Effects of Synthetic Acaricides and Nosema ceranae (Microsporidia: Nosematidae) on Molecules Associated with Chemical Communication and Recognition in Honey Bees
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Experiment I—Effect of Nosema ceranae Infection and Ethanol
2.2.2. Experiment II—Effect of Acaricides
2.2.3. Experiment III—Effect of the Combination of Nosema ceranae Infection and Coumaphos
2.3. Recorded Variables
2.3.1. Survival and Diet Consumption
2.3.2. Nosema ceranae Development
2.3.3. EO and CHC Quantification
3. Statistical Analyses
4. Results
4.1. Experiment I—Effect of Nosema ceranae Infection on EO Production and CHC Profile
4.1.1. Nosema ceranae Development
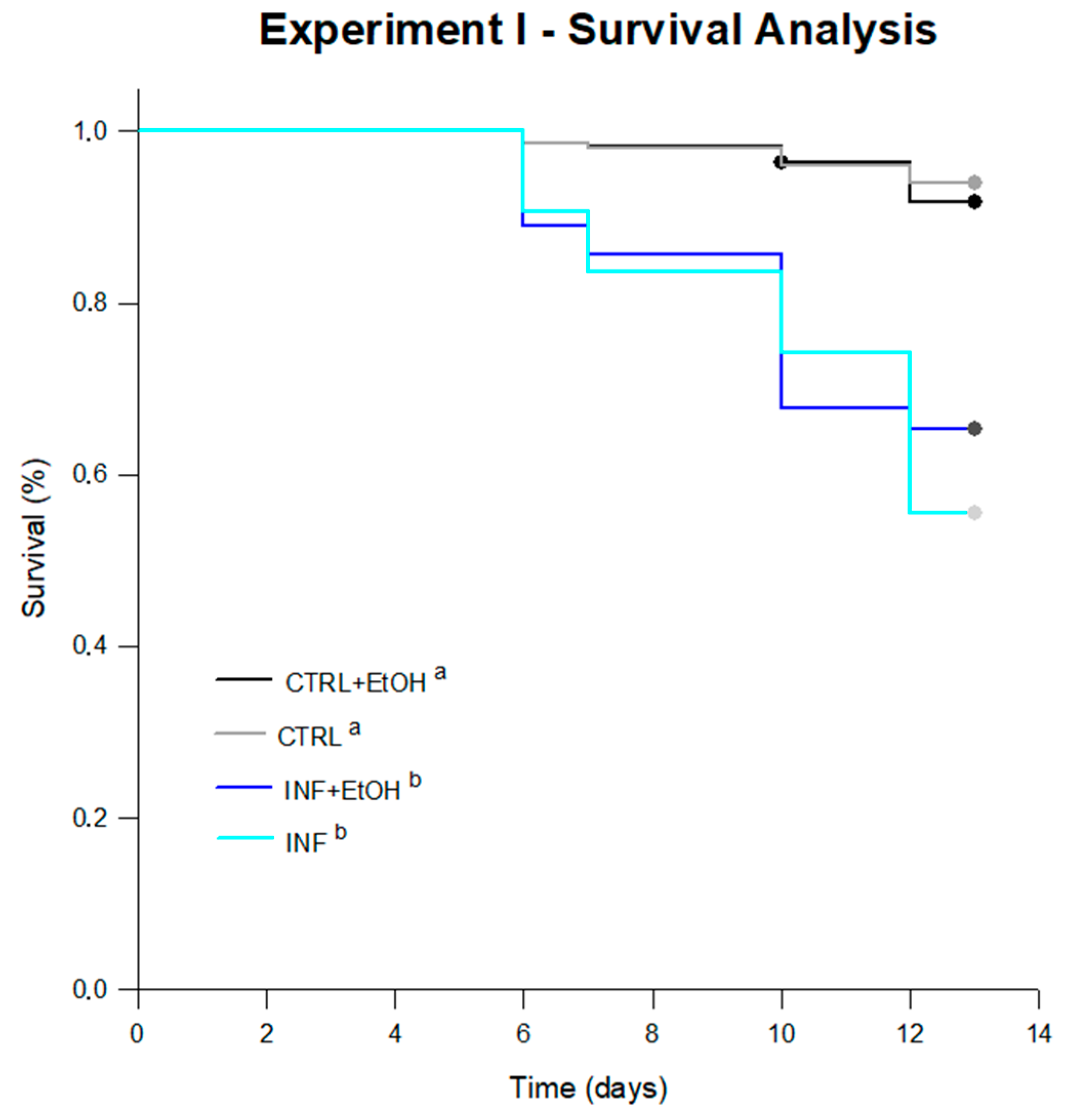
4.1.2. Survival and Diet Consumption
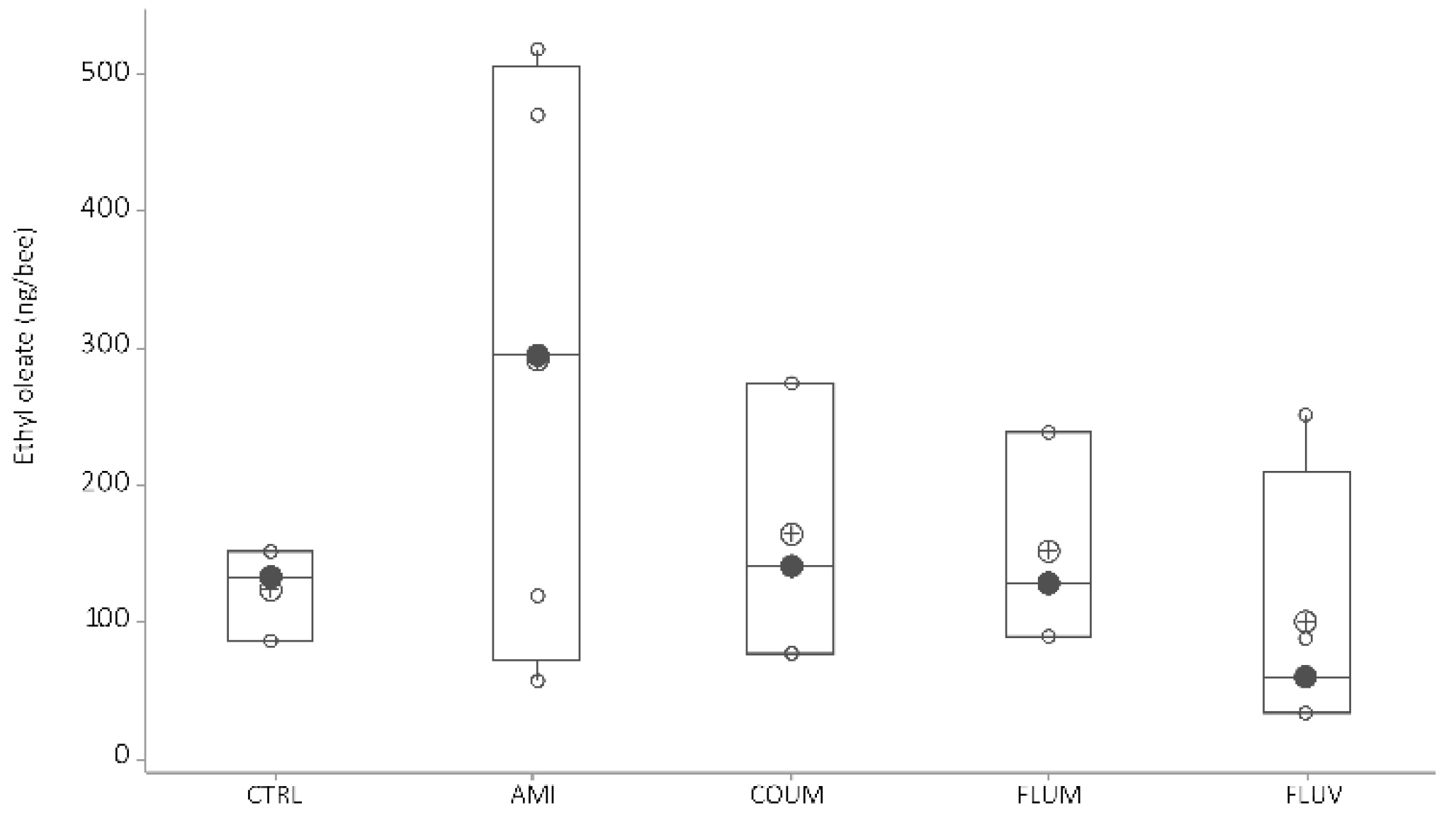
4.1.3. Ethyl Oleate
4.1.4. Cuticular Hydrocarbons
4.2. Experiment II–Effect of Acaricides on EO Production and CHC Profile
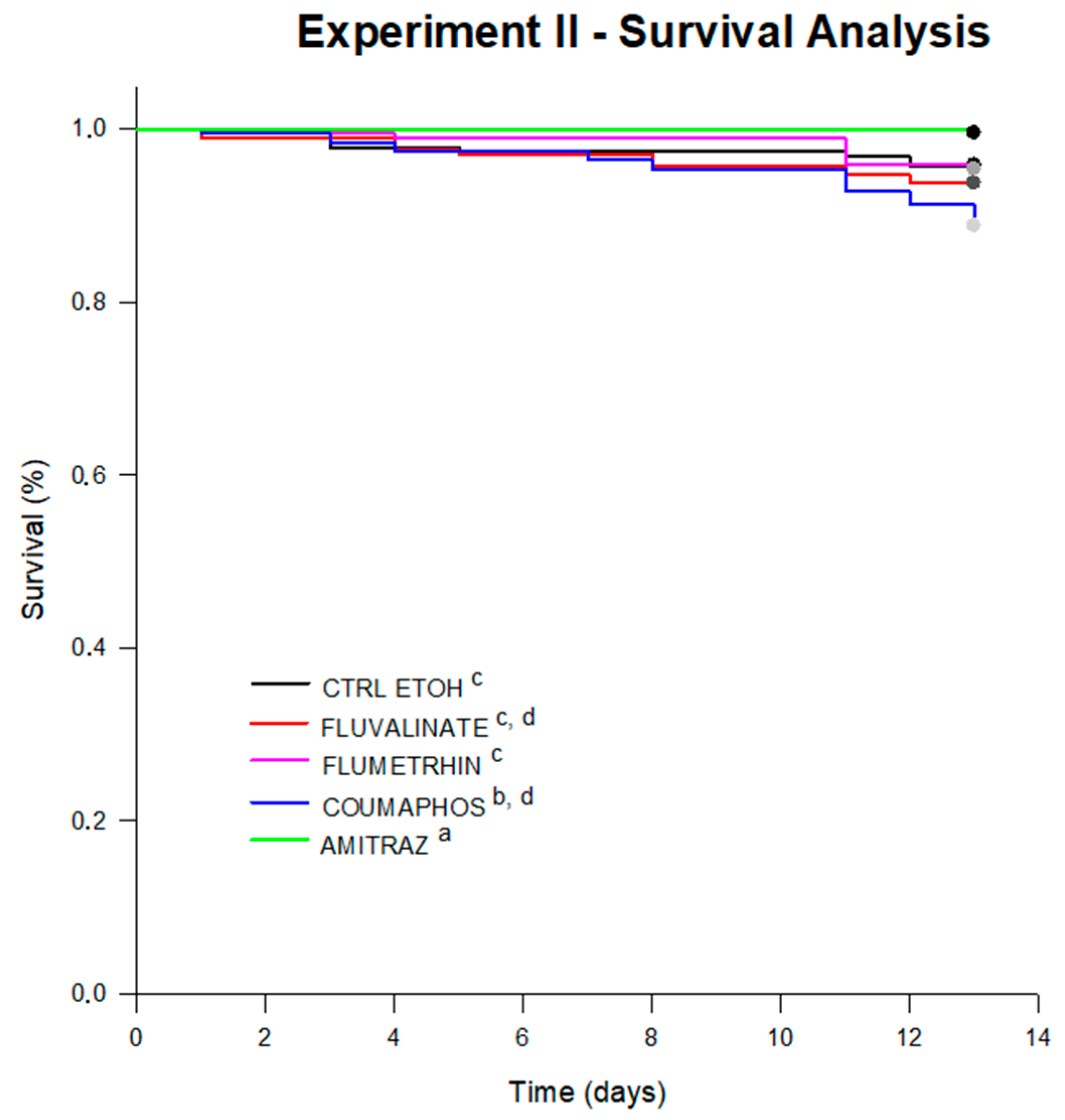
4.2.1. Survival and Diet Consumption
4.2.2. Cuticular Hydrocarbons
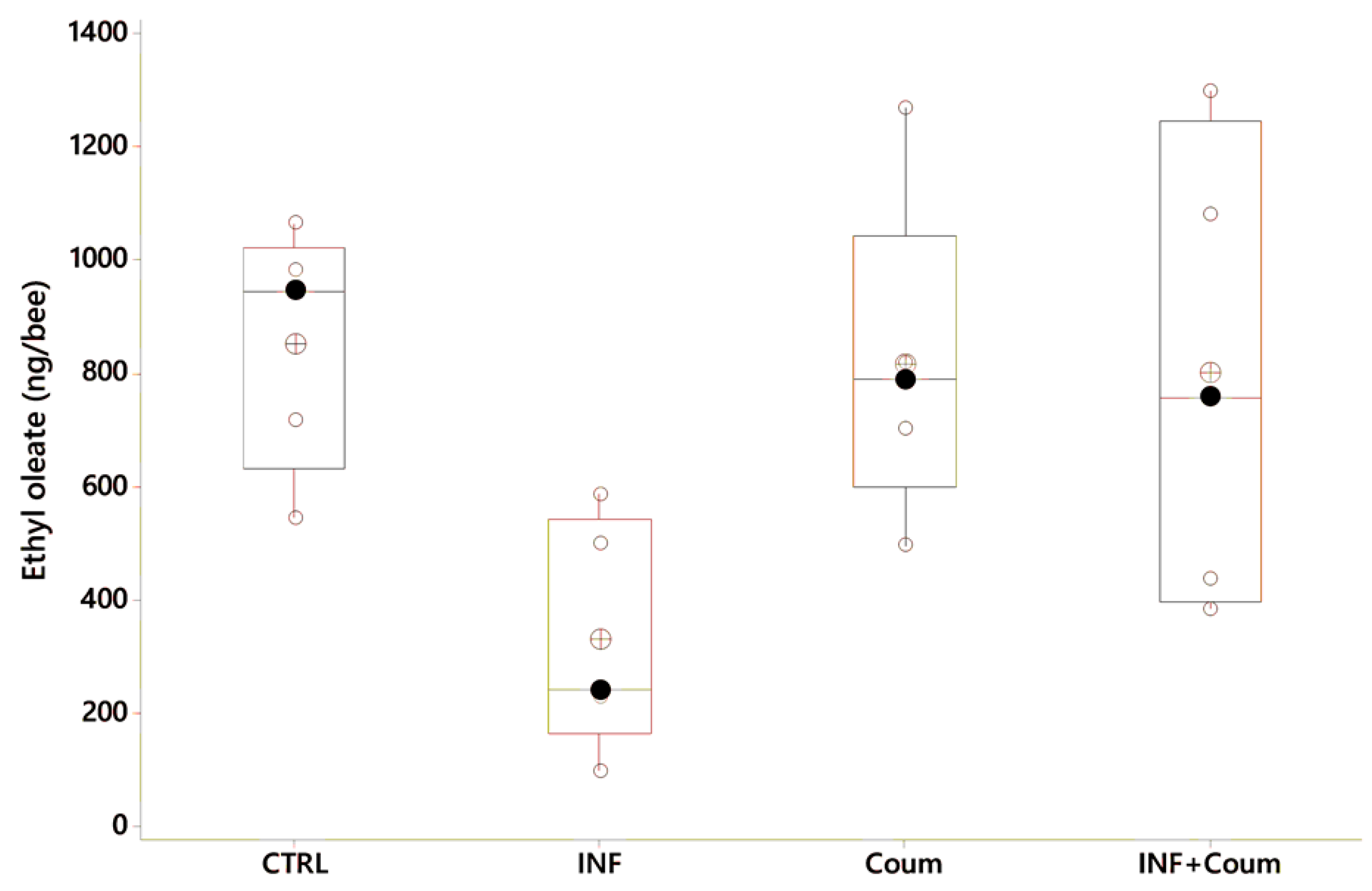
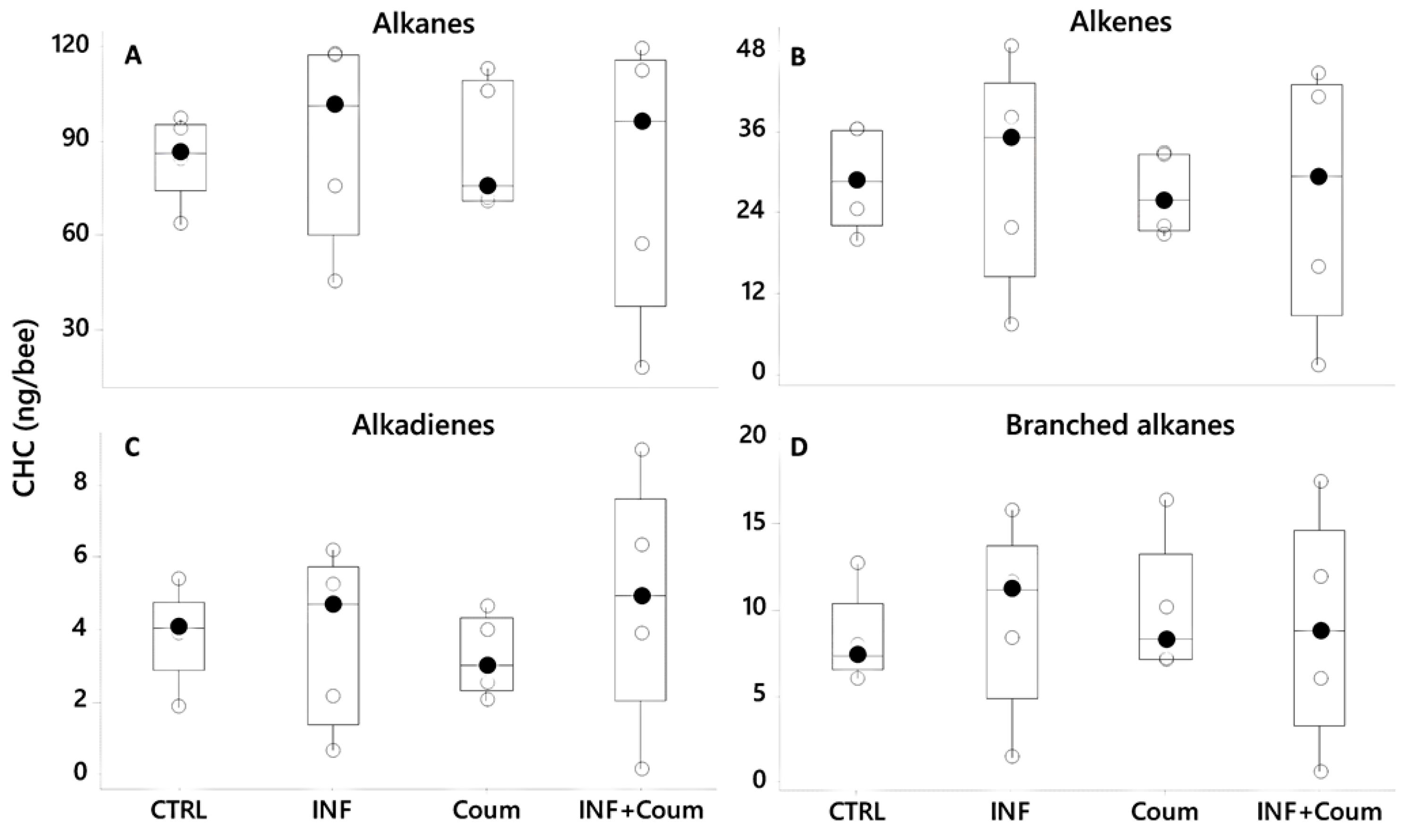
4.3. Experiment III—Effect of Nosema ceranae Infection and Coumaphos on EO Production and CHC Profile
4.3.1. Nosema ceranae Development
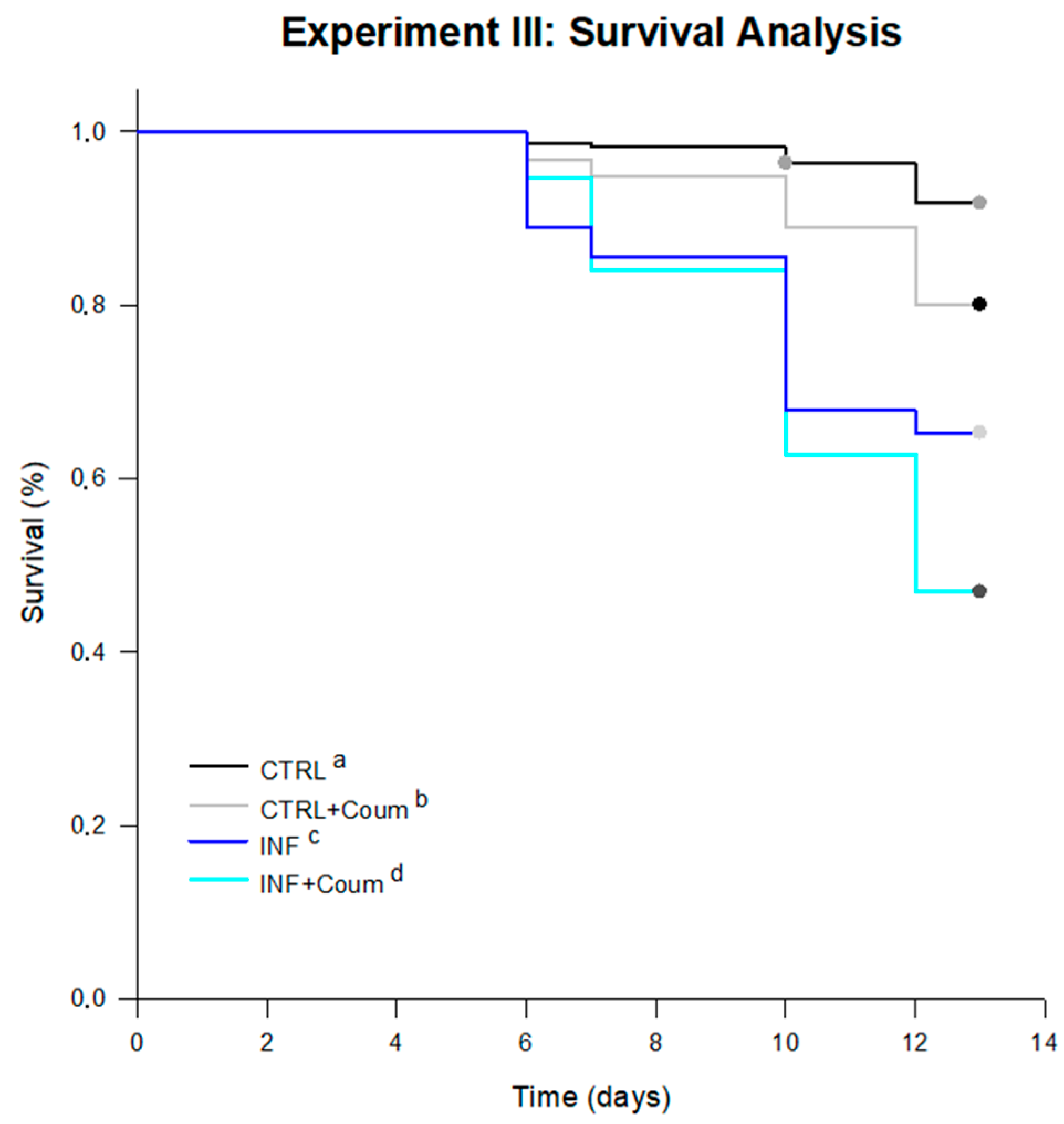
4.3.2. Survival and Diet Consumption
4.3.3. Ethyloleate
4.3.4. Cuticular Hydrocarbons
5. Discussion
5.1. Effect of Nosema ceranae Infection
5.2. Effect of Acaricides
5.3. Effect of Nosema ceranae Infection and Coumaphos
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drummond, F.; Ballman, E.S.; Eitzer, B.D.; Du Clos, B.; Dill, J. Exposure of Honey Bee (Apis mellifera L.) Colonies to Pesticides in Pollen, A Statewide Assessment in Maine. Environ. Èntomol. 2018, 47, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M. Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 2017, 49, 131–150. [Google Scholar] [CrossRef]
- Higes, M.; Meana, A.; Bartolomé, C.; Botías, C.; Martín-Hernández, R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef]
- Soklič, M.; Gregorc, A. Comparison of the two microsporidia that infect honey bees—A review. Agriculture 2016, 13, 49–56. [Google Scholar] [CrossRef][Green Version]
- Hernández, R.M.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [PubMed]
- Burnham, A.J. Scientific Advances in Controlling Nosema ceranae (Microsporidia) Infections in Honey Bees (Apis mellifera). Front. Veter. Sci. 2019, 6, 79. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Huang, W.-F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invertebr. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; Vanengelsdorp, D. Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to the Gut Pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef]
- Garrido, P.M.; Porrini, M.P.; Antúnez, K.; Branchiccela, B.; Martínez-Noël, G.M.; Zunino, P.; Salerno, G.; Eguaras, M.; Ieno, E.N. Sublethal effects of acaricides and Nosema ceranae infection on immune related gene expression in honeybees. Veter. Res. 2016, 47, 51. [Google Scholar] [CrossRef]
- Alaux, C.; Maisonnasse, A.; Le Conte, Y. Pheromones in a Superorganism. Anxiety 2010, 83, 401–423. [Google Scholar] [CrossRef]
- Ali, M.F.; Morgan, E.D. Chemical Communication in Insect Communities: A Guide To Insect Pheromones With Special Emphasis On Social Insects. Biol. Rev. 1990, 65, 227–247. [Google Scholar] [CrossRef]
- Ayasse, M.; Paxton, R.J.; Tengö, J. Mating behavior and chemical communication in the order hymenoptera. Annu. Rev. Èntomol. 2001, 46, 31–78. [Google Scholar] [CrossRef] [PubMed]
- Slessor, K.N.; Winston, M.L.; Le Conte, Y. Pheromone Communication in the Honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Greene, M. Cuticular hydrocarbon cues in the formation and maintenance of insect social groups. In Insect Hydrocarbons; Cambridge University Press: Cambridge, UK, 2010; pp. 244–253. [Google Scholar]
- Dussaubat, C.; Maisonnasse, A.; Alaux, C.; Tchamitchan, S.; Brunet, J.-L.; Plettner, E.; Belzunces, L.P.; Le Conte, Y. Nosema spp. Infection Alters Pheromone Production in Honey Bees (Apis mellifera). J. Chem. Ecol. 2010, 36, 522–525. [Google Scholar] [CrossRef]
- Salvy, M.; Martin, C.; Bagnères, A.-G.; Provost, É.; Roux, M.; Le Conte, Y.; Clément, J. Modifications of the cuticular hydrocarbon profile of Apis mellifera worker bees in the presence of the ectoparasitic mite Varroa jacobsoni in brood cells. Parasitology 2001, 122, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Kather, R.; Drijfhout, F.P.; Martin, S.J. Task Group Differences in Cuticular Lipids in the Honey Bee Apis mellifera. J. Chem. Ecol. 2011, 37, 205–212. [Google Scholar] [CrossRef]
- Rademacher, E.; Harz, M. Oxalic acid for the control of varroosis in honey bee colonies—A review. Apidologie 2006, 37, 98–120. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Scholl, J.; Naug, D. Olfactory discrimination of age-specific hydrocarbons generates behavioral segregation in a honeybee colony. Behav. Ecol. Sociobiol. 2011, 65, 1967–1973. [Google Scholar] [CrossRef]
- McDonnell, C.M.; Alaux, C.; Parrinello, H.; Desvignes, J.P.; Crauser, D.; Durbesson, E.; Beslay, D.; Le Conte, Y. Ecto- and endoparasite induce similar chemical and brain neurogenomic responses in the honey bee (Apis mellifera). BMC Ecol. 2013, 13, 25. [Google Scholar] [CrossRef]
- Murray, Z.L.; A Keyzers, R.; Barbieri, R.F.; Digby, A.P.; Lester, P.J. Two pathogens change cuticular hydrocarbon profiles but neither elicit a social behavioural change in infected honey bees, Apis mellifera (Apidae: Hymenoptera). Austral Èntomol. 2016, 55, 147–153. [Google Scholar] [CrossRef]
- Biganski, S.; Kurze, C.; Müller, M.Y.; Moritz, R.F.A. Social response of healthy honeybees towards Nosema ceranae-infected workers: Care or kill? Apidologie 2018, 49, 325–334. [Google Scholar] [CrossRef]
- Leoncini, I.; Crauser, D.; Robinson, G.E.; Le Conte, Y. Worker-worker inhibition of honey bee behavioural development independent of queen and brood. Insectes Sociaux 2004, 51, 392–394. [Google Scholar] [CrossRef]
- Pankiw, T. Brood Pheromone Modulation of Pollen Forager Turnaround Time in the Honey Bee (Apis mellifera L.). J. Insect Behav. 2007, 20, 173–180. [Google Scholar] [CrossRef]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef]
- Atienza, J.; Jiménez, J.; Bernal, J.; Martín, M. Supercritical fluid extraction of fluvalinate residues in honey. Determination by high-performance liquid chromatography. J. Chromatogr. A 1993, 655, 95–99. [Google Scholar] [CrossRef]
- Chauzat, M.-P.; Carpentier, P.; Martel, A.-C.; Bougeard, S.; Cougoule, N.; Porta, P.; Lachaize, J.; Madec, F.; Aubert, M.; Faucon, J.-P. Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ. Èntomol. 2009, 38, 514–523. [Google Scholar] [CrossRef]
- Martel, A.-C.; Zeggane, S.; Aurières, C.; Drajnudel, P.; Faucon, J.-P.; Aubert, M. Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar® or Asuntol®50. Apidologie 2007, 38, 534–544. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Vanengelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity—USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Fries, I. Contribution to the Study of Nosema Disease (Nosema apis Z.) in Honey Bee (Apis mellifera L.) Colonies; Swedish University of Agricultural Sciences: Upsala, Sweden, 1998. [Google Scholar]
- Branco, M.R.; Kidd, N.A.; Pickard, R.S. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie 2006, 37, 452–461. [Google Scholar] [CrossRef]
- Fries, I.; Aarhus, A.; Hansen, H.; Korpela, S. Comparison of diagnostic methods for detection of low infestation levels of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp. Appl. Acarol. 1991, 10, 279–287. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Orantes-Bermejo, J. Acaricides and their residues in Spanish commercial beeswax. Pest Manag. Sci. 2010, 66, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Porrini, M.P.; Garrido, P.M.; Eguaras, M.J. Individual feeding of honey bees: Modification of the Rinderer technique. J. Apic. Res. 2013, 52, 194–195. [Google Scholar] [CrossRef]
- Cole, R. The application of the “triangulation” method to the purification of Nosema spores from insect tissues. J. Invertebr. Pathol. 1970, 15, 193–195. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of Colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef]
- Cantwell, G. Standard methods for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Pub. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Linstrom, P.; Mallard, W. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [CrossRef]
- Alaux, C.; Brunet, J.-L.; Dussaubat, C.; Mondet, F.; Tchamitchian, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.-L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef]
- Porrini, M.P.; Sarlo, E.G.; Medici, S.K.; Garrido, P.M.; Porrini, D.P.; Damiani, N.; Eguaras, M.J. Nosema ceranae development in Apis mellifera: Influence of diet and infective inoculum. J. Apic. Res. 2011, 50, 35–41. [Google Scholar] [CrossRef]
- Ptaszyńska, A.; Borsuk, G.; Mułenko, W.; Olszewski, K. Impact of ethanol on Nosema spp. infected bees. Med. Weter. 2013, 69, 736–741. [Google Scholar]
- Dussaubat, C.; Sagastume, S.; Gómez-Moracho, T.; Botías, C.; García-Palencia, P.; Martín-Hernández, R.; Le Conte, Y.; Higes, M. Comparative study of Nosema ceranae (Microsporidia) isolates from two different geographic origins. Veter. Microbiol. 2013, 162, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Cook, S.C. Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int. J. Parasitol. 2018, 48, 433–444. [Google Scholar] [CrossRef]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Sinpoo, C.; Paxton, R.J.; Disayathanoowat, T.; Krongdang, S.; Chantawannakul, P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect Physiol. 2018, 105, 1–8. [Google Scholar] [CrossRef]
- Richard, F.-J.; Aubert, A.; Grozinger, C.M. Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol. 2008, 6, 50. [Google Scholar] [CrossRef]
- Van Buren, N.W.M.; Mariën, A.G.H.; Oudejans, R.C.H.M.; Velthuis, H.H.W. Perizin, an acaricide to combat the mite Varroa jacobsoni: Its distribution in and influence on the honeybee Apis mellifera. Physiol. Èntomol. 1992, 17, 288–296. [Google Scholar] [CrossRef]
- Teeters, B.S.; Johnson, R.M.; Ellis, M.D.; Siegfried, B.D. Using video-tracking to assess sublethal effects of pesticides on honey bees (Apis mellifera L.). Environ. Toxicol. Chem. 2012, 31, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, T.; Spivak, M.; Weaver, D.; Weaver, B.; Glenn, T. Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J. Econ. Èntomol. 2002, 95, 28–35. [Google Scholar] [CrossRef]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J.; Vanengelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef]
- Garrido, P.M.; Antúnez, K.; Martin, M.; Porrini, M.P.; Zunino, P.; Eguaras, M.J. Immune-related gene expression in nurse honey bees (Apis mellifera) exposed to synthetic acaricides. J. Insect Physiol. 2013, 59, 113–119. [Google Scholar] [CrossRef]
- Dahlgren, L.; Johnson, R.M.; Siegfried, B.D.; Ellis, M.D. Comparative Toxicity of Acaricides to Honey Bee (Hymenoptera: Apidae) Workers and Queens. J. Econ. Èntomol. 2012, 105, 1895–1902. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four Common Pesticides, Their Mixtures and a Formulation Solvent in the Hive Environment Have High Oral Toxicity to Honey Bee Larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome Analyses of the Honeybee Response to Nosema ceranae and Insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Doublet, V.; Labarussias, M.; De Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Silva-Zacarin, E.C.; Carvalho, S.M.; Kramberger, D.; Teixeira, É.W.; Malaspina, O. Effects of Nosema ceranae and thiametoxam in Apis mellifera: A comparative study in Africanized and Carniolan honey bees. Chemosphere 2016, 147, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Lodesani, M.; Costa, C.; Serra, G.; Colombo, R.; Sabatini, A.G. Acaricide residues in beeswax after conversion to organic beekeeping methods. Apidologie 2008, 39, 324–333. [Google Scholar] [CrossRef]
- Medici, S.; Maggi, M.D.; Sarlo, E.G.; Ruffinengo, S.; Marioli, J.M.; Eguaras, M. The presence of synthetic acaricides in beeswax and its influence on the development of resistance in Varroa destructor. J. Apic. Res. 2015, 54, 267–274. [Google Scholar] [CrossRef]
- Boi, M.; Serra, G.; Colombo, R.; Lodesani, M.; Massi, S.; Costa, C. A 10 year survey of acaricide residues in beeswax analysed in Italy. Pest Manag. Sci. 2015, 72, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Barrios, L.; Nanetti, A.; Meana, A.; Martín-Hernández, R.; Garrido-Bailón, E.; Higes, M. Nosema spp. parasitization decreases the effectiveness of acaricide strips (Apivar®) in treating varroosis of honey bee (Apis mellifera iberiensis) colonies. Environ. Microbiol. Rep. 2011, 4, 57–65. [Google Scholar] [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® Treatments against Nosema ceranae Infection in Apis mellifera Investigated by Two qPCR Methods. Veter. Sci. 2020, 7, 125. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Porrini, M.P.; Garrido, P.M.; Porrini, D.P.; Eguaras, M.E. Nosema ceranae dynamics in productive honey bee hives. In preparation.




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porrini, M.P.; Garrido, P.M.; Umpiérrez, M.L.; Porrini, L.P.; Cuniolo, A.; Davyt, B.; González, A.; Eguaras, M.J.; Rossini, C. Effects of Synthetic Acaricides and Nosema ceranae (Microsporidia: Nosematidae) on Molecules Associated with Chemical Communication and Recognition in Honey Bees. Vet. Sci. 2020, 7, 199. https://doi.org/10.3390/vetsci7040199
Porrini MP, Garrido PM, Umpiérrez ML, Porrini LP, Cuniolo A, Davyt B, González A, Eguaras MJ, Rossini C. Effects of Synthetic Acaricides and Nosema ceranae (Microsporidia: Nosematidae) on Molecules Associated with Chemical Communication and Recognition in Honey Bees. Veterinary Sciences. 2020; 7(4):199. https://doi.org/10.3390/vetsci7040199
Chicago/Turabian StylePorrini, Martín Pablo, Paula Melisa Garrido, María Laura Umpiérrez, Leonardo Pablo Porrini, Antonella Cuniolo, Belén Davyt, Andrés González, Martín Javier Eguaras, and Carmen Rossini. 2020. "Effects of Synthetic Acaricides and Nosema ceranae (Microsporidia: Nosematidae) on Molecules Associated with Chemical Communication and Recognition in Honey Bees" Veterinary Sciences 7, no. 4: 199. https://doi.org/10.3390/vetsci7040199
APA StylePorrini, M. P., Garrido, P. M., Umpiérrez, M. L., Porrini, L. P., Cuniolo, A., Davyt, B., González, A., Eguaras, M. J., & Rossini, C. (2020). Effects of Synthetic Acaricides and Nosema ceranae (Microsporidia: Nosematidae) on Molecules Associated with Chemical Communication and Recognition in Honey Bees. Veterinary Sciences, 7(4), 199. https://doi.org/10.3390/vetsci7040199





