Clinical and Correlated Responses among Steroid Hormones and Oxidant/Antioxidant Biomarkers in Pregnant, Non-Pregnant and Lactating CIDR-Pre-Synchronized Dromedaries (Camelus dromedarius)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Guidelines
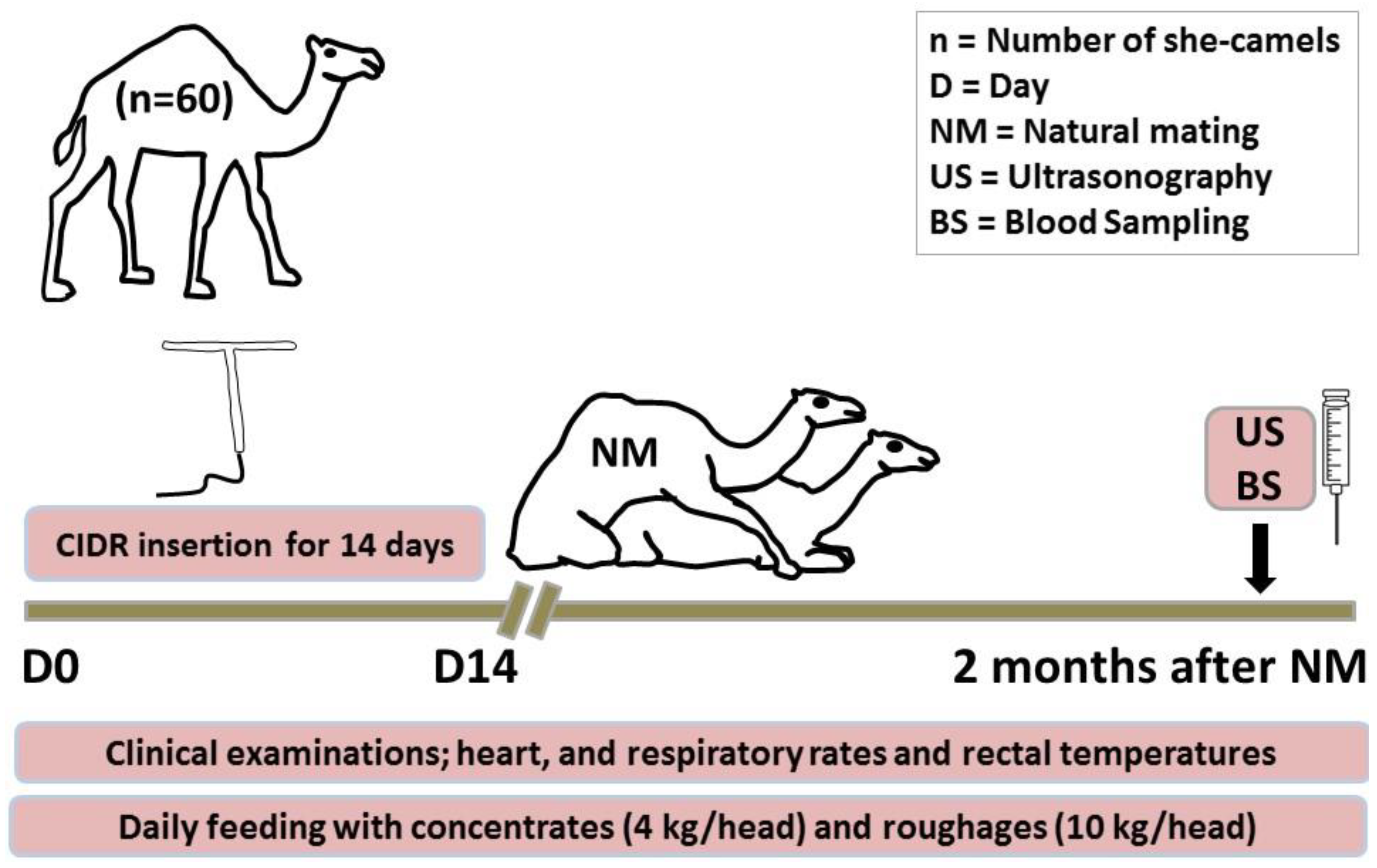
2.2. Animals and Therapeutic Strategy
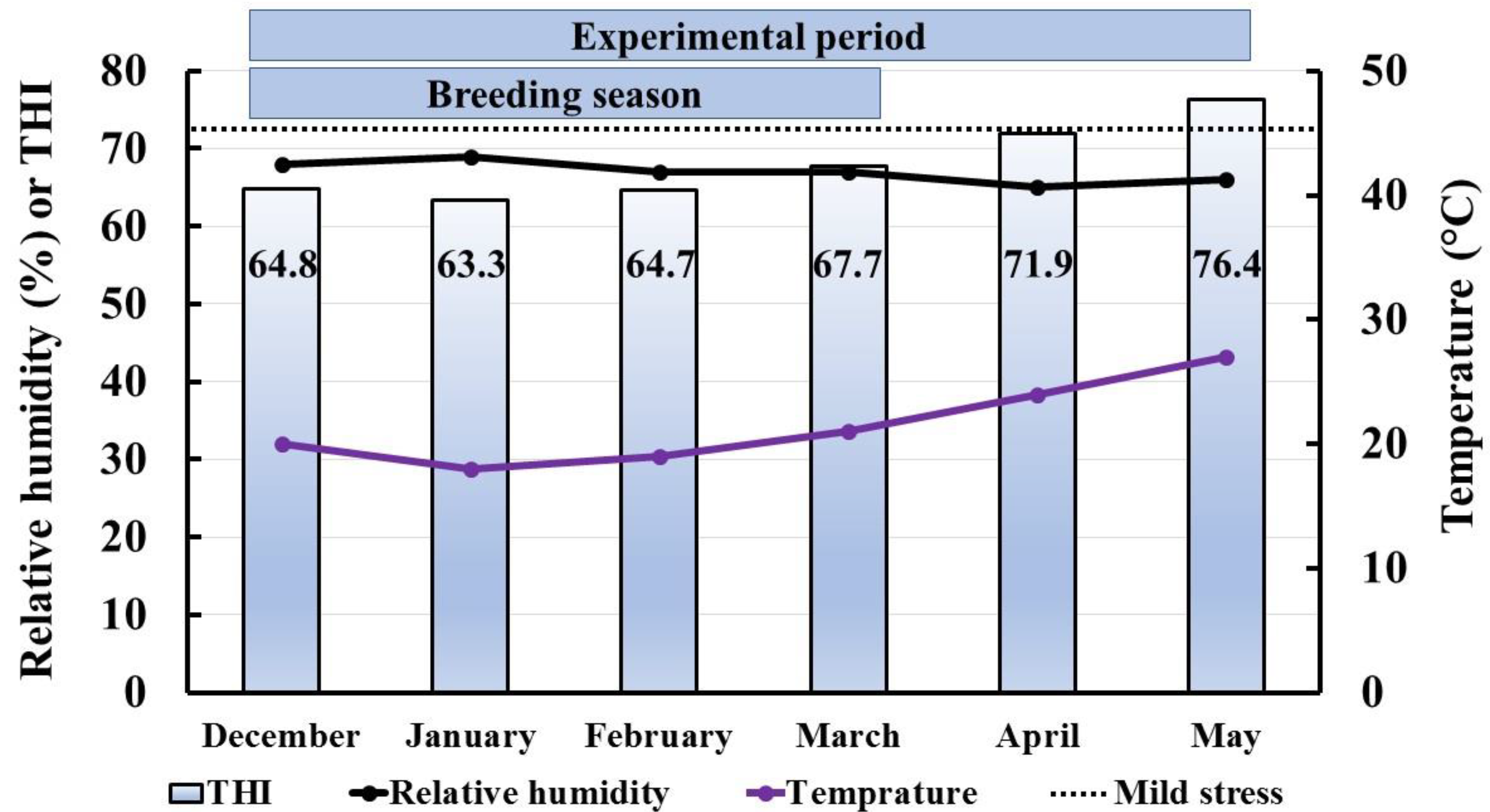
2.3. Study Location
2.4. Samples
2.5. Clinical Examination
2.6. Hormonal Analysis
2.7. Oxidant and Antioxidants Biomarkers Assays
2.8. Liver Functions and Lipid Profile Indices
2.9. Kidney Functions Biomarkers
2.10. Mineral Metabolism Indicators
2.11. Statistical Analysis
3. Results
3.1. Conception Rates and Clinical Parameters
3.2. Serum Concentrations of Steroid Hormones
3.3. Serum Concentrations of Oxidants and Antioxidants Biomarkers
3.4. Serum Concentrations of Liver Functions and Lipid Profile Indices
3.5. Serum Concentrations of Kidney Functions Biomarkers
3.6. Serum Concentrations of Mineral Metabolism
3.7. Correlation between Steroid Hormones and Oxidant/Antioxidant Biomarkers
3.8. Correlation between Steroid Hormones and Liver Functions/Lipid Profile Indices
3.9. Correlation between Steroid Hormones and Renal Functions Biomarkers
3.10. Correlation between Steroid Hormones and Minerals Parameters
4. Discussion
4.1. Clinical Examination and Conception Rates
4.2. Serum Concentrations of Steroid Hormones
4.3. Serum Concentrations of Oxidants and Antioxidants Biomarkers
4.4. Serum Concentrations of Liver Functions and Lipid Profile Indices
4.5. Serum Concentrations of Kidney Functions Biomarkers
4.6. Serum Concentrations of Mineral Metabolism
4.7. Correlation between Steroid Hormones and Oxidant/Antioxidant Biomarkers
4.8. Correlation between Steroid Hormones and Liver Functions and Lipid Profile Indices
4.9. Correlation between Steroid Hormones and Renal Functions Biomarkers
4.10. Correlation between Steroid Hormones and Minerals Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaughan, J.B. Which physiological adaptation allows camels to tolerate high heat load–and what more can we learn? J. Camelid Sci. 2011, 4, 85–88. [Google Scholar]
- Tibary, A.; El Allali, K. Dromedary camel: A model of heat resistant livestock animal. Theriogenology 2020, 154, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Faye, B. Role, distribution and perspective of camel breeding in third millennium economies. Emir. J. Food Agric. 2015, 27, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Marai, I.; Zeidan, A.; Abdel-Samee, A.; Abizaid, A.; Fadiel, A. Camels’ reproductive and physiological performance traits as affected by environmental conditions. Trop. Subtrop. Agroecosyst 2009, 10, 129–149. [Google Scholar]
- Al-Eknah, M.M. Reproduction in old world camels. Anim. Reprod. Sci. 2000, 60, 583–592. [Google Scholar] [CrossRef]
- El-Hassanien, E.E.; El-Bahrawy, K.A.; Fateh El-bab, A.Z.; Zeitoun, M.M. Sexual behavior and semen physical traits of desert male camels in rut. J. Egypt. Vet. Med. Assoc. 2004, 64, 305–321. [Google Scholar]
- Skidmore, J.A. Reproductive physiology in female old-world camelids. Anim. Reprod. Sci. 2011, 124, 148–154. [Google Scholar] [CrossRef]
- El-Bahrawy, K.A.; Khalifa, M.A.; Rateb, S.A. Recent advances in Dromedary camel reproduction. Emir. J. Food Agric. 2015, 27, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Abo El-Maaty, A.M.; Mohamed, R.H.; Abd El Hameed, A.R.; Hozyen, H.F.; Ali, A.H. Ovarian hormones and antioxidant biomarkers in dromedary camels synchronized with new and re-used controlled intravaginal drug release (CIDR)/GPG (Ovsynch) program during breeding season. Trop. Anim. Health Prod. 2019, 51, 1619–1625. [Google Scholar] [CrossRef]
- Abo El-Maaty, A.M.; Mohamed, R.H.; Hozyen, H.F.; El-Kattan, A.M.; Mahmoud, M.A.; Ali, A.H. Effect of exogenous progesterone treatment on ovarian steroid hormones and oxidant and antioxidant biomarkers during peak and low breeding seasons in Dromedary she-camel. Vet. World 2019, 12, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swelum, A.A.; Alowaimer, A.N. The efficacy of controlled internal drug release (CIDR) in synchronizing the follicular wave in dromedary camels (Camelus dromedarius) during the breeding season. Theriogenology 2015, 84, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.A.; Rateb, S.A.; El-Bahrawy, K.A. Fixed-time induction of ovulation in camels superovulated by different eCG modalities during the transition period in Egypt Superovulation in camels during the transition period. Trop. Anim. Health Prod. 2016, 48, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Gitto, E.; Reiter, R.J.; Karbownik, M.; Tan, D.X.; Gitto, P.; Barberi, S.; Barberi, I. Causes of oxidative stress in the pre- and perinatal period. Biol. Neonate 2002, 81, 146–157. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.K.; Singh, O.P.; Pandey, V.; Verma, P.K. Oxidative Stress and Antioxidant Status during Transition Period in Dairy Cows Asian-Aust. J. Anim. Sci. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.N.; Armstrong, D. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Celi, P. The role of oxidative stress in small ruminants’ health and production. R. Bras. Zootec. 2010, 39, 348–363. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Casella, S.; Giannetto, C.; Bazzano, M.; Giudice, E.; Fazio, F. Oxidative stress associated with road transportation in ewes. Small Rumin. Res. 2013, 112, 235–238. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Alhidary, I.A.; Abdelrahman, M.M.; Khan, R.U.; Harron, R.M. Antioxidant status and immune responses of growing camels supplemented a long acting multi-trace minerals rumen bolus. Ital. J. Anim. Sci. 2016, 15, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Shoieb, S.M.; Ibrahim, H.M.M.; Sayed-Ahmed, M.; El-khodery, S.A. Antioxidant Trace Elements and Oxidative Stress Levels Associated with Pasteurellosis in Camel-Calves (Camelus dromedarius). J. Vet. Sci. Technol. 2016, 7, 393. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hamid, I.S. Blood constituents, antioxidant activities and hormonal profile in she-camels (Camelus dromedarius) during different physiological statuses in the Northwestern Coast of Egypt. Int. J. Vet. Sci. 2021, 10, 247–258. [Google Scholar] [CrossRef]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2008, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Kale, M.; Rathore, N.; Johnand, S.; Bhatnagar, D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: A possible involvement of reactive oxygen species. Toxicol. Lett. 1999, 105, 197–205. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Omidi, A.; Montazertorbati, M.B.; Sajedi, Z.H. Lipid profile and thyroid hormone status in the last trimester of pregnancy in single humped camels (Camelus dromedarius). Trop. Anim. Health Prod. 2014, 46, 609–614. [Google Scholar] [CrossRef]
- Omidi, A.; Fathi, M.H.; Asiaban, M. Elevated levels of blood urea nitrogen and creatinine in the last trimester of pregnancy of dromedary camels (Camelus dromedarius) Iran. J. Vet. Res. 2015, 9, 249–255. [Google Scholar] [CrossRef]
- Ouajd, S.; Kamel, B. Physiological particularities of dromedary (Camelus dromedarius) and experimental implications. Scand. J. Lab. Anim. Sci. 2009, 36, 19–29. [Google Scholar] [CrossRef]
- Woreta, T.A.; Alqahtani, S.A. Evaluation of abnormal liver tests. Med. Clin. N. Am. 2014, 98, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Monjezi, S.; Mohammadpour, A.A.; Behnamrasouli, M. Histometrical study of the different parts of ureter in one-humped camel. Comp. Clin. Pathol. 2014, 23, 561–567. [Google Scholar] [CrossRef]
- Abere, S.A.; Oguzor, N.S. Adaptation of animals to arid ecological conditions. World J. Zool. 2011, 6, 209–214. [Google Scholar]
- Kamili, A.; Bengoumi, M.; Oukessou, M.; Faye, B.; Lefebvre, H.P. Assessment of glomerular filtration rate in normally hydrated and dehydrated dromedary camel by plasma exogenous creatinine clearance test. Emirates J. Food Agric. 2013, 25, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a crosssectional study and review of literature. J. Cachexia Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef]
- Bani, I.Z.A.; Al-Majali, A.M.; Amireh, F.; Al-Rawashreh, O.F. Metabolic profile in goat does in late pregnancy with and without subclinical pregnancy toxemia. Vet. Clin. Pathol. 2008, 37, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Hagawane, S.D.; Shinde, S.B.; Rajuru, S.B. Haematological and blood biochemical profile in lactating Buffaloes in and around Parbbani city. Vet. World 2009, 2, 467–469. [Google Scholar]
- Habte, M.; Eshetu, M.; Maryo, M.; Andualem, D.; Legesse, A.; Admassu, B. The influence of weather conditions on body temperature, milk composition and yields of the free-ranging dromedary camels in Southeastern rangelands of Ethiopia. Cogent Food Agric. 2021, 7, 1930932. [Google Scholar] [CrossRef]
- Fowler, M.E. Medicine and Surgery of Camelids, 3rd ed.; Blackwell Publishing Ltd.: Ames, IO, USA, 2010; pp. 89–109, 408. [Google Scholar]
- Abdel-Rahman, H.M.A.; Ibrahim, M.A.; Elmetwaly, H.A. Hormonal profile, antioxidant status and some biochemical parameters during pregnancy and periparturient period in Dromedary she camel. Egypt. J. Vet. Sci. 2017, 48, 81–94. [Google Scholar]
- Hassan, H.Y.; Gadallah, S.; Kamr, A.; Abdelazeim, A. Serum iron, calcium, phosphorus and magnesium concentrations and their effects on hemato-immune dynamics in diseased camels (Camelus dromedarius). EC Vet. Sci. 2019, 4, 1–11. [Google Scholar]
- Muhammad, B.F.; Aliyu, D.; Njidda, A.A.; Madigawa, I.L. Some haematological, biochemical and hormonal profile of pregnant and non-pregnant she-camels (Camelus dromedarius) raised in a Sudan savanna zone of Nigeria. J. Camel Pract. Res. 2011, 18, 73–77. [Google Scholar]
- Hamad, B.; Aggad, H.; Hadef, L.; Adaika, A. Effect of cold and hot seasons on thermoregulation and hemogram blood parameters of dromedary camel (Camelus dromedarius) in Algeria. Livestock Res. Rural Dev. 2017, 29, 1–8. [Google Scholar]
- Ayoub, M.A.; El-Khouly, A.A.; Mohamed, T.M. Some hematological and biochemical parameters and steroid hormone levels in the one-humped camel during different physiological conditions. Emir. J. Agric. Sci. 2003, 15, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.P.; Khanna, N.D.; Agarwal, V.K.; Dwaraknath, P.K. Circulating levels of estrogen and progesterone in female camels (Camelus dromedarius) during pregnancy. Theriogenology 1987, 28, 849–859. [Google Scholar] [CrossRef]
- El-Wishy, A.B.; Hemeida, N.A.; Omar, M.A.; Mobarak, A.M.; ElSayed, M.A. Functional changes in the pregnant camel with special reference to fetal growth. Br. Vet. J. 1981, 137, 527–537. [Google Scholar] [CrossRef]
- Homeida, A.M.; Khalil, M.G.R.; Taha, A.A. Plasma concentrations of progesterone, oestrogens, testosterone and LH activity during the estrous cycle in camel (Camelus dromedarius). J. Reprod. Fert. 1988, 83, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Quzy, I.; Suhel, A.; Purohit, G.N. Hormonal management of ovarian activity in breeding camels two months ahead of the natural breeding season. Camel Int. J. Vet. Sci. 2013, 1, 37–49. [Google Scholar] [CrossRef]
- Anderson, D.E.; Grubb, T.; Silveira, F. The effect of short duration transportation on serum cortisol response in alpacas (LIama pacos). Vet. J. 1999, 157, 189–191. [Google Scholar] [CrossRef]
- Riquelme, R.A.; LIanos, J.A.; McGarrigle, H.H.; Sanhueza, E.M.; Hanson, M.A.; Giussani, D.A. Chemo reflex contribution to adrenocortical function during acute hypoxemia in the llama fetus at 0.6 to 0.7 of gestation. Endocrinology 1998, 139, 2567–2570. [Google Scholar] [CrossRef]
- Saeb, M.; Baghshani, H.; Nazifi, S.; Saeb, S. Physiological response of dromedary camels to road transportation in relation to circulating levels of cortisol, thyroid hormones and some serum biochemical parameters. Trop. Anim. Health Prod. 2010, 42, 55. [Google Scholar] [CrossRef]
- Ebissy, E.A.; El-Sayed, A.A.; Mohamed, R.H. Hematological and biochemical profile in female camels (Camelus dromedarius) during the transition period. Slov. Vet. Res. 2019, 56 (Suppl. 22), 571–577. [Google Scholar] [CrossRef] [Green Version]
- Suganya, G.; Gomathy, V.S. Hormone profile of Tellicherry goats during periparturient period. Tamilnadu. J. Vet. Anim. Sci. 2009, 5, 211–221. [Google Scholar]
- Tharwat, M.; Ali, A.; Al-Sobayil, F.; Selim, L.; Abbas, H. Hematobiochemical profile in female camels (Camelus dromedarius) during the periparturient period. J. Camel Pract. Res. 2015, 22, 101–106. [Google Scholar] [CrossRef]
- Saleh, M.A.; Al-Salahy, M.B.; Sanousi, S.A. Oxidative stress in blood of camels (Camelus dromedaries) naturally infected with Trypanosoma evansi. Vet. Parasitol. 2009, 10, 192–199. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.M.; Elmoslemany, A.M. Cardiac and oxidative stress biomarkers in Trypanosoma evansi infected camels: Diagnostic and prognostic prominence. Parasitology 2015, 142, 767–772. [Google Scholar] [CrossRef] [PubMed]
- El-Bahr, S.M.; El-Deeb, W.M. Trypanosoma evansi in naturally infected dromedary camels: Lipid profile, oxidative stress parameters, acute phase proteins and proinflammatory cytokines. Parasitology 2016, 143, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Kdliwadmath, M.V.; Kodliwadmath, S.M. Study of oxidative stress and enzymatic antioxidants in normal pregnancy. Indian J. Clin. Biochem. 2007, 22, 135–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S. Lipid peroxidation in pregnancy. Hypertens. Pregnancy 1994, 13, 1–32. [Google Scholar] [CrossRef]
- Jarikre, T.A.; Ohore, G.O.; Oyagbemi, A.A.; Emikpe, B.O. Evaluation of oxidative stress in caprine bronchoalveolar lavage fluid of pneumonic and normal lungs. Intern. J. Vet. Sci. Med. 2017, 5, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Dedar, R.K.; Patil, N.V. Oxidative stress in pregnant and lactating camels. J. Camel Pract. Res. 2013, 20, 1–4. [Google Scholar]
- Trotta, R.J.; Sullivan, S.J.; Stem, A.I. Lipid peroxidation and haemoglobin degradation in red blood cell exposed to t-butylhydroperoxide. Effect of HMP shunt as medicated by glutathione and ascorbate. Biochem. J. 1982, 204, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Kamr, A.; Gadallah, S.; Arbaga, A.; Hassan, H.Y. Oxidant and antioxidant biomarkers and the risk factor of age on their concentrations in pneumonic Arabian camels (Camelus dromedarius). J. Camelid Sci. 2020, 13, 40–48. [Google Scholar]
- Panda, N.; Kaur, H.; Mohanty, T.K. Reproductive Performance of Dairy Buffaloes Supplemented with Varying Levels of Vitamin E. Asian-Aust. J. Anim. Sci. 2006, 19, 19–25. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ferdous, J.; Rahman, M.K.; Akter, S.; Hassan, M.M.; Islam, A. Reference values for hematological and serum biochemical parameters of dromedary camel (Camelus dromedarius) in sub-tropical climate of Bangladesh. Adv. Anim. Vet. Sci. 2019, 7, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Abdalmula, A.M.; Benashour, F.M.; Shmela, M.; Alnagar, F.A.; Abograra, I.; Buker, A.O. Blood Profile in Normal One Humped Dromedary (Camelus dromedarius) Camels in Libya. Part 3: Effect of Sex Variation on Biochemical and Haematological Blood Profile. Int. J. Sci. Basic Appl. Res. 2019, 48, 9–24. [Google Scholar]
- Saeed, A.; Khan, I.A.; Hussein, M.M. Change in biochemical profile of pregnant camels (Camelus dromedarius) at term. Comp. Clin. Path. 2009, 18, 139–143. [Google Scholar] [CrossRef]
- Kelanmer, R.; Antoine-Moussiaux, N.; Abu-Median, A.A.K.; Hanzen, C.H.; Kaidi, R. Effect of nutrition on reproductive performance during the peripartum period of female camel (Camelus dromedarius) in Algeria. J. Anim. Vet. Advan. 2018, 14, 192–196. [Google Scholar]
- Mohamed, H.; Hussein, A. Studies on Normal Haematological and Serum Biochemical Values of the ‘Hijin’ Racing Camels (Camelus dromedarius) in Kuwait. Vet. Res. Commun. 1999, 23, 241–248. [Google Scholar] [CrossRef]
- El-tohamy, M.M.; Salama, A.; Yousef, A.A. Blood constituents in relation to the reproductive state in she-camel (Camelus dromedarius). Beitr Trop Landwirtsch Vet. 1986, 24, 425–430. [Google Scholar]
- Saleh, M.A.; El-Sokkary, G.H.; AbdelRazik, A.R. Circulating steroids and proteins in Egyptian oasis pregnant camels (Camelus dromedaries). J. Camel Pract. Res. 2000, 7, 9–13. [Google Scholar]
- Jainudee, M.R.; Hafez, E.S.E. Gestation Prenatal Physiology and Parturition. In Reproduction in Farm Animals; Hafez, E.S.E., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1994; pp. 247–283. [Google Scholar]
- Janku, L.; Pavlata, L.; Mišurová, L.; Filípek, J.; Pechová, A.; Dvořák, R. Levels of protein fractions in blood serum of periparturient goats. Acta Vet. Brno. 2011, 80, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dong, H.; Thompson, D.C.; Shertzer, H.G.; Nebert, D.W.; Vasiliou, V. Glutathione defense mechanism in liver injury: In-sights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2008; pp. 882–888. [Google Scholar]
- National Research Council. Nutrient Requirements of Small Ruminants; National Academy Press: Washington, DC, USA, 2007.
- Fudge, N.J.; Kovacs, C.S. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 2010, 151, 886–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukder, S.; Kerrisk, K.L.; Ingenhoff, L.; Gabai, G.; Garcia, S.C.; Celi, P. Changes in plasma oxidative stress biomarkers in dairy cows after oestrus synchronisation with controlled internal drug release CIDR. and prostaglandin F2α PGF2α. Anim. Reprod. Sci. 2014, 54, 1490–1496. [Google Scholar] [CrossRef]
- Kaya, S. Evaluation of Certain Oxidative Stress Parameters in Heifers that were Administered Short Term PRID. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 569–573. [Google Scholar]
- Kupczyński, R.; Chudoba-Drozdowska, B. Values of selected biochemical parameters of cows’blood during their drying-off and the beginning of lactation. Electron. J. Pol. Agric. Univ. 2002, 5, 1. [Google Scholar]
- Ali, A.; Tharwat, M.; Al-sobayil, F.A. Hormonal, biochemical, and hematological profiles in female camels (Camelus dromedarius) affected with reproductive disorders. Anim. Reprod. Sci. 2010, 118, 372–376. [Google Scholar] [CrossRef] [PubMed]
| NPREG | LACT | PREG | Reference Values | |
|---|---|---|---|---|
| Temperature (°C) | 37.66 ± 0.48 a | 37.27 ± 0.32 a | 37.82 ± 0.18 a | (37.2 ± 0.77) 1 or (37.52 ± 0.09) 2 |
| Pulse (Beats/min) | 30.56 ± 3.01 b | 28.16 ± 2.31 b | 36.02 ± 4.15 a | (32–36) 3 or (24–48/min) 4 |
| Respiration (Breaths/min) | 10.66 ± 1.88 b | 12.61 ± 3.12 b | 20.72 ± 2.03 a | (12.55 ± 0.30) 2 or (8–18) 5 |
| Rumen motility (Movements/2 min) | 3.65 ± 0.66 a | 3.15 ± 0.33 a | 3.80 ± 0.71 a | (4.25 ± 0.14) 2 or (4.3 ± 0.14) 6 |
| NPREG | LACT | PREG | Reference Values | |
|---|---|---|---|---|
| P4 (ng/mL) | 0.93 ± 0.21 c | 2.30 ± 0.32 b | 4.29 ± 0.53 a | (0.29 ± 0.26–0.33 ± 0.44) 1 |
| E2 (pg/mL) | 180.38 ± 50.61 b | 123.63 ± 20.37 c | 265.5 ± 51.91 a | (1.29 ± 1.44–2.66 ± 1.98) 1 |
| Cortisol (nmol/L) | 42.32 ± 8.96 b | 44.63 ± 7.73 b | 74.25 ± 15.28 a | (38.17 ± 3.99) 2 |
| NPREG | LACT | PREG | Reference Values | |
|---|---|---|---|---|
| MDA (μmol/L) | 11.35 ± 1.63 b | 12.50 ± 1.80 b | 28.85 ± 1.72 a | (13.2 ± 0.6) 1 or (13.89 ± 0.94) 2 or (10·23–11·62) 3 |
| SOD (U/mL) | 4.09 ± 0.14 b | 3.89 ± 0.16 b | 4.52 ± 0.17 a | (5.0 ± 0.4) 1 or (5·11–6·33) 3 or (5.52 ± 0.72) 4 |
| TAC (mmol/L) | 3.40 ± 0.20 a | 3.06 ± 0.14 b | 1.79 ± 0.09 c | (1.53 ± 0.48) 4 or (0.81 ± 0.02) 5 |
| CAT (U/L) | 24.46 ± 0.99 a | 21.98 ± 1.09 b | 17.07 ± 1.17 c | (15.7 ± 0.4) 1 or (15.87 ± 0.84) 2 or (18.80 ± 0.63) 4 |
| GSH (mg/dL) | 37.68 ± 2.35 a | 31.23 ± 4.80 b | 17.29 ± 1.63 c | (8.19 ± 0.53) 4 |
| NPREG | LACT | PREG | Reference Values | |
|---|---|---|---|---|
| Glucose (mmol/L) | 9.47 ± 0.82 a | 8.76 ± 1.14 a | 9.89 ± 1.37 a | (5.01–8.03) 1 or (6.36 ± 0.35) 2 or (4.88–6.97) 3 |
| Total proteins (g/L) | 58.66 ± 2.70 b | 56.42 ± 2.72 a | 55.61 ± 2.99 a | (59.7–106.7) 1 or (61.20 ± 4.30) 2 or (53–78) 4 |
| Albumins (g/L) | 27.04 ± 2.85 a | 24.58 ± 1.69 a | 26.23 ± 2.52 a | (12.2–77.5) 1 or (38.30 ± 2.10) 2 or (30.80 ± 1.38) 3 |
| Globulins (g/L) | 34.09 ± 3.74 a | 29.68 ± 1.08 b | 29.38 ± 1.02 b | (18.42–23.87) 3 or (16–29) 5 |
| AST (U/L) | 106.13 ± 6.73 c | 117.88 ± 586 b | 126.13 ± 7.12 a | (84.1–161.8) 1 or (34–148) 4 |
| ALP (U/L) | 35.5 ± 7.63 c | 52.26 ± 4.50 b | 76 ± 8.42 a | (40–176) 4 or (41–92) 5 |
| GGT (U/L) | 16.95 ± 1.58 c | 25.25 ± 8.96 b | 39.69 ± 2.15 a | (12–28) 4 or (7–29) 5 |
| NPREG | LACT | PREG | Reference Values | |
|---|---|---|---|---|
| BUN (mmol/L) | 6.42 ± 0.56 b | 9.78 ± 3.26 a | 10.60 ± 4.02 a | (13.98 ± 1.09) 1 or (2.50–9.64) 2 |
| Cr (μmol/L) | 72.29 ± 9.15 b | 76.80 ± 13.85 b | 93.93 ± 10.09 a | (114.92–265.20) 2 or (97.2–221) 3 |
| CK (U/L) | 105.99 ± 5.81 b | 107.88 ± 4.39 b | 113.38 ± 4.44 a | (70–250) 2 or (100.36–159·76) 4 |
| NPREG | LACT | PREG | Reference Values | |
|---|---|---|---|---|
| Ca (mmol/L) | 2.28 ± 0.38 a | 2.21 ± 0.09 a | 2.08 ± 0.21 a | (1.80–3.13) 1 or (1.43–2.12) 2 or (2.63 ± 0.07) 3 |
| P (mmol/L) | 1.54 ± 0.30 b | 1.57 ± 0.26 b | 1.92 ± 0.25 a | (1.10–2.29) 1 or (1.43–2.12) 2 or (3.53 ± 0.08) 3 |
| Mg (mmol/L) | 0.94 ± 0.08 a | 1.09 ± 0.11 a | 1.02 ± 0.09 a | (0.74–1.72) 1 or (1.07–1.01) 2 or (1.07 ± 0.03) 3 |
| P4 (ng/mL) | E2 (pg/mL) | Cortisol (nmol/L) | MDA (μmol/L) | SOD (U/mL) | TAC (mmol/L) | CAT (U/L) | GSH (mg/dL) | |
|---|---|---|---|---|---|---|---|---|
| P4 | −0.1019 | −0.4394 ** | −0.5116 ** | −0.6291 ** | 0.2936 | 0.1703 | 0.1908 | |
| (0.6358) | (0.0317) | (0.0106) | (0.0010) | (0.1637) | (0.4263) | (0.3718) | ||
| E2 | 0.5821 ** | 0.7590 ** | 0.57059 ** | −0.7703 ** | −0.7371 ** | −0.8106 ** | ||
| (0.0028) | (0.0002) | (0.00360) | (0.0001) | (0.0004) | (0.0002) | |||
| Cortisol | −0.8060 ** | 0.6514 ** | −0.7663 ** | −0.7499 ** | −0.7398 ** | |||
| (0.0001) | (0.0006) | (0.0001) | (0.0002) | (0.0004) | ||||
| MDA | 0.83926 ** | −0.9201 ** | −0.8534 ** | −0.8624 ** | ||||
| (0.0003) | (0.0002) | (0.0001) | (0.0006) | |||||
| SOD | −0.7371 ** | −0.6095 ** | −0.6855 ** | |||||
| (0.0004) | (0.0016) | (0.0002) | ||||||
| TAC | 0.9198 ** | 0.8873 ** | ||||||
| (0.0002) | (0.0008) | |||||||
| CAT | 0.8674 ** | |||||||
| (0.0004) | ||||||||
| GSH | ||||||||
| P4 (ng/mL) | E2 (pg/mL) | Cortisol (nmol/L) | Glucose (mmol/L) | TP (g/L) | Albumins (g/L) | Globulins (g/L) | AST (U/L) | ALP (U/L) | GGT (U/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| P4 | 0.0723 | −0.1012 | 0.3344 | −0.2784 | 0.5081 * | −0.0885 | −0.03903 | |||
| (0.1878) | (0.6379) | (0.1102) | (0.1878) | (0.0113) | (0.6810) | (0.8563) | ||||
| E2 | 0.1472 | −0.4968 * | 0.2550 | −0.6352 ** | −0.3715 * | −0.6997 ** | −0.8219 ** | |||
| (0.4926) | (0.0135) | (0.2291) | (0.0085) | (0.0439) | (0.0001) | (0.0008) | ||||
| Cortisol | 0.2241 | −0.1862 | 0.3381 | −0.2665 | −0.4431 * | −0.5923 ** | −0.6433 ** | |||
| (0.2926) | (0.3836) | (0.1061) | (0.2081) | (0.0301) | (0.0023) | (0.0070) | ||||
| Glucose | −0.1618 | 0.0338 | −0.0603 | −0.3569 | −0.3613 | −0.3447 | ||||
| (0.4410) | (0.8753) | (0.7795) | (0.0869) | (0.0828) | (0.0990) | |||||
| TP | 0.4298 * | 0.4437 * | −0.4315 * | 0.5015 * | −0.3569 | |||||
| (0.0361) | (0.0299) | (0.0352) | (0.0125) | (0.0869) | ||||||
| Albumins | −0.5276 ** | 0.1573 | −0.2825 | −0.3332 | ||||||
| (0.0080) | (0.4628) | (0.1811) | (0.1117) | |||||||
| Globulins | −0.1116 | 0.3988 | 0.5712 ** | |||||||
| (0.6037) | (0.0536) | (0.0036) | ||||||||
| AST | 0.4298 * | 0.4437 * | ||||||||
| (0.0361) | (0.0299) | |||||||||
| ALP | 0.9009 ** | |||||||||
| (0.0002) | ||||||||||
| GGT | ||||||||||
| P4 (ng/mL) | E2 (pg/mL) | Cortisol (nmol/L) | BUN (mmol/L) | Cr (μmol/L) | CK (U/L) | |
|---|---|---|---|---|---|---|
| P4 | −0.5141 * | −0.4100 * | −0.1148 | |||
| (0.0102) | (0.0466) | (0.5934) | ||||
| E2 | 0.0181 | 0.5278 ** | −0.4815 * | |||
| (0.9331) | (0.0080) | (0.0172) | ||||
| Cortisol | 0.0129 | 0.5921 ** | −0.2811 | |||
| (0.9525) | (0.0023) | (0.1833) | ||||
| BUN | 0.1295 | −0.0104 | ||||
| (0.5464) | (0.9617) | |||||
| Cr | −0.3594 | |||||
| (0.0845) | ||||||
| CK | ||||||
| P4 (ng/mL) | E2 (pg/mL) | Cortisol (nmol/L) | Ca (mmol/L) | P (mmol/L) | Mg (mmol/L) | |
|---|---|---|---|---|---|---|
| P4 | 0.3342 | −0.2719 | −0.4823 * | |||
| (0.110412) | (0.1987) | (0.0170) | ||||
| E2 | −0.1825 | 0.5805 ** | −0.1231 | |||
| (0.39350) | (0.0029) | (0.5665) | ||||
| Cortisol | −0.3060 | 0.4165 * | −0.0446 | |||
| (0.1459) | (0.0429) | (0.8362) | ||||
| Ca | 0.0618 | 0.2237 | ||||
| (0.7741) | (0.2935) | |||||
| P | 0.4603 * | |||||
| (0.0236) | ||||||
| Mg | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, R.H.; Khalphallah, A.; Nakada, K.; Elmeligy, E.; Hassan, D.; Ebissy, E.A.; Ghandour, R.A.; Mousa, S.A.; Hassaneen, A.S.A. Clinical and Correlated Responses among Steroid Hormones and Oxidant/Antioxidant Biomarkers in Pregnant, Non-Pregnant and Lactating CIDR-Pre-Synchronized Dromedaries (Camelus dromedarius). Vet. Sci. 2021, 8, 247. https://doi.org/10.3390/vetsci8110247
Mohamed RH, Khalphallah A, Nakada K, Elmeligy E, Hassan D, Ebissy EA, Ghandour RA, Mousa SA, Hassaneen ASA. Clinical and Correlated Responses among Steroid Hormones and Oxidant/Antioxidant Biomarkers in Pregnant, Non-Pregnant and Lactating CIDR-Pre-Synchronized Dromedaries (Camelus dromedarius). Veterinary Sciences. 2021; 8(11):247. https://doi.org/10.3390/vetsci8110247
Chicago/Turabian StyleMohamed, Ragab H., Arafat Khalphallah, Ken Nakada, Enas Elmeligy, Dalia Hassan, Eman A. Ebissy, Rehab A. Ghandour, Sabry A. Mousa, and Ahmed S. A. Hassaneen. 2021. "Clinical and Correlated Responses among Steroid Hormones and Oxidant/Antioxidant Biomarkers in Pregnant, Non-Pregnant and Lactating CIDR-Pre-Synchronized Dromedaries (Camelus dromedarius)" Veterinary Sciences 8, no. 11: 247. https://doi.org/10.3390/vetsci8110247
APA StyleMohamed, R. H., Khalphallah, A., Nakada, K., Elmeligy, E., Hassan, D., Ebissy, E. A., Ghandour, R. A., Mousa, S. A., & Hassaneen, A. S. A. (2021). Clinical and Correlated Responses among Steroid Hormones and Oxidant/Antioxidant Biomarkers in Pregnant, Non-Pregnant and Lactating CIDR-Pre-Synchronized Dromedaries (Camelus dromedarius). Veterinary Sciences, 8(11), 247. https://doi.org/10.3390/vetsci8110247






