Seroprevalence of Mycobacterium avium subsp. paratuberculosis in Dairy Cattle in Khartoum State, Sudan
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
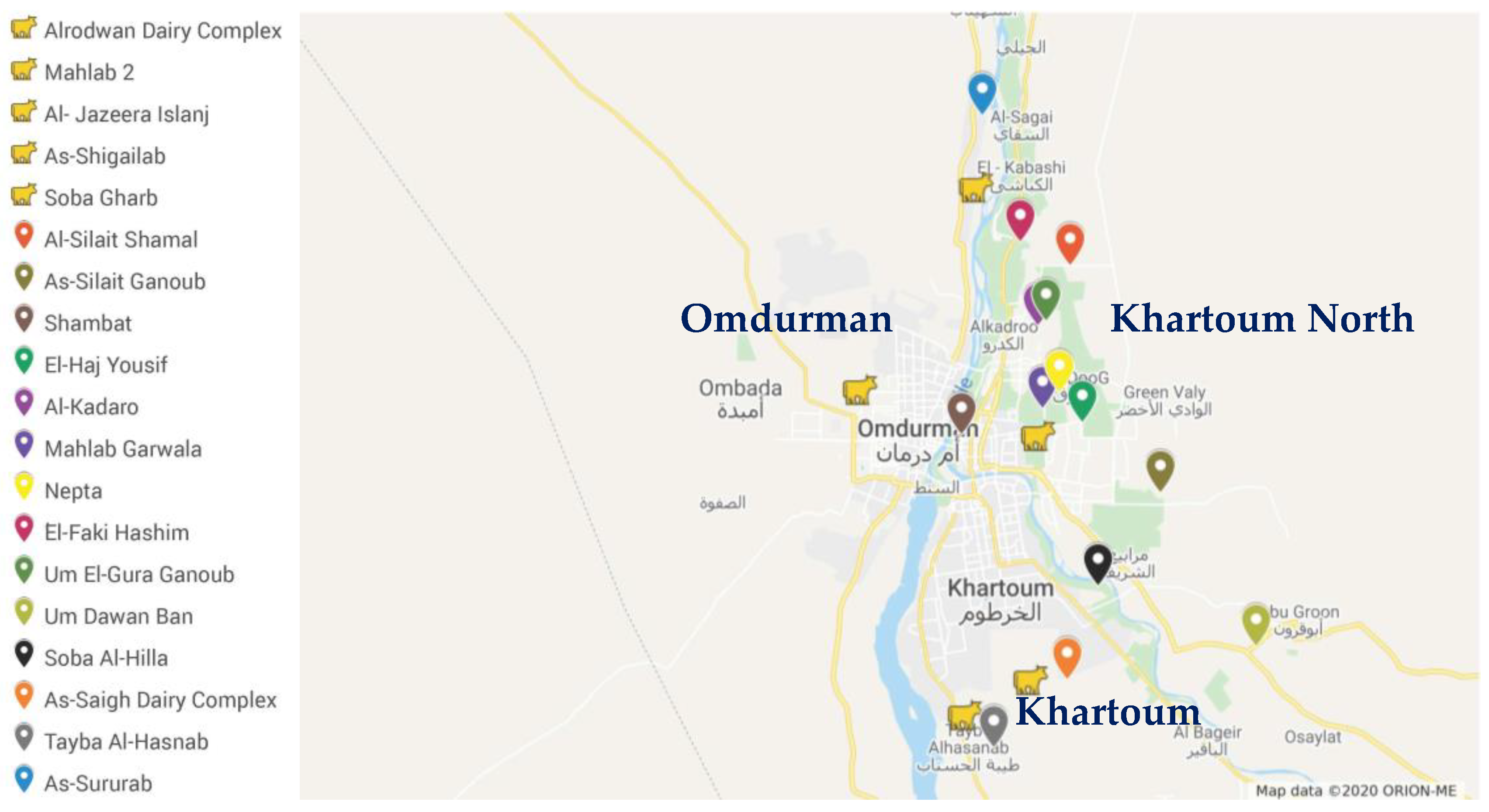
2.2. Study Population
2.3. Enzyme Linked Immuno Sorbent Assay (ELISA) Procedure
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fawzy, A.; Zschöck, M.; Ewers, C.; Eisenberg, T. Genotyping methods and molecular epidemiology of Mycobacterium avium subsp. paratuberculosis (MAP). Int. J. Vet. Sci. Med. 2018, 6, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rathnaiah, G.; Zinniel, D.K.; Bannantine, J.P.; Stabel, J.R.; Gröhn, Y.T.; Collins, M.T.; Barletta, R.G. Pathogenesis, Molecular Genetics, and Genomics of Mycobacterium avium subsp. paratuberculosis, the Etiologic Agent of Johne’s Disease. Front. Vet. Sci. 2017, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Mycobacterium avium subspecies paratuberculosis: A possible causative agent in human morbidity and risk to public health safety. Open Vet. J. 2018, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Ridler, A.; Wilson, P.R.; Heuer, C. Control of clinical paratuberculosis in New Zealand pastoral livestock. N. Z. Vet. J. 2017, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Immune-based diagnosis of paratuberculosis. In Paratuberculosis, Organism, Disease and Control; Behr, M.A., Collins, D.M., Eds.; CABI Head Office: Wallingford, Oxfordshire, UK, 2010; pp. 284–291. [Google Scholar]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef]
- Behr, M.A. Paratuberculosis and Crohn’s disease. In Paratuberculosis, Organism, Disease and Control; Behr, M.A., Collins, D.M., Eds.; CABI Head Office: Wallingford, Oxfordshire, UK, 2010; pp. 40–50. [Google Scholar]
- Barkema, H.W.; Hesselink, J.W.; McKenna, S.; Benedictus, G.; Groenendaal, H. Global prevalence and economics of infection with Mycobacterium avium subsp. paratuberculosis in ruminants. In Paratuberculosis: Organism, Disease, Control; CABI Publishing: Wallingford, Oxfordshire, UK, 2010; pp. 10–21. [Google Scholar]
- Whittington, R.J.; Begg, D.J.; De Silva, K.; Purdie, A.C.; Dhand, N.K.; Plain, K.M. Case definition terminology for paratuberculosis (Johne’s disease). BMC Vet. Res. 2017, 13, 1–13. [Google Scholar] [CrossRef]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P. Veterinary Medicine, 10th ed.; Saunders: Philadelphia, PA, USA, 2007. [Google Scholar]
- Whittington, R.J.; Marsh, I.B.; Saunders, V.; Grant, I.R.; Juste, R.; Sevilla, I.A.; Manning, E.J.; Whitlock, R.H. Culture phenotypes of genomically and geographically diverse Mycobacterium avium subsp. paratuberculosis isolates from different hosts. J. Clin. Microbiol. 2011, 49, 1822–1830. [Google Scholar] [CrossRef]
- Bannantine, J.P.; Paustian, M.L.; Kapur, V.; Eda, S. Proteome and antigen of Mycobacterium avium subsp. paratuberculosis. In Paratuberculosis Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CABI Head Office: Wallingford, Oxfordshire, UK, 2010; pp. 94–109. [Google Scholar]
- Fernández-Silva, J.; Correa-Valencia, N.M.; Ramírez, N.F. Systematic review of the prevalence of paratuberculosis in cattle, sheep, and goats in Latin America and the Caribbean. Trop. Anim. Heal. Prod. 2014, 46, 1321–1340. [Google Scholar] [CrossRef]
- Imada, J.; Kelton, F.D.; Barkema, H.W. Epidemiology, Global Prevalence and Economics of the Infection. In Paratuberculosis, Organism, Disease, Control, 2nd ed.; Behr, M.A., Stevenson, K., Kapur, V., Eds.; CABI Publishing: Wallingford, Oxfordshire, UK, 2020; pp. 1–13. [Google Scholar]
- Lee, K.W.; Jung, B.Y. Seroprevalence of Mycobacterium avium subspecies paratuberculosis in cattle in Korea. Vet. Rec. 2009, 165, 661–662. [Google Scholar] [CrossRef]
- Sun, W.-W.; Lv, W.-F.; Cong, W.; Meng, Q.-F.; Wang, C.-F.; Shan, X.-F.; Qian, A.-D. Mycobacterium avium subspecies paratuberculosis and Bovine Leukemia Virus Seroprevalence and Associated Risk Factors in Commercial Dairy and Beef Cattle in Northern and Northeastern China. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Gurung, R.B.; Begg, D.J.; Whittington, R.J. A national serosurvey to determine the prevalence of paratuberculosis in cattle in Bhutan following detection of clinical cases. Vet. Med. Sci. 2018, 4, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Singh, A.V.; Singh, R.; Sharma, S.; Shukla, N.; Misra, S.; Singh, P.; Sohal, J.S.; Kumar, H.; Patil, P.K.; et al. Sero-prevalence of Bovine Johne’s disease in buffaloes and cattle population of North India using indigenous ELISA kit based on native Mycobacterium avium subspecies paratuberculosis ‘Bison type’ genotype of goat origin. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Prince, A.; Hassan, A.A.; Fayed, A.; Zschöck, M.; Naga, M.; Omar, M.; Salem, M.; El-Sayed, A. Epidemiological studies on Johne’s disease in ruminants and Crohn’s disease in humans in Egypt. Int. J. Vet. Sci. Med. 2013, 1, 79–86. [Google Scholar] [CrossRef][Green Version]
- Okuni, J.B.; Loukopoulos, P.; Reinacher, M.; Ojok, L. Seroprevalence of Mycobacterium avium subspecies paratuberculosis Antibodies in Cattle from Wakiso, Mpigi and Luwero Districts in Uganda. Int. J. Anim. Vet. Adv. 2011, 3, 156–160. [Google Scholar]
- Mpenda, F.N.; Buza, J. Seroprevalence of bovine paratuberculosis in Arusha, Northern Tanzania. Am. J. Res. Comm. 2014, 2, 97–104. [Google Scholar]
- Chaudhary, A.Q.; Fawi, M.T.; Obeid, H.M. Johne’s disease in goats in the Sudan. Vet. Rec. 1964, 76, 246–247. [Google Scholar]
- Fawi, M.T.; Obeid, H.M. A note on Johne’s disease among cattle in the Sudan. Bull. Epiz. Dis. Afr. 1964, 12, 437. [Google Scholar]
- Mohammed, K.B.; El-Eragi, A.; Zakia, A.M. Seroprevalence of Bovine Paratuberculosis Specific Antibodies in Khartoum and Al-Jazeera States, Sudan. J. Anim. Vet. Adv. 2010, 9, 2098–2101. [Google Scholar] [CrossRef]
- Mortier, R.A.; Barkema, H.W.; De Buck, J. Susceptibility to and diagnosis of Mycobacterium avium subspecies paratuberculosis infection in dairy calves: A review. Prev. Vet. Med. 2015, 121, 189–198. [Google Scholar] [CrossRef]
- Okuni, J.B.; Hansen, S.; Eltom, K.H.; Eltayeb, E.; Amanzada, A.; Omega, J.A.; Czerny, C.-P.; El Wahed, A.A.; Ojok, L. Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms 2020, 8, 1007. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Enevoldsen, C.; Gröhn, Y.T. The Mycobacterium avium subsp. paratuberculosis ELISA response by parity and stage of lactation. Prev. Vet. Med. 2002, 54, 1–10. [Google Scholar] [CrossRef]
| Area | Animal Level | Herd Level | ||||
|---|---|---|---|---|---|---|
| No. Tested | Positive | No. Tested | Positive | |||
| No. | % | No. | % | |||
| Khartoum | 51 * | 2 | 3.9 | 11 | 2 | 18.2 |
| Khartoum North | 78 | 4 | 5.1 | 15 | 2 | 13.3 |
| Omdurman | 45 | 5 | 11.1 | 9 | 3 | 22.2 |
| Total | 175 | 11 | 6.3 | 37 | 7 | 18.9 |
| Result | Age | Body Condition | Herd Size | Sex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >3 | <3 | Total | Emaciated | Good | Total | L | M | S | Total | Female | Male | Total | ||
| Negative | Count | 119 | 45 | 164 | 28 | 136 | 164 | 44 | 81 | 39 | 164 | 137 | 27 | 164 |
| % of total | 68.0 | 25.7 | 93.7 | 16 | 77.7 | 93.7 | 24.9 | 46.3 | 22.3 | 93.7 | 78.3 | 15.4 | 93.7 | |
| Positive | Count | 10 | 1 | 11 | 0 | 11 | 11 | 2 | 6 | 3 | 11 | 10 | 1 | 11 |
| % of total | 5.7 | 0.6 | 6.3 | 0.0 | 6.3 | 6.3 | 1.2 | 3.4 | 1.7 | 6.3 | 5.7 | 0.6 | 6.3 | |
| Total | Count | 129 | 46 | 175 | 28 | 147 | 175 | 46 | 87 | 42 | 175 | 147 | 28 | 175 |
| % of total | 73.7 | 26.3 | 100.0 | 16.0 | 84.0 | 100.0 | 26.3 | 49.7 | 24.0 | 100.0 | 84.0 | 16.0 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmagzoub, W.A.; Adam, N.M.; Idris, S.M.; Mukhtar, M.E.; Abdelaziz, S.A.; Okuni, J.B.; Ojok, L.; Abd El Wahed, A.; Eltayeb, E.; Gameel, A.A.; et al. Seroprevalence of Mycobacterium avium subsp. paratuberculosis in Dairy Cattle in Khartoum State, Sudan. Vet. Sci. 2020, 7, 209. https://doi.org/10.3390/vetsci7040209
Elmagzoub WA, Adam NM, Idris SM, Mukhtar ME, Abdelaziz SA, Okuni JB, Ojok L, Abd El Wahed A, Eltayeb E, Gameel AA, et al. Seroprevalence of Mycobacterium avium subsp. paratuberculosis in Dairy Cattle in Khartoum State, Sudan. Veterinary Sciences. 2020; 7(4):209. https://doi.org/10.3390/vetsci7040209
Chicago/Turabian StyleElmagzoub, Wisal A., Nabawia M. Adam, Sanaa M. Idris, Mohamed E. Mukhtar, Sanaa A. Abdelaziz, Julius B. Okuni, Lonzy Ojok, Ahmed Abd El Wahed, ElSagad Eltayeb, Ahmed A. Gameel, and et al. 2020. "Seroprevalence of Mycobacterium avium subsp. paratuberculosis in Dairy Cattle in Khartoum State, Sudan" Veterinary Sciences 7, no. 4: 209. https://doi.org/10.3390/vetsci7040209
APA StyleElmagzoub, W. A., Adam, N. M., Idris, S. M., Mukhtar, M. E., Abdelaziz, S. A., Okuni, J. B., Ojok, L., Abd El Wahed, A., Eltayeb, E., Gameel, A. A., & Eltom, K. H. (2020). Seroprevalence of Mycobacterium avium subsp. paratuberculosis in Dairy Cattle in Khartoum State, Sudan. Veterinary Sciences, 7(4), 209. https://doi.org/10.3390/vetsci7040209






