K.J.; Dunn, J.; Bergevin, M.D.; et al. Comparative efficacy of blanket versus selective dry-cow therapy: A systematic review and pairwise meta-analysis. Anim. Health Res. Rev. 2019, 20, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Niemi, R.E.; Hovinen, M.; Vilar, M.J.; Simojoki, H.; Rajala-Schultz, P.J. Dry cow therapy and early lactation udder health problems—Associations and risk factors. Prev. Vet. Med. 2021, 188, 105268. [Google Scholar] [CrossRef]
- Rowe, S.M.; Godden, S.M.; Nydam, D.V.; Gorden, P.J.; Lago, A.; Vasquez, A.K.; Royster, E.; Timmerman, J.; Thomas, M.J. Randomized controlled trial investigating the effect of 2 selective dry-cow therapy protocols on udder health and performance in the subsequent lactation. J. Dairy Sci. 2020, 103, 6493–6503. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; van den Heuvel, K.W.H.; Lam, T.J.G.M.; Scherpenzeel, C.G.M.; van Schaik, G. Monitoring udder health on routinely collected census data: Evaluating the short- to mid-term consequences of implementing selective dry cow treatment. J. Dairy Sci. 2021, 104, 2280–2289. [Google Scholar] [CrossRef]
- Lozada-Soto, E.; Maltecca, C.; Anderson, K.; Tiezzi, F. Analysis of Milk leukocyte differential measures for use in management practices for decreased mastitis incidence. J. Dairy Sci. 2020, 103, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, D.; Lipkens, Z.; Piepers, S.; De Vliegher, S. Investigation of differential somatic cell count as a potential new supplementary indicator to somatic cell count for identification of intramammary infection in dairy cows at the end of the lactation period. Prev Vet. Med. 2019, 172, 104803. [Google Scholar] [CrossRef]
- Zecconi, A.; Meroni, G.; Sora, V.; Mattina, R.; Cipolla, M.; Zanini, L. Total and differential cell counts as a tool to identify intramammary infections in cows after calving. Animals 2021, 11, 727. [Google Scholar] [CrossRef]
- Zecconi, A.; Vairani, D.; Cipolla, M.; Rizzi, N.; Zanini, L. Assessment of Subclinical Mastitis Diagnostic Accuracy by Differential Cell Count in Individual Cow Milk. Ital. J. Anim. Sci. 2018, 18, 460–465. [Google Scholar] [CrossRef]
- Sargeant, J.M.; O’Connor, A.M.; Gardner, I.A.; Dickson, J.S.; Torrence, M.E.; Dohoo, I.R.; Lefebvre, S.L.; Morley, P.S.; Ramirez, A.; Snedeker, K. The REFLECT statement: Reporting Guidelines for Randomized Controlled Trials in Livestock and Food Safety: Explanation and elaboration. J. Food Prot. 2010, 73, 579–603. [Google Scholar] [CrossRef]
- Costa, A.; Goi, A.; Penasa, M.; Nardino, G.; Posenato, L.; De Marchi, M. Variation of immunoglobulins G, A, and M and bovine serum albumin concentration in Holstein cow colostrum. Animal 2021, 15, 100299. [Google Scholar] [CrossRef]
- International Committee for Animal Recording (ICAR). Section 2—Guidelines for Dairy Cattle Milk Recording. Available online: https://www.icar.org/Guidelines/02-Overview-Cattle-Milk-Recording.pdf (accessed on 20 August 2021).
- Leitner, G.; Eligulashvily, R.; Krifucks, O.; Perl, S.; Saran, A. Immune cell differentiation in mammary gland tissues and milk of cows chronically infected with Staphylococcus aureus. J. Vet. Med. Ser. B 2003, 50, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Godden, S.M.; Royster, E.; Timmerman, J.; Rapnicki, P.; Green, H. Evaluation of an Automated Milk Leukocyte Differential Test and the California Mastitis Test for Detecting Intramammary Infection in Early- and Late-Lactation Quarters and Cows. J. Dairy Sci. 2017, 100, 6527–6544. [Google Scholar] [CrossRef]
- National Mastitis Council. Laboratory and Field Handbook on Bovine Mastitis; Natl. Mastitis Counc., Inc.: Madison, MA, USA, 1999. [Google Scholar]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of somatic cell count and mastitis: An overview. Asian-Australas. J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential somatic cell count—A Novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef] [Green Version]
- Kirsanova, E.; Heringstad, B.; Lewandowska-Sabat, A.; Olsaker, I. Alternative subclinical mastitis traits for genetic evaluation in dairy cattle. J. Dairy Sci. 2019, 102, 5323–5329. [Google Scholar] [CrossRef] [Green Version]
- De Haas, Y.; Barkema, H.W.; Veerkamp, R.F. The effect of pathogen-specific clinical mastitis on the lactation curve for somatic cell count. J. Dairy Sci. 2002, 85, 1314–1323. [Google Scholar] [CrossRef]
- Kirkeby, C.; Toft, N.; Schwarz, D.; Farre, M.; Nielsen, S.S.; Zervens, L.; Hechinger, S.; Halasa, T. Differential somatic cell count as an additional indicator for intramammary infections in dairy cows. J. Dairy Sci. 2020, 103, 1759–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimie, E.; Ebrahimi, F.; Ebrahimi, M.; Tomlinson, S.; Petrovski, K.R. A large-scale study of indicators of sub-clinical mastitis in dairy cattle by attribute weighting analysis of milk composition features: Highlighting the predictive power of lactose and electrical conductivity. J. Dairy Res. 2018, 85, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bovenhuis, H.; Penasa, M. Changes in milk lactose content as indicators for longevity and udder health in Holstein cows. J. Dairy Sci. 2020, 103, 11574–11584. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Kirkeby, C. Differential somatic cell count: Value for udder health management. Front. Vet. Sci. 2020, 7, 1153. [Google Scholar] [CrossRef]
- Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Boichard, D.; Rupp, R. Invited review: Low milk somatic cell count and susceptibility to mastitis. J. Dairy Sci. 2018, 101, 6703–6714. [Google Scholar] [CrossRef] [Green Version]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. Food Anim. 2012, 28, 165–185. [Google Scholar] [CrossRef]
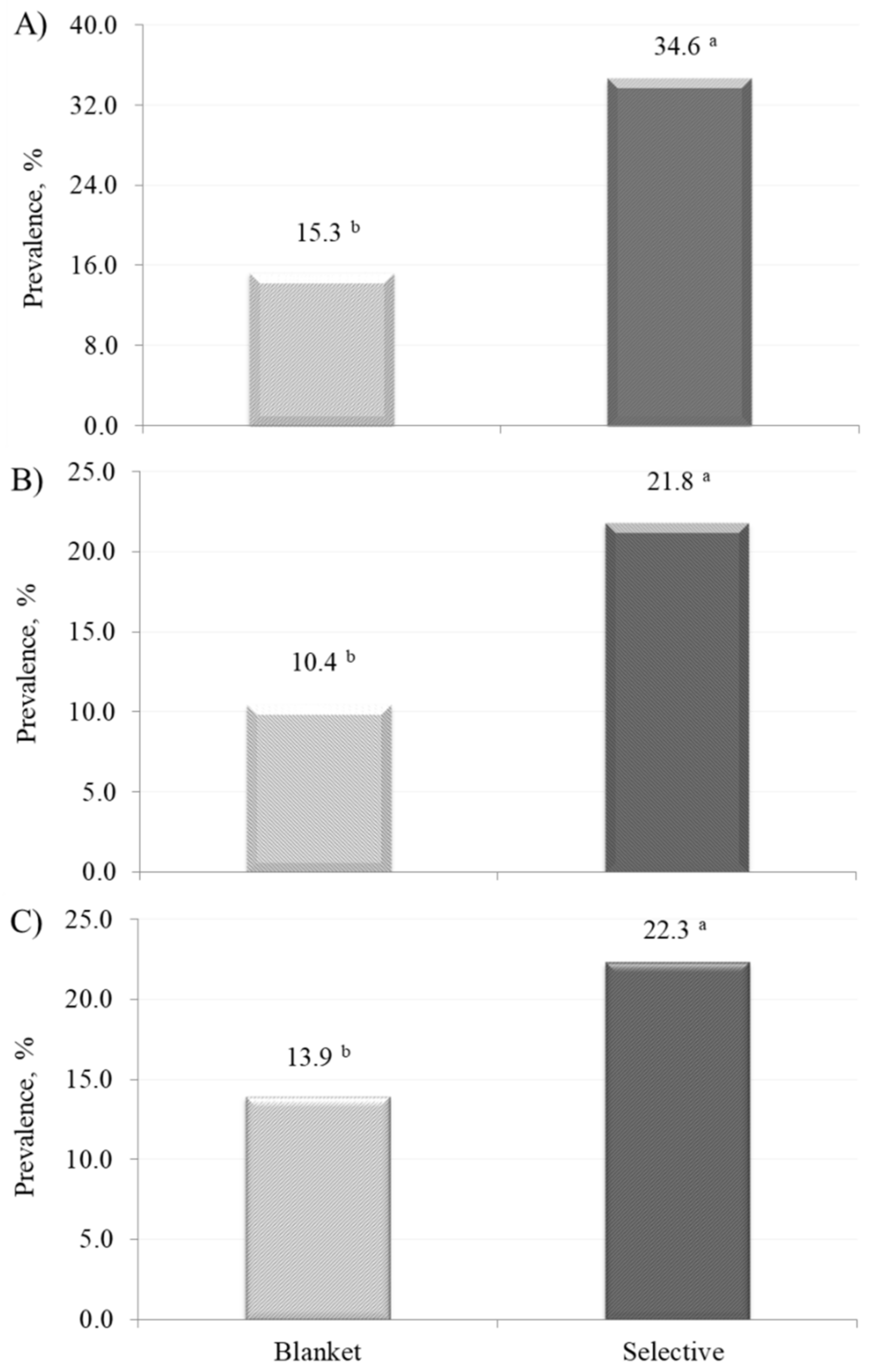
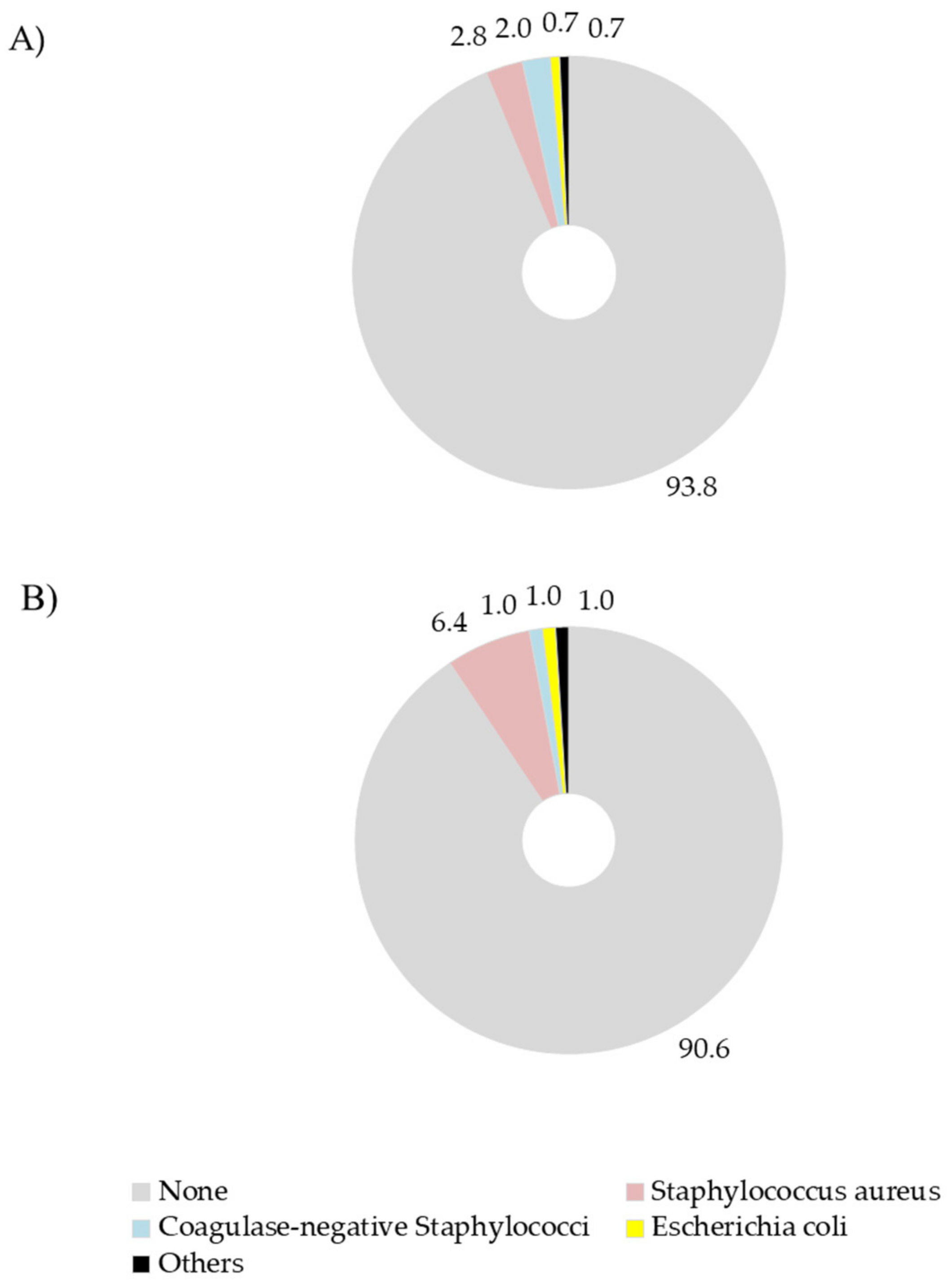
| Item | BDCT | SDCT |
|---|---|---|
| Number (n) | ||
| Overall cows | 98 | 102 |
| Cows after restrictions | 41 | 58 |
| Quarters after restrictions | 144 | 202 |
| Quarters/cow | 3.51 | 3.48 |
| Frequency (%) | ||
| Parity 1 | 77.6 | 75.6 |
| Parity 2 | 15.5 | 17.1 |
| Later parities (3 + 4) | 6.9 | 7.3 |
| Dry period length (d) | ||
| Average | 65.95 | 63.90 |
| Standard deviation | 6.13 | 9.14 |
| Trait | Before Drying Off | After Drying Off | ||
|---|---|---|---|---|
| BDCT | SDCT | BDCT | SDCT | |
| SCS | 1.71 (1.08) | 1.97 (1.23) | 1.82 (1.79) | 2.57 (1.93) |
| NS | 4.41 (0.96) | 4.58 (1.12) | 4.75 (1.81) | 5.33 (2.09) |
| MS | 3.58 (1.04) | 3.95 (1.36) | 3.84 (1.51) | 4.46 (1.71) |
| LS | 3.81 (0.98) | 4.02 (1.06) | 3.95 (1.55) | 4.39 (1.52) |
| PMNS | 1.37 (1.03) | 1.52 (1.18) | 1.52 (1.79) | 2.14 (2.01) |
| DSCC | 0.78 (0.19) | 0.74 (0.20) | 0.76 (0.23) | 0.75 (0.21) |
| Trait | BDCT | SDCT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCS | NS | MS | LS | PMNS | SCS | NS | MS | LS | PMNS | |
| NS | 0.95 | 0.96 | ||||||||
| MS | 0.83 | 0.72 | 0.81 | 0.72 | ||||||
| LS | 0.89 | 0.81 | 0.65 | 0.85 | 0.80 | 0.65 | ||||
| PMNS | 0.97 | 0.97 | 0.71 | 0.91 | 0.97 | 0.97 | 0.70 | 0.88 | ||
| DSCC | 0.10 ns | 0.17 | −0.18 | 0.21 | 0.23 | 0.14 ns | 0.20 ns | −0.04 ns | 0.19 | 0.19 |
| Trait 1 | p-Value | BDCT | SDCT | ||
|---|---|---|---|---|---|
| SCS | 0.004 | 1.85 b | (0.23) | 2.68 a | (0.18) |
| NS | 0.012 | 4.73 b | (0.25) | 5.48 a | (0.19) |
| MS | 0.048 | 3.91 b | (0.23) | 4.47 a | (0.17) |
| LS | 0.040 | 3.95 b | (0.20) | 4.45 a | (0.15) |
| PMNS | 0.009 | 1.53 b | (0.23) | 2.25 a | (0.17) |
| DSCC | 0.785 | 0.76 a | (0.03) | 0.75 a | (0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; De Marchi, M.; Sagrafoli, D.; Lanzi, H.; Amatiste, S.; Boselli, C.; Giacinti, G. Milk Somatic Cell Count and Polymorphonuclear Cells in Healthy Quarters of Cows That Underwent Blanket and Selective Dry Therapy: An Italian Case Study. Vet. Sci. 2021, 8, 298. https://doi.org/10.3390/vetsci8120298
Costa A, De Marchi M, Sagrafoli D, Lanzi H, Amatiste S, Boselli C, Giacinti G. Milk Somatic Cell Count and Polymorphonuclear Cells in Healthy Quarters of Cows That Underwent Blanket and Selective Dry Therapy: An Italian Case Study. Veterinary Sciences. 2021; 8(12):298. https://doi.org/10.3390/vetsci8120298
Chicago/Turabian StyleCosta, Angela, Massimo De Marchi, Daniele Sagrafoli, Hillary Lanzi, Simonetta Amatiste, Carlo Boselli, and Giuseppina Giacinti. 2021. "Milk Somatic Cell Count and Polymorphonuclear Cells in Healthy Quarters of Cows That Underwent Blanket and Selective Dry Therapy: An Italian Case Study" Veterinary Sciences 8, no. 12: 298. https://doi.org/10.3390/vetsci8120298
APA StyleCosta, A., De Marchi, M., Sagrafoli, D., Lanzi, H., Amatiste, S., Boselli, C., & Giacinti, G. (2021). Milk Somatic Cell Count and Polymorphonuclear Cells in Healthy Quarters of Cows That Underwent Blanket and Selective Dry Therapy: An Italian Case Study. Veterinary Sciences, 8(12), 298. https://doi.org/10.3390/vetsci8120298






