Genetic Effect and Growth Curve Parameter Estimation under Heat Stress in Slow-Growing Thai Native Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
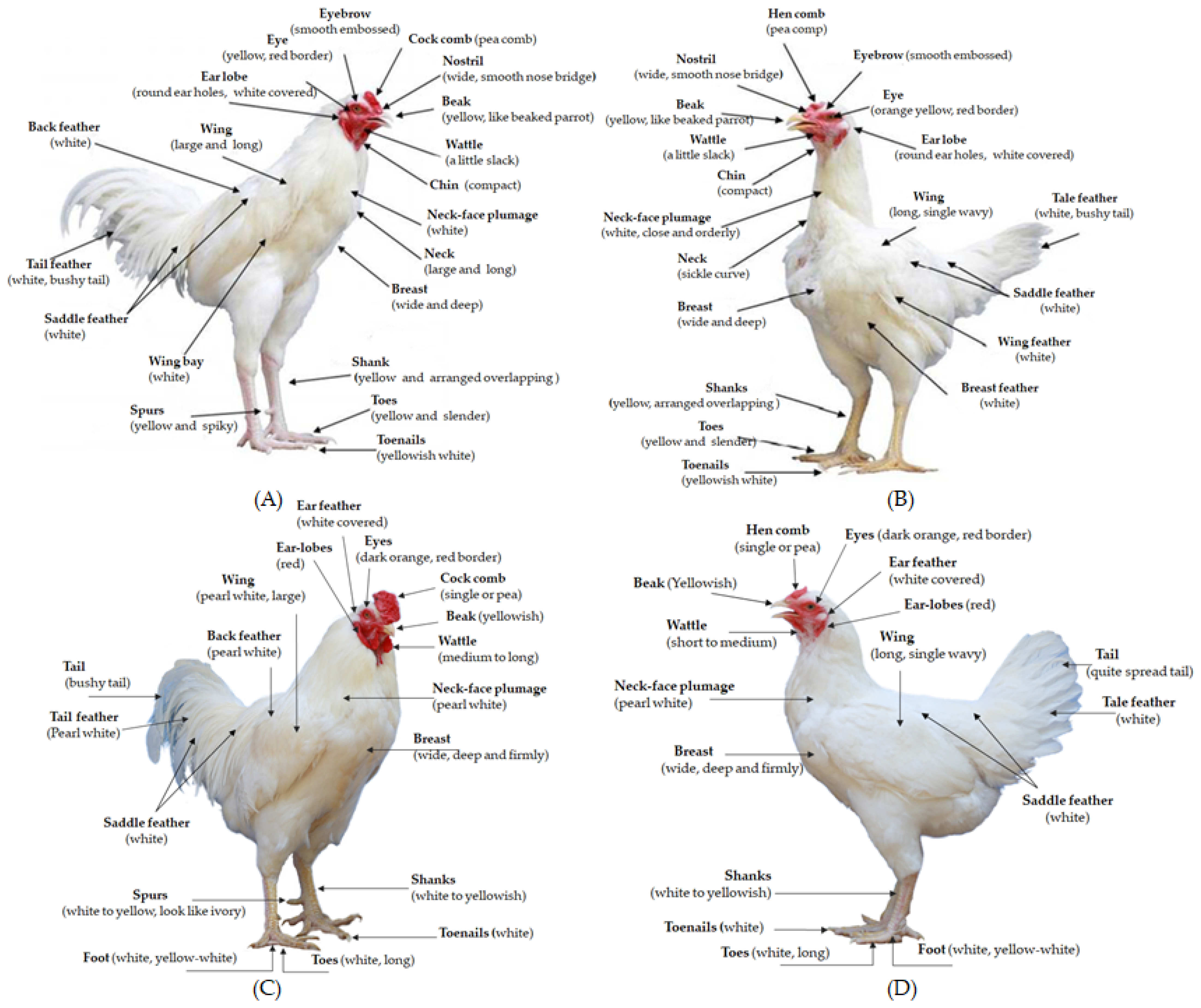
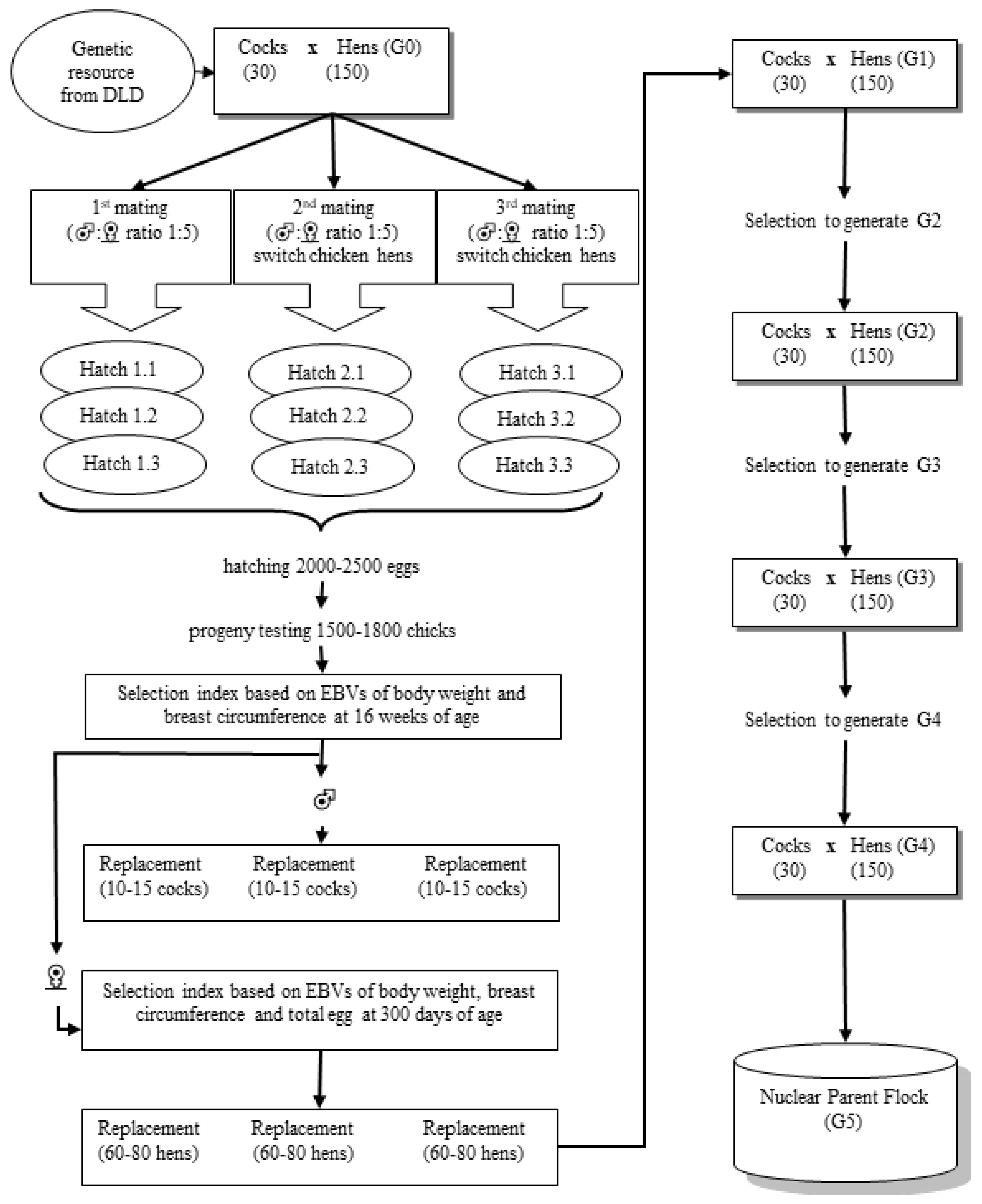
2.2. Animals and Management
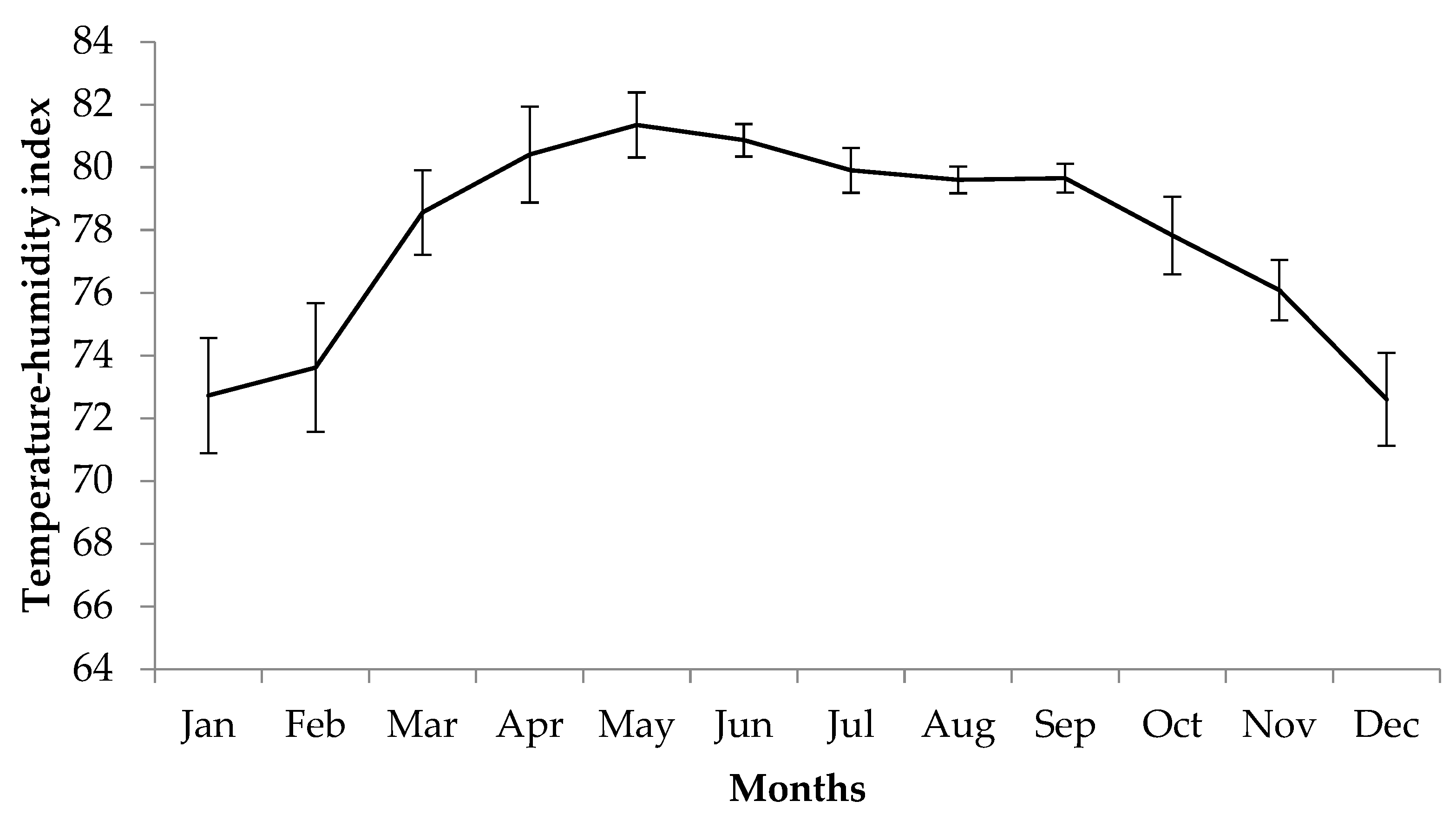
2.3. Climate Conditions
2.4. Data and Statistical Analysis
- for the von Bertalanffy function,
- for the Gompertz function, and
- for the logistic function.
2.5. Estimation of Genetic Parameters
3. Results
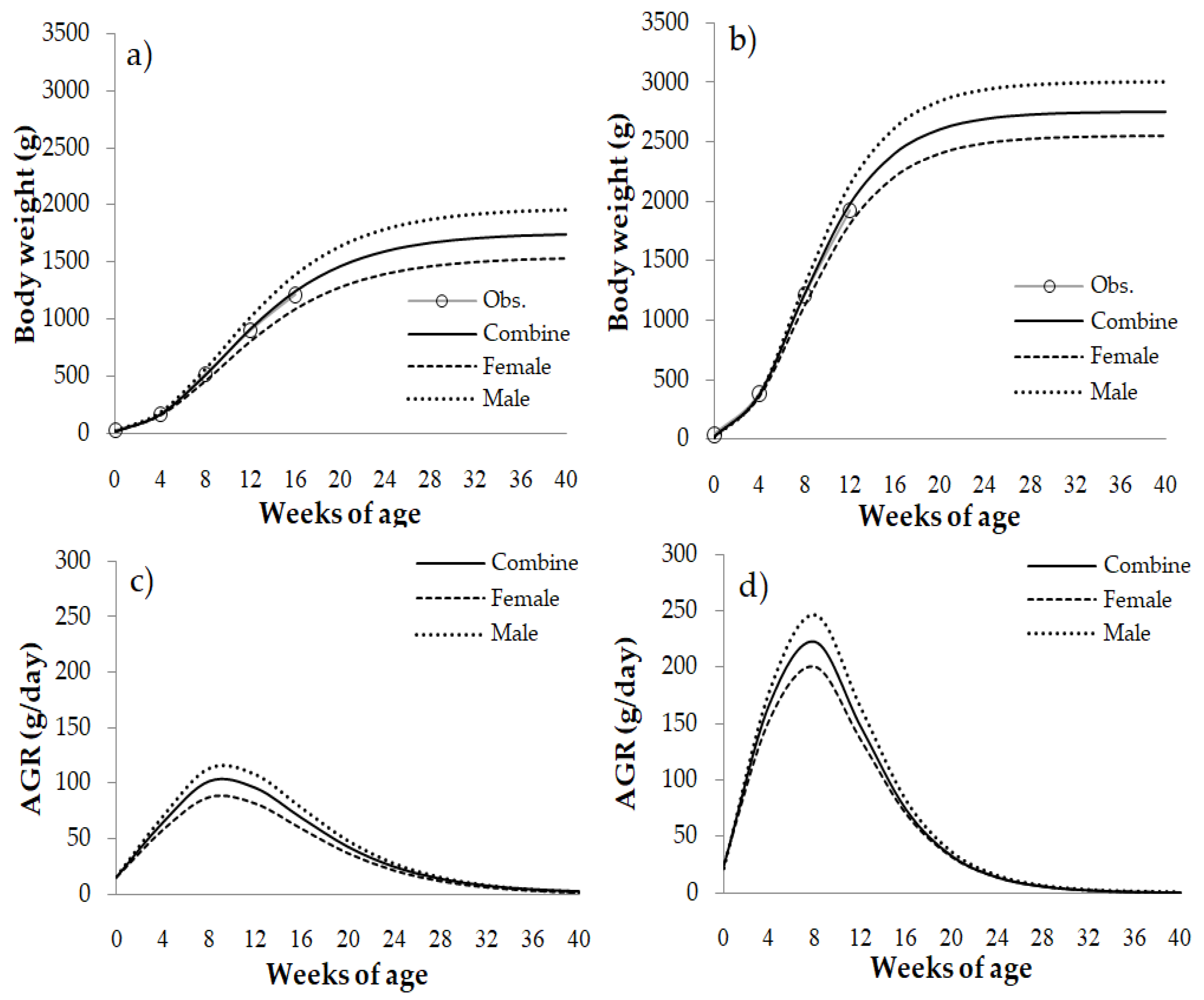
3.1. Fitting Model and Growth Curve Parameters
3.2. The Characteristics of the Growth Curve Based on the Gompertz Function
3.3. Estimated Genetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Nogales Baena, S.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference. Animals 2021, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- Padhi, M.K. Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica 2016, 2016, 2604685. [Google Scholar] [CrossRef] [PubMed]
- FAO. Domestic Animal Diversity Information System (DAD-IS): Data; FAO: Rome, Italy, 2021. [Google Scholar]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Aengwanich, W. Effects of high environmental temperature on blood indices of Thai indigenous chickens, Thai indigenous chickens crossbred and Broilers. Int. J. Poult. Sci. 2007, 6, 427–430. [Google Scholar] [CrossRef][Green Version]
- Duangjinda, M.; Tunim, S.; Duangdaen, C.; Boonkum, W. Hsp70 genotypes and heat tolerance of commercial and native chickens reared in hot and humid conditions. Braz. J. Poult. Sci. 2017, 19, 7–18. [Google Scholar] [CrossRef]
- Yahav, S. Regulation of body temperature: Strategies and mechanisms. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: New York, NY, USA, 2015; pp. 869–905. [Google Scholar]
- Suganya, T.; SenTHIkumar, S.; Deepa, K.; Amutha, R. Nutritional management to alleviate heat stress in broilers. Int. J. Sci. Environ. Technol. 2015, 4, 661–666. [Google Scholar]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A natural antioxidant for prevention of heat-induced oxidative stress in poultry. Worlds Poult. Sci. J. 2018, 74, 89–100. [Google Scholar] [CrossRef]
- Decuypere, E.; Huybrechts, L.M.; Kuhn, E.R.; Tixier-Boichard, M.; Merat, P. Physiological alterations associated with the chicken sex-linked dwarfing gene. Crit. Rev. Poult. Biol. 1991, 3, 191–221. [Google Scholar]
- Yalcin, S.; Testik, A.; Ozkan, S.; Settar, P.; Celen, F.; Cahaner, A. Performance of naked neck and normal broilers in hot, warm, and temperate climates. Poult. Sci. 1997, 76, 930–937. [Google Scholar] [CrossRef]
- Lin, H.; Jiao, H.C.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006, 62, 71–86. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Garcia, A.A.P., Jr.; Filho, R.V.F.; Souza, T.M.; de Oliveira, E.R.; de Oliveira, E.B.; do Nascimento, C.S.; Meneghetti, C.; Wenceslau, A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS ONE 2017, 12, e0186083. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I.; Hoogenboom, G. Genetic component of heat stress in dairy cattle, development of heat index function. J. Dairy Sci. 2000, 83, 2120–2125. [Google Scholar] [CrossRef]
- Abbas, A.A.; Yosif, A.A.; Shukur, A.M.; Ali, F.H. Effect of genotypes, storage periods and feed additives in broiler breeder diets on embryonic and hatching indicators and chicks blood parameters. Sci. Agri. 2014, 7, 44–48. [Google Scholar]
- Al-Samarai, F.R. Growth curve of commercial broiler as predicted by different nonlinear functions. Am. J. Appl. Sci. Res. 2015, 1, 6–9. [Google Scholar]
- Mignon-Grasteau, S.; Beaumont, C.; Le Biham–Duval, E.; Poivey, J.P.; De Rochembeau, H.; Ricard, F.H. Genetic parameters of growth curve parameters in male and female chickens. Br. Poult. Sci. 1999, 40, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gang, F.Y.; Zhen, Y.S. A study on the growth curve of and maximum prot from layer-type cockerel chicks. Br. Poult. Sci. 1997, 38, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.M. Ornithology; Beijing Normal University Press: Beijing, China, 1995; pp. 281–296. [Google Scholar]
- National Oceanic and Atmospheric Administration. Livestock Hot Weather Stress; US Government Printing Office: Washington, DC, USA, 1976.
- Wilson, B.J. Growth curves: Their analysis and use. In Growth and Poultry Meat Production; Boorman, K.N., Wilson, B.J., Eds.; British Poultry Science Ltd.: Edinburgh, UK, 1977; pp. 89–115. [Google Scholar]
- SAS User’s Guide Statistic; SAS Institute: Cary, NC, USA, 2018.
- Arando, A.; González-Ariza, A.; Lupi, T.M.; Nogales, S.; León, J.M.; NavasGonzález, F.J.; Delgado, J.V.; Camacho, M.E. Comparison of non-linear models to describe the growth in the Andalusian turkey breed. Ital. J. Anim. Sci. 2021, 20, 1156–1167. [Google Scholar] [CrossRef]
- González Ariza, A.; Baena, S.N.; Lupi, T.M.; Arbulu, A.A.; NavasGonzález, F.J.; Jurado, J.M.L.; Bermejo, J.V.D.; Vallejo, M.E.C. Characterisation of biological growth curves of different varieties of an endangered native hen breed kept under free range conditions. Ital. J. Anim. Sci. 2021, 20, 806–813. [Google Scholar] [CrossRef]
- Selvaggi, M.; Laudadio, V.; Dario, C.; Tufarelli, V. Modelling growth curves in a nondescript Italian Chicken Breed: An opportunity to improve genetic and feeding strategies. J. Poult. Sci. 2015, 52, 288–294. [Google Scholar] [CrossRef]
- Duangjinda, M.; Misztal, I.; Tsurata, S. BLUPF90-PCPAK 2.5: User’s Manual; The University of Georgia and Khon Kaen University: Athens, GA, USA, 2005. [Google Scholar]
- Ravagnolo, O.; Misztal, I. Genetic component of heat stress in dairy cattle, parameter estimation. J. Dairy Sci. 2000, 83, 2126–2130. [Google Scholar] [CrossRef]
- Saeed, M.; Abbas, G.; Alagawanyd, M.; Kambohe, A.A.; El-Hackd, M.E.A.; Khafagaf, A.F.; Chaoa, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef]
- Tirawattanawanich, C.; Chantakru, S.; Nimitsantiwong, W.; Tongyai, S. The effects of tropical environmental conditions on the stress and immune responses of commercial broilers, Thai indigenous chickens, and crossbred chickens. J. Appl. Poult. Res. 2011, 20, 409–420. [Google Scholar] [CrossRef]
- Dottavio, A.M.; Álvarez, M.; Canet, Z.E.; Font, M.T.; Di Masso, R.J. Growth pattern of experimental hybrids for free range broiler production. Rev. Arg. Prod. Anim. 2007, 27, 75–82. [Google Scholar]
- Dourado, L.R.B.; Sakomura, N.K.; Nascimento, D.C.N.; Dorigam, J.C.; Marcato, S.M.; Fernandes, J.B.K. Growth and performance of naked neck broiler reared in free–range system. Ciênc. Agrotec. 2009, 33, 875–881. [Google Scholar] [CrossRef]
- Moharrery, A.; Mirzaei, M. Growth characteristics of commercial broiler and native chickens as predicted by different growth functions. J. Anim. Feed Sci. 2014, 23, 82–89. [Google Scholar] [CrossRef][Green Version]
- Eleroğlu, H.; Yıldırım, A.; Sekeroğlu, A.; Çoksöyler, F.N.; Duman, M. Comparison of growth curves by growth models in slow–growing chicken genotypes raised the organic system. Int. J. Agric. Biol. 2014, 16, 529–535. [Google Scholar]
- Osei-Amponsah, R.; Kayang, B.B.; Naazie, A.; Barchia, I.M.; Arthur, P.F. Evaluation of models to describe temporal growth in local chickens of Ghana. Iran J. Appl. Anim. Sci. 2014, 4, 855–861. [Google Scholar]
- Yang, Y.; Mekki, D.M.; Lv, S.J.; Wang, L.Y.; Yu, J.H.; Wang, J.Y. Analysis of fitting growth models in Jinghai mixed-sex yellow chicken. Int. J. Poult. Sci. 2006, 5, 517–521. [Google Scholar]
- Tongsiri, S.; Jeyaruban, G.; Hermesch, S.; van der Werf, J.; Li, L.; Chormai, T. Genetic parameters and inbreeding effects for production traits of Thai native chickens. Asian-Australas. J. Anim. Sci. 2019, 32, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Manjula, P.; Park, H.B.; Seo, D.; Choi, N.; Jin, S.; Ahn, S.J.; Heo, K.N.; Kang, B.S.; Lee, J.H. Estimation of heritability and genetic correlation of body weight gain and growth curve parameters in Korean native chicken. Asian-Australas. J. Anim. Sci. 2018, 31, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, I.A.; Oni, O.O.; Nwagu, B.I.; Adeyinka, F.D. Genetic parameter estimates for Body weights of Naked Neck broiler chickens. Int. J. Poult. Sci. 2006, 5, 589–592. [Google Scholar]
- Saatchi, M.; Omed, H.; Dewi, I.A. Genetic parameter from univariate and bivariate analysis of egg and weight trait in Japanese quails. Poult. Sci. 2006, 85, 185–190. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to ho climate and strategies to alleviate heat stress in livestock production. Animal 2011, 6, 707. [Google Scholar] [CrossRef] [PubMed]
- FaTHI, M.M.; Galal, A.; El-Safty, S.; Mahrous, M. Naked neck and frizz legends for improving chickens raised under high ambient temperature: Growth performance and egg production. Worlds Poult. Sci. J. 2013, 69, 813. [Google Scholar] [CrossRef]
- Mutibvu, T.; Chimonyo, M.; Halimani, T.E. Physiological responses of slow-growing chickens under diurnally cycling temperature in a hot environment. Poul. Sci. J. 2017, 19, 567. [Google Scholar] [CrossRef]
- Syafwan, S.; Kwakkel, R.P.; Verstegen, M.W.A. Heat stress and feeding strategies in meat-type chickens. Worlds Poult. Sci. J. 2011, 67, 653–674. [Google Scholar] [CrossRef]
- Settar, P.; Yalcin, S.; Turkmut, L.; Ozkan, S.; Cahanar, A. Season by genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999, 78, 1353–1358. [Google Scholar] [CrossRef]
- Al-Batshan, H.A. Performance and heat tolerance of broilers as affected by genotype and high ambient temperature. Asian-Australas. J. Anim. Sci. 2002, 15, 1502–1506. [Google Scholar] [CrossRef]
- Usala, M.; Macciotta, N.P.P.; Bergamaschi, M.; Maltecca, C.; Fix, J.; Schwab, C.; Shull, C.; Tiezzi, F. Genetic parameters for tolerance to heat stress in crossbred swine carcass traits. Front. Genet. 2021, 11, 1821. [Google Scholar] [CrossRef] [PubMed]

| Functions | von Bertalanffy | Gompertz | Logistic |
|---|---|---|---|
| Characteristics | |||
| Age at inflection point (IPA) | (ln3)/ | ln()/ | ln()/ |
| Weight at inflection point (IPW) | 8/27, | /e, | /2 |
| Maximum growth rate (MGR) | 3IPW/2 | IPW | IPW/2 |
| Absolute growth rate (AGR) |
| Functions | Sex | Parameters | Model fit | Growth Inflection | Maximum Growth Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MSE | Pseudo-R2 | AIC | IPA (wk) | IPW (g) | MGR (g/wk) | |||||
| Thai native (Kai Shee) | ||||||||||
| von Bertalanffy | Male | 2495.1 | 0.88 | 0.10 | 54,871 | 0.94 | 188,357 | 9.5 | 739.3 | 112.1 |
| Female | 2004.9 | 0.82 | 0.09 | 17,621 | 0.95 | 176,916 | 9.4 | 594.0 | 84.8 | |
| Combine | 2252.4 | 0.85 | 0.10 | 38,083 | 0.94 | 376,789 | 9.5 | 667.4 | 98.9 | |
| Gompertz | Male | 1979.2 | 4.50 | 0.16 | 54,722 | 0.97 | 188,310 | 9.4 | 728.1 | 116.4 |
| Female | 1550.2 | 4.13 | 0.15 | 17,519 | 0.96 | 176,810 | 9.1 | 570.3 | 88.5 | |
| Combine | 1760.6 | 4.39 | 0.16 | 37,948 | 0.96 | 376,664 | 9.3 | 647.7 | 103.0 | |
| logistic | Male | 1515.7 | 27.01 | 0.34 | 55,044 | 0.92 | 188,412 | 9.7 | 757.9 | 128.7 |
| Female | 1185.7 | 22.54 | 0.33 | 17,703 | 0.96 | 177,000 | 9.4 | 592.9 | 97.8 | |
| Combine | 1340.4 | 26.24 | 0.34 | 38,206 | 0.94 | 376,904 | 9.6 | 670.2 | 114.4 | |
| Thai synthetic (Kaimook e-san1) | ||||||||||
| von Bertalanffy | Male | 3524.5 | 0.98 | 0.16 | 47,033 | 0.94 | 54,485 | 6.9 | 1044.3 | 244.4 |
| Female | 3029.1 | 0.92 | 0.15 | 33,891 | 0.96 | 53,785 | 6.9 | 897.5 | 198.6 | |
| Combine | 3205.9 | 0.96 | 0.16 | 47,851 | 0.95 | 110,132 | 6.8 | 949.9 | 221.4 | |
| Gompertz | Male | 3012.5 | 5.07 | 0.23 | 46,195 | 0.97 | 54,426 | 7.2 | 1108.2 | 250.1 |
| Female | 2554.6 | 4.67 | 0.22 | 33,243 | 0.97 | 53,716 | 7.1 | 939.8 | 203.7 | |
| Combine | 2752.5 | 4.90 | 0.22 | 47,047 | 0.96 | 110,024 | 7.1 | 1012.6 | 226.4 | |
| logistic | Male | 2345.5 | 33.02 | 0.47 | 46,256 | 0.92 | 54,389 | 7.5 | 1172.8 | 274.5 |
| Female | 1982.5 | 28.09 | 0.45 | 33,412 | 0.94 | 53,680 | 7.4 | 991.3 | 223.9 | |
| Combine | 2150.4 | 30.69 | 0.46 | 47,311 | 0.93 | 109,951 | 7.4 | 1075.2 | 248.7 | |
| Parameter Estimates | Thai Native Breed | Thai Synthetic Breed |
|---|---|---|
| 478.58 | 455.20 | |
| 22.70 | 23.52 | |
| −80.56 | −84.38 | |
| 1223.95 | 1352.30 | |
| 74.03 | 94.10 | |
| −190.82 | −222.44 | |
| 249.660 | 428.32 | |
| (SE) | 0.23 ± 0.01 | 0.18 ± 0.03 |
| (SE) | 0.61 ± 0.01 | 0.58 ± 0.02 |
| −0.77 | −0.82 | |
| −0.63 | −0.62 | |
| Rate of decline of AGR | ||
| Male (g/THI) | −10.10 | −20.53 |
| Female (g/THI) | −6.61 | −11.35 |
| Average (g/THI) | −8.36 | −15.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonkum, W.; Duangjinda, M.; Kananit, S.; Chankitisakul, V.; Kenchaiwong, W. Genetic Effect and Growth Curve Parameter Estimation under Heat Stress in Slow-Growing Thai Native Chickens. Vet. Sci. 2021, 8, 297. https://doi.org/10.3390/vetsci8120297
Boonkum W, Duangjinda M, Kananit S, Chankitisakul V, Kenchaiwong W. Genetic Effect and Growth Curve Parameter Estimation under Heat Stress in Slow-Growing Thai Native Chickens. Veterinary Sciences. 2021; 8(12):297. https://doi.org/10.3390/vetsci8120297
Chicago/Turabian StyleBoonkum, Wuttigrai, Monchai Duangjinda, Srinuan Kananit, Vibuntita Chankitisakul, and Wootichai Kenchaiwong. 2021. "Genetic Effect and Growth Curve Parameter Estimation under Heat Stress in Slow-Growing Thai Native Chickens" Veterinary Sciences 8, no. 12: 297. https://doi.org/10.3390/vetsci8120297
APA StyleBoonkum, W., Duangjinda, M., Kananit, S., Chankitisakul, V., & Kenchaiwong, W. (2021). Genetic Effect and Growth Curve Parameter Estimation under Heat Stress in Slow-Growing Thai Native Chickens. Veterinary Sciences, 8(12), 297. https://doi.org/10.3390/vetsci8120297






