Risk Factors and Severity of Gastrointestinal Parasites in Selected Small Ruminants from Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Consent
2.2. Study Area
2.3. Sample Size and Study Design
2.4. Sample and Data Collection
2.5. Laboratory Examinations
2.5.1. Detection of GIPs and Evaluation of Fecal Egg/Oocyst Count
2.5.2. Evaluation of Anemia
2.6. Statistical Analysis
3. Results
3.1. The Overall Prevalence and Spectrum of Parasites Detected among Small Ruminants from Negeri Sembilan, Malaysia
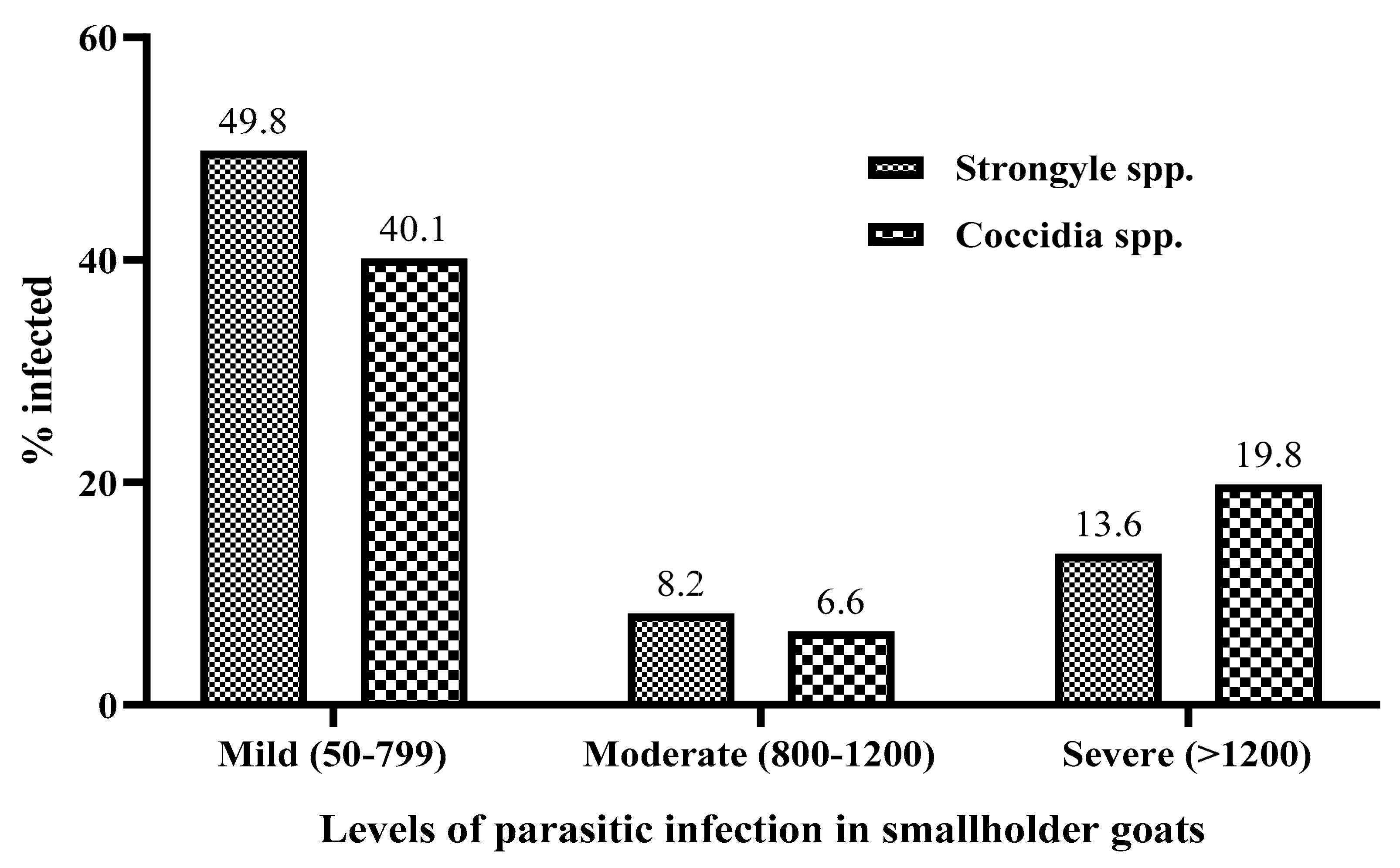
3.2. The Intensity of Parasitic Infection among Small Ruminants from Negeri Sembilan, Malaysia
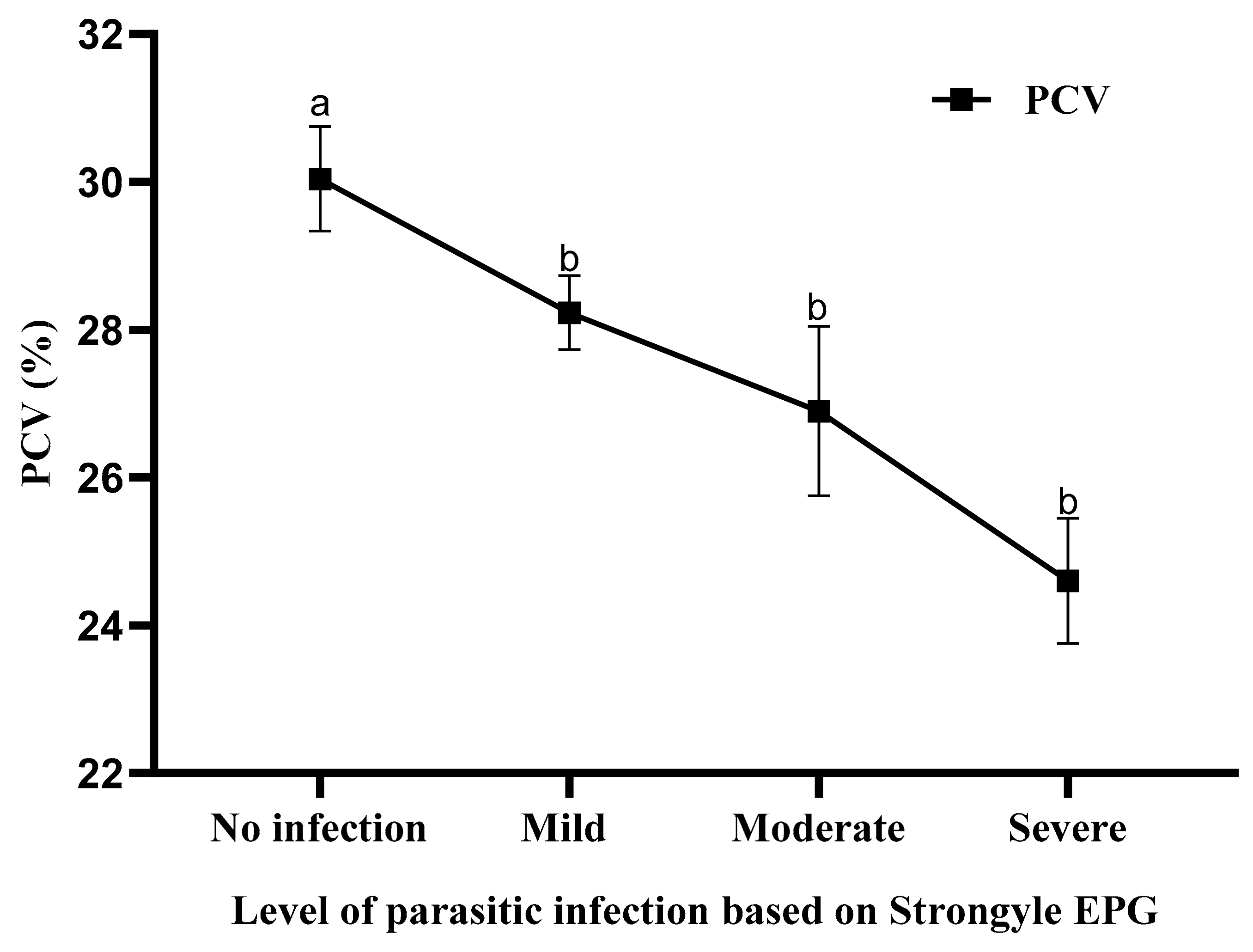
3.3. Effect of Different Levels of Strongyle Infection on the PCV of Small Ruminants from Negeri Sembilan, Malaysia
3.4. Risk Factors of GIP Infection Among Small Ruminants from Negeri Sembilan, Malaysia
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loh, T.C. Livestock production and the feed industry in Malaysia. Protein Sources Anim. Feed Ind. 2002, 329–339. [Google Scholar]
- Chandrawathani, P.; Adnan, M.; Waller, P.J. Anthelmintic resistance in sheep and goat farms on Peninsular Malaysia. Vet. Parasitol. 1999, 82, 305–310. [Google Scholar] [CrossRef]
- Jesse, F.F.A.; Adamu, L.; Hero, M.; Jamal, A.B.; Osman, A.Y.; Haron, A.W.; Awang, D.N.; Roslim, N. Parasitic Gastro-Enteritis (PGE) Concurrent with Eperythrozoonosis in a Goat: A Case Report. IOSR J. Agric. Vet. Sci. Ver. 2013, 4, 63–66. [Google Scholar] [CrossRef]
- Urquhart, G.M.; Armour, J.; Duncan, J.L.; Dunn, A.M.; Jennings, F.W. Veterinary Parasitology, 2nd ed.; Blackwell Science: Oxford, UK, 1996; ISBN 0632040513. [Google Scholar]
- Bhat, S.A.; Mir, M.U.R.; Qadir, S.; Allaie, I.M.; Khan, H.M.; Husain, I.; Sheikh, B.A. Prevalence of gastrointestinal parasitic infections in Sheep of Kashmir valley of India. Vet. World 2012, 5, 667–671. [Google Scholar] [CrossRef]
- Paul, B.T.; Biu, A.A.; Ahmed, G.M.; Mohammed, A.; Philip, M.H.; Yusuf, J. Point Prevalence and Intensity of Gastrointestinal Parasite Ova/ Oocyst and Its Association with Body Condition Score (BCS) of Sheep and Goats in Maiduguri, Nigeria. J. Adv. Vet. Parasitol. 2016, 3, 81–88. [Google Scholar] [CrossRef]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Baillière Tindall: London, UK, 1982; ISBN 0-7020-0820-6. [Google Scholar]
- Yusof, A.M.; Isa, M.L.M. Prevalence of gastrointestinal nematodiasis and coccidiosis in goats from three selected farms in Terengganu, Malaysia. Asian Pac. J. Trop. Biomed. 2016, 6, 735–739. [Google Scholar] [CrossRef]
- Gibbons, L.M.; Khalil, L.F. A key for the identification of genera of the nematode family Trichostrongylidae Leiper, 1912. J. Helminthol. 1982, 56, 185–233. [Google Scholar] [CrossRef]
- Nisbet, A.J.; Meeusen, E.N.; González, J.F.; Piedrafita, D.M. Immunity to Haemonchus contortus and Vaccine Development. Adv. Parasitol. 2016, 93, 353–396. [Google Scholar] [CrossRef]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The Pathophysiology, Ecology and Epidemiology of Haemonchus contortus Infection in Small Ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar] [CrossRef]
- Regassa, F.; Sori, T.; Dhuguma, R.; Kiros, Y. Epidemiology of Gastrointestinal Parasites of Ruminants in Western Oromia, Ethiopia. Int. J. Appl. Res. Vet. Med. 2006, 4, 51–57. [Google Scholar]
- Gharekhani, J.; Sadeghi-Dehkordi, Z.; Bahrami, M. Prevalence of Coccidiosis in Broiler Chicken Farms in Western Iran. J. Vet. Med. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ann Zainalabidin, F.; Raimy, N.; Hazmi Yaacob, M.; Musbah, A.; Bathmanaban, P.; Asmar Ismail, E.; Che Mamat, Z.; Zahari, Z.; Iswadi Ismail, M.; Panchadcharam, C. The Prevalence of Parasitic Infestation of Small Ruminant Farms in Perak, Malaysia. Trop. Life Sci. Res. 2015, 26, 1–8. [Google Scholar]
- Biu, A.A.; Onyiche, E.T.; Mohammed, A.; Paul, B.T.; Adegoke, M.A.; Ngulde, S.I. Prevalence of Strongyle ova in Goats and comparative studies of some faecal culture techniques. J. Appl. Sci. Environ. Manag. 2018, 22, 153. [Google Scholar] [CrossRef][Green Version]
- Nwosu, C.O.; Madu, P.P.; Richards, W.S. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Vet. Parasitol. 2007, 144, 118–124. [Google Scholar] [CrossRef]
- Owhoeli, O.; Elele, K.; Gboeloh, L.B. Prevalence of Gastrointestinal Helminths in Exotic and Indigenous Goats Slaughtered in Selected Abattoirs in Port Harcourt, South-South, Nigeria. Chin. J. Biol. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Dorny, P.; Symoens, C.; Jalila, A.; Vercruysse, J.; Sani, R. Strongyle infections in sheep and goats under the traditional husbandry system in peninsular Malaysia. Vet. Parasitol. 1995, 56, 121–136. [Google Scholar] [CrossRef]
- Sani, R.A.; Adnan, M.; Cheah, T.S.; Chandrawathani, P. Worm control for small ruminants in goats in Serdang, West Malaysia. J. Vet. Malays. 2004, 16, 1–8. [Google Scholar]
- Chandrawathani, P.; Nurulaini, R.; Adnan, M.; Premalaatha, B.; Khadijah, S.; Jamnah, O.; Zaini, C.M.; Khor, S.K.; Zawida, Z. A survey of parasitic infection on small ruminant farms in Kinta and Hilir Perak districts, Perak, Malaysia. Trop. Biomed. 2009, 26, 11–15. [Google Scholar]
- Nor-Azlina, A.A.; Sani, R.A.; Ariff, O.M. Management Practices Affecting Helminthiasis in Goats. Pertanika J. Trop. Agric. Sci. 2011, 34, 295–301. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science: Oxford, UK, 2005; ISBN 978-1-405-15627-1. [Google Scholar]
- Mohammed, K.; Abba, Y.; Ramli, N.S.B.; Marimuthu, M.; Omar, M.A.; Abdullah, F.F.J.; Sadiq, M.A.; Tijjani, A.; Chung, E.L.T.; Lila, M.A.M. The use of FAMACHA in estimation of gastrointestinal nematodes and total worm burden in Damara and Barbados Blackbelly cross sheep. Trop. Anim. Health Prod. 2016, 48, 1013–1020. [Google Scholar] [CrossRef]
- Russel, A. Body condition scoring of sheep and goats. Practice 1984, 6, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M.; Burke, J.M.; Terrill, T.H.; Miller, J.E.; Getz, W.R.; Mobini, S.; Valencia, E.; Williams, M.J.; Williamson, L.H.; Larsen, M.; et al. Validation of the FAMACHA© eye color chart for detecting clinical anemia in sheep and goats on farms in the southern United States. Vet. Parasitol. 2004, 123, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Schalm’s Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1986. [Google Scholar]
- Jackson, P.G.G.; Cockcroft, P.D. Clinical Examination of Farm Animals; Blackwell Science Ltd.: Oxford, UK, 2007; ISBN 9780470752425. [Google Scholar]
- Sergeant, E.S. Epitools Epidemiological Calculators. Available online: https://epitools.ausvet.com.au/ (accessed on 15 September 2020).
- Ikeme, M.M.; Iskander, F.; Chong, L.C. Seasonal changes in the prevalence of Haemonchus and Trichostrongylus hypobiotic larvae in tracer goats in Malaysia. Trop. Anim. Health Prod. 1987, 19, 184–190. [Google Scholar] [CrossRef]
- Jesse, F.F.A.; Jazid, N.H.B.A.; Mohammed, K.; Tijjani, A.; Chung, E.L.T.; Abba, Y.; Sadiq, M.; Saharee, A.A. Hemotropic Mycoplasma ovis infection in goats with concurrent gastrointestinal parasitism in Malaysia. J. Adv. Vet. Anim. Res. 2015, 2, 464. [Google Scholar] [CrossRef]
- Jesse, F.F.; Abba, Y.; Peter, I.D.; Bitrus, A.A.; Hambali, I.U.; Jamaluddin, N.L.; Haron, A.W. Clinical Management of Parasitic Gastroenteritis (PGE) Concurrent with Mycoplasmosis and Orf in Sheep. Adv. Anim. Vet. Sci. 2017, 5, 358–361. [Google Scholar]
- Souza, U.A.; Oberrather, K.; Fagundes-Moreira, R.; Almeida, B.A.; Valle, S.D.; Girotto-Soares, A.; Soares, J.F. First molecular detection of Mycoplasma ovis (Hemotropic mycoplasmas) from Sheep in Brazil. Rev. Bras. Parasitol. Veterinária 2019, 28, 360–366. [Google Scholar] [CrossRef]
- Paul, B.T.; Jesse, F.F.A.; Chung, E.L.T.; Che-amat, A.; Mohd Lila, M.A.; Hashi, H.A.; Norsidin, M.J. Review of clinical aspects, epidemiology and diagnosis of haemotropic Mycoplasma ovis in small ruminants: Current status and future perspectives in tropics focusing on Malaysia. Trop. Anim. Health Prod. 2020, 52, 18. [Google Scholar] [CrossRef]
- Dey, A.R.; Begum, N.; Alim, M.A.; Malakar, S.; Islam, M.T.; Alam, M.Z. Gastrointestinal nematodes in goats in Bangladesh: A large-scale epidemiological study on the prevalence and risk factors. Parasite Epidemiol. Control 2020, 9, e00146. [Google Scholar] [CrossRef]
- Shah-Fischer, M.; Say, R.R. Manual of Tropical Veterinary Parasitology; CAB International: Wallingford, UK, 1989. [Google Scholar]
- Waller, P.J. From discovery to development: Current industry perspectives for the development of novel methods of helminth control in livestock. Vet. Parasitol. 2006, 139, 1–14. [Google Scholar] [CrossRef]
- Waller, P.J. Management and control of nematode parasites of small ruminants in the face of total anthelmintic failure. Trop. Biomed. 2004, 21, 7–13. [Google Scholar]
- Tsotetsi, A.M.; Mbati, P.A. Parasitic helminths of veterinary importance in cattle, sheep and goats on communal farms in the northeastern Free State, South Africa. J. S. Afr. Vet. Assoc. 2003, 74, 45–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zvinorova, P.I.; Halimani, T.E.; Muchadeyi, F.C.; Matika, O.; Riggio, V.; Dzama, K. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin. Res. 2016, 143, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Egbe-Nwiyi, T.N.; Paul, B.T.; Cornelius, A.C. Coprological detection of equine nematodes among slaughtered donkeys (Equus asinus) in Kaltungo, Nigeria. Vet. World 2019, 12, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Idika, I.K.; Iheagwam, C.N.; Ezemonye, C.N.; Nwosu, C.O. Gastrointestinal Nematodes and Body Condition Scores of Goats Slaughtered in Nsukka, Nigeria. Niger. Vet. J. 2012, 33, 440–447. [Google Scholar]
- Hansen, J.; Perry, B. Techniques for parasite assays and identification in faecal samples. In Epidemiology Diagnosis and Control of Helminth Parasites of Ruminants; International Laboratory for Research on Animal Diseases: Nairobi, Kenya; FAO: Rome, Italy, 1994; pp. 1–15. [Google Scholar]
- Jalila, A.; Dorny, P.; Sani, R.; Salim, N.B.; Vercruysse, J. Coccidial infections of goats in Selangor, peninsular Malaysia. Vet. Parasitol. 1998, 74, 165–172. [Google Scholar] [CrossRef]
- Foreyt, W.J. Coccidiosis and cryptosporidiosis in sheep and goats. Vet. Clin. N. Am. Food Anim. Pract. 1990, 6, 655–670. [Google Scholar] [CrossRef]
- Ruiz, A.; González, J.F.; Rodríguez, E.; Martín, S.; Hernández, Y.I.; Almeida, R.; Molina, J.M. Influence of climatic and management factors on Eimeria infections in goats from semi-arid zones. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Heal. 2006, 53, 399–402. [Google Scholar] [CrossRef]
- Diop, G.; Yanagida, T.; Hailemariam, Z.; Menkir, S.; Nakao, M.; Sako, Y.; Ba, C.T.; Ito, A. Genetic characterization of Moniezia species in Senegal and Ethiopia. Parasitol. Int. 2015, 64, 256–260. [Google Scholar] [CrossRef]
- Ermilov, S.G. Additions to the oribatid mite fauna of Malaysia, with description of a new species of the genus Lohmannia (Acari, Oribatida, Lohmanniidae). Acarina 2016, 24, 159–165. [Google Scholar] [CrossRef][Green Version]
- Ermilov, S.G.; Kalúz, S. New faunistic and taxonomic data on oribatid mites (Acari: Oribatida) of Malaysia. Biologia (Bratisl) 2020. [Google Scholar] [CrossRef]
- Roepstorff, A.; Nansen, P. Epidemiology, Diagnosis and Control of Helminth Parasites of Swine FAO Animal Health Manual No. 3; Food and Agriculture Organization: Copenhagen, Denmark, 1998; ISBN 1170-229X. [Google Scholar]
- Dugassa, J.; Hussein, A.; Kebede, A.; Mohammed, C. Prevalence and Associated Risk Factors of Gastrointestinal Nematodes of Sheep and Goats in ZiwayDugda District, Eastern Arsi Zone of Oromia Regional State, Ethiopia. ARC J. Anim. Vet. Sci. 2018, 4, 6–14. [Google Scholar] [CrossRef]
- Odoi, A.; Gathuma, J.M.; Gachuiri, C.K.; Omore, A. Risk factors of gastrointestinal nematode parasite infections in small ruminants kept in smallholder mixed farms in Kenya. BMC Vet. Res. 2007, 3. [Google Scholar] [CrossRef] [PubMed]

| Factor | Examined | Prevalence (%) | 95% CI * |
|---|---|---|---|
| Breed | |||
| Saanen goat | 33 | 33 (100) | 89.57–100.00 |
| Boer goat | 224 | 176 (78.6) | 72.74–83.44 |
| Gender | |||
| Male | 68 | 41(60.3) | 48.41–71.07 |
| Female | 189 | 168 (88.9) | 83.61–92.62 |
| Age | |||
| Young | 54 | 25 (46.3) | 33.69–59.40 |
| Adult | 203 | 184 (90.6) | 85.84–93.93 |
| Physiological Status | |||
| Immature | 52 | 25 (48.1) | 35.11–61.32 |
| Mating stock | 148 | 131 (88.5) | 82.37–92.70 |
| Lactating | 30 | 29 (96.7) | 83.33–99.41 |
| Pregnant | 27 | 24 (88.9) | 71.94–96.15 |
| Body Condition Score | |||
| Fat | 22 | 18 (81.8) | 61.49–92.69 |
| Average | 201 | 162 (80.6) | 74.58–85.47 |
| Thin | 34 | 29 (85.3) | 69.87–93.55 |
| FAMACHA© Score | |||
| Severely anemic | 07 | 06 (85.7) | 48.68–97.43 |
| Mildly anemic | 88 | 73 (83.0) | 73.76–89.39 |
| Anemic | 160 | 130 (81.3) | 74.50–86.54 |
| Non-anemic | 02 | 00 (0.0) | 0.00–65.76 |
| 1 PCV Categories | |||
| Non-Anemic | 211 | 171 (81.0) | 75.21–85.76 |
| Anemic | 46 | 38 (82.6) | 69.28–90.92 |
| Flocks | |||
| A-Lenggeng | 91 | 87 (95.6) | 89.23–98.28 |
| B-Senawang | 55 | 48 (87.3) | 75.98–93.69 |
| C-Seremban | 75 | 45 (60.0) | 48.69–70.34 |
| D-Mendom | 36 | 29 (80.6) | 64.98–90.25 |
| Production Purpose | |||
| Dairy | 33 | 33 (100) | 89.57–100 |
| Meat | 224 | 176 (78.6) | 72.74–83.44 |
| Overall | 257 | 209 (81.32) | 76.11–85.61 |
| Categories of Infection | Positive | Prevalence (%) | 95% CI * |
|---|---|---|---|
| Strongyle eggs | 43 | 16.7 | 12.66–21.78 |
| Eimeria oocysts | 11 | 4.3 | 2.41–7.50 |
| Strongyle + Eimeria | 130 | 50.6 | 44.50–56.64 |
| Strongyle + Eimeria + Moniezia | 18 | 7.0 | 4.47–10.79 |
| Strongyle + Moniezia | 02 | 0.8 | 0.21–2.80 |
| Strongyle + Trichuris | 01 | 0.4 | 0.07–2.17 |
| Eimeria + Moniezia | 03 | 1.2 | 0.40–3.38 |
| Strongyle + Eimeria + Trichuris | 01 | 0.4 | 0.07–2.17 |
| Factor | EPG (Mean ± SE) | OPG (Mean ± SE) |
|---|---|---|
| Breed | ||
| Saanen goat | 1345.83 ± 322.71 a | 7691.38 ± 2848.90 1 |
| Boer goat | 697.19 ± 73.99 b | 1072.89 ± 122.63 2 |
| Gender | ||
| Male | 403.23 ± 120.86 a | 6520.31 ± 2435.04 1 |
| Female | 858.50 ± 1108.07 b | 1199.64 ± 263.12 2 |
| Age | ||
| Young | 726.47 ± 234.84 | 13980.00 ± 5026.60 1 |
| Adult | 787.43 ± 82.90 | 1062.18 ± 1517.32 2 |
| Physiological Status | ||
| Immature | 726.47 ± 234.84 | 13980.00 ± 5026.60 1 |
| Mating stock | 707.46 ± 101.79 | 1194.95 ± 161.64 2 |
| Lactating | 1177.59 ± 227.75 | 862.00 ± 195.31 2 |
| Pregnant | 695.83 ± 131.60 | 631.82 ± 204.85 2 |
| Body Condition Score | ||
| Fat | 526.47 ± 112.22 a | 733.33 ± 243.91 |
| Average | 676.43 ± 69.37 a | 2350.00 ± 653.83 |
| Thin | 1488.89 ± 362.63 b | 2258.33 ± 957.76 |
| FAMACHA© Score | ||
| Severely anemic | 1416.67 ± 483.51 | 3150.00 ± 2426.16 1 |
| Mildly Anemic | 793.44 ± 143.14 | 4182.54 ± 1366.88 1 |
| Anemic | 743.16 ± 94.40 | 954.81 ± 124.26 2 |
| 1 PCV Categories | ||
| Non-Anemic (PCV > 22%) | 702.38 ± 83.95 a | 2302.86 ± 594.33 |
| Anemic (PCV ≤ 22%) | 1097.30 ± 193.18 b | 1709.68 ± 1069.38 |
| Flocks | ||
| A-Lenggeng | 951.45 ± 162.26 a | 3639.10 ± 1111.78 |
| B-Senawang | 713.33 ± 78.04 | 863.83 ± 130.75 |
| C-Seremban | 317.86 ± 52.38 b | 693.48 ± 261.01 |
| D-Mendom | 1169.64 ± 256.64 a | 1521.74 ± 531.43 |
| Production purpose | ||
| Dairy | 1345.322.71 ± 322.71 a | 7691.38 ± 2848.90 1 |
| Meat | 697.19 ± 73.99 b | 1072.89 ± 122.63 2 |
| Factor | Number | Positive | χ2 | p |
|---|---|---|---|---|
| Breed | ||||
| Saanen goat | 33 | 33 (100) | 8.695 | 0.003 * |
| Boer goat | 224 | 176 (78.6) | ||
| Gender | ||||
| Male | 68 | 41(60.3) | 26.921 | 0.000 * |
| Female | 189 | 168 (88.9) | ||
| Age | ||||
| Young | 54 | 25 (46.3) | 55.221 | 0.000 * |
| Adult | 203 | 184 (90.6) | ||
| Body Condition Score | ||||
| Fat | 22 | 18 (81.8) | 0.426 | 0.808 |
| Average | 201 | 162 (80.6) | ||
| Thin | 34 | 29 (85.3) | ||
| Physiological Status | ||||
| Immature | 52 | 25 (48.1) | 48.547 | 0.000 * |
| Mating stock | 148 | 131 (88.5) | ||
| Lactating | 30 | 29 (96.7) | ||
| Pregnant | 27 | 24 (88.9) | ||
| FAMACHA© Score | ||||
| Severely anemic | 07 | 06 (85.7) | 8.952 | 0.030 * |
| Mildly anemic | 88 | 73 (83.0) | ||
| Anemic | 160 | 130 (81.3) | ||
| Non-anaemic | 02 | 00 (0.0) | ||
| 1 PCV Categories | ||||
| Non-anaemic | 211 | 171 (81.0) | 0.061 | 0.805 |
| Anaemic | 46 | 38 (82.6) | ||
| Flocks | ||||
| A-Lenggeng | 91 | 87 (95.6) | 35.967 | 0.000 * |
| B-Senawang | 55 | 48 (87.3) | ||
| C-Seremban | 75 | 45 (60.0) | ||
| D-Mendom | 36 | 29 (80.6) | ||
| Production Purpose | ||||
| Dairy | 33 | 33 (100) | 8.695 | 0.003 * |
| Meat | 224 | 176 (78.6) |
| Risk Factor | β | SE | df | p | AOR | 95% CI |
|---|---|---|---|---|---|---|
| Gender (Female) | 1.16 | 0.42 | 1 | 0.005 * | 3.1882 | 1.41–7.19 |
| Age (Adult) | 2.39 | 0.42 | 1 | 0.000 * | 11.007 | 4.81–25.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, B.T.; Jesse, F.F.A.; Chung, E.L.T.; Che’Amat, A.; Mohd Lila, M.A. Risk Factors and Severity of Gastrointestinal Parasites in Selected Small Ruminants from Malaysia. Vet. Sci. 2020, 7, 208. https://doi.org/10.3390/vetsci7040208
Paul BT, Jesse FFA, Chung ELT, Che’Amat A, Mohd Lila MA. Risk Factors and Severity of Gastrointestinal Parasites in Selected Small Ruminants from Malaysia. Veterinary Sciences. 2020; 7(4):208. https://doi.org/10.3390/vetsci7040208
Chicago/Turabian StylePaul, Bura Thlama, Faez Firdaus Abdullah Jesse, Eric Lim Teik Chung, Azlan Che’Amat, and Mohd Azmi Mohd Lila. 2020. "Risk Factors and Severity of Gastrointestinal Parasites in Selected Small Ruminants from Malaysia" Veterinary Sciences 7, no. 4: 208. https://doi.org/10.3390/vetsci7040208
APA StylePaul, B. T., Jesse, F. F. A., Chung, E. L. T., Che’Amat, A., & Mohd Lila, M. A. (2020). Risk Factors and Severity of Gastrointestinal Parasites in Selected Small Ruminants from Malaysia. Veterinary Sciences, 7(4), 208. https://doi.org/10.3390/vetsci7040208





