Effect of Different Doses of Atipamezole on Reversal of Medetomidine-Induced Tear-Flow Decrease in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Treatment
2.3. Measurement
2.4. Statistical Analysis
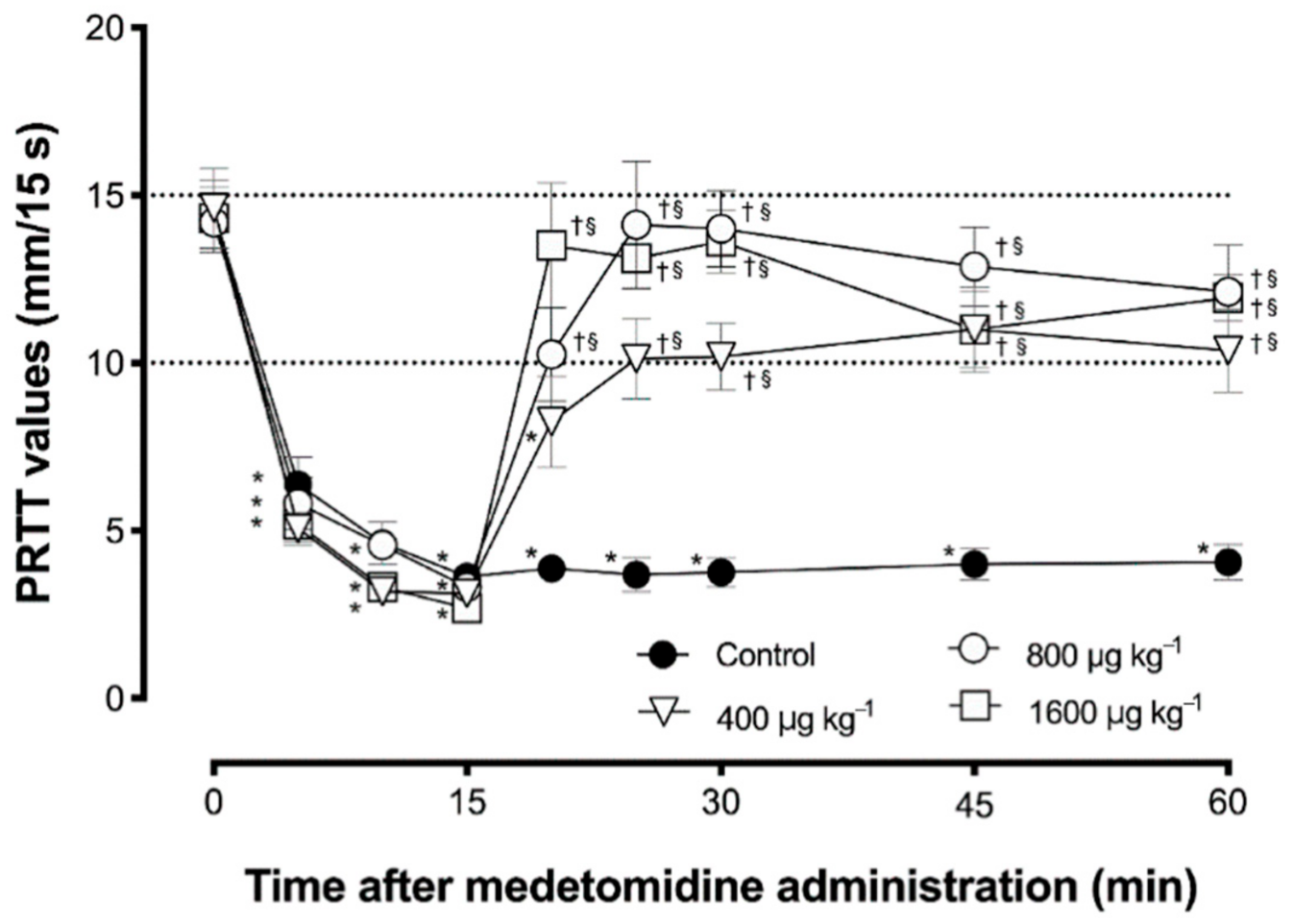
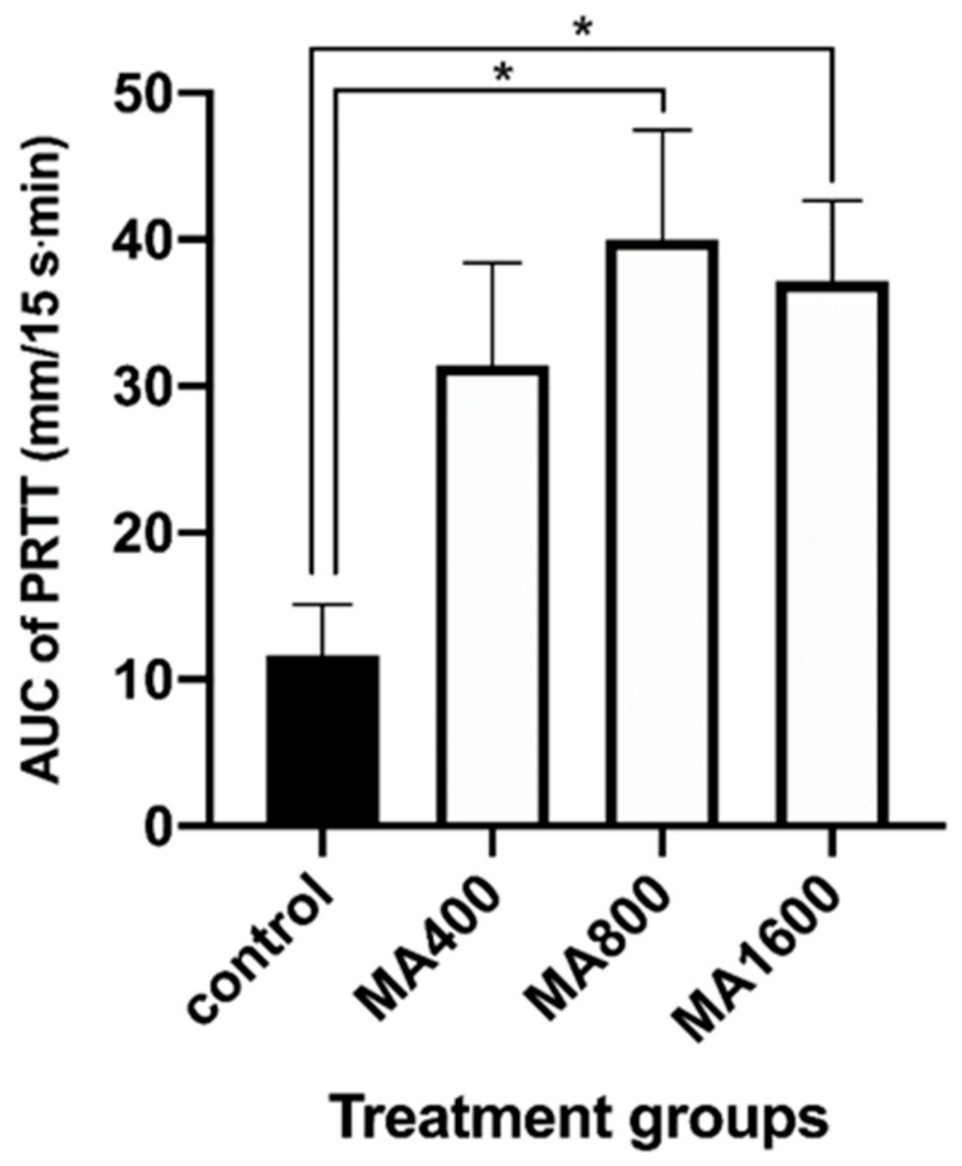
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinclair, M.D. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 2003, 44, 885–897. [Google Scholar] [PubMed]
- Bryant, C.E.; England, G.C.; Clarke, K.W. Comparison of the sedative effects of medetomidine and xylazine in horses. Vet. Rec. 1991, 129, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.G.; Schumacher, J.; Orosz, S.E.; Ramsay, E.C. Sedative Effects of Medetomidine in Pigeons (Columba livia). J. Avian Med. Surg. 2001, 15, 95–100. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Nishimura, R.; Sasaki, N.; Ishiguro, T.; Tamura, H.; Takeuchi, A. Sedative effects of medetomidine in pigs. J. Vet. Med. Sci. 1992, 54, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Hikasa, Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in healthy cats. Can. J. Vet. Res. 2008, 72, 278–286. [Google Scholar]
- Ambrisko, T.D.; Hikasa, Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can. J. Vet. Res. 2002, 66, 42–49. [Google Scholar]
- Vaisanen, M.; Raekallio, M.; Kuusela, E.; Huttunen, P.; Leppaluoto, J.; Kirves, P.; Vainio, O. Evaluation of the perioperative stress response in dogs administered medetomidine or acepromazine as part of the preanesthetic medication. Am. J. Vet. Res. 2002, 63, 969–975. [Google Scholar] [CrossRef]
- Lamont, L.; Burton, S.; Caines, D.; Masaoud, E.; Troncy, E. Effects of 2 different medetomidine infusion rates on selected neurohormonal and metabolic parameters in dogs. Can. J. Vet. Res. 2012, 76, 143–148. [Google Scholar]
- Sanchez, R.F.; Mellor, D.; Mould, J. Effects of medetomidine and medetomidine-butorphanol combination on Schirmer tear test 1 readings in dogs. Vet. Ophthalmol. 2006, 9, 33–37. [Google Scholar] [CrossRef]
- Abdelhakiem, M.A.H.; Elmeligy, E.; Al-lethie, A. Effect of Xylazine HCl and/or Ketamine HCl on the Tear Production in Clinically Healthy Dogs. Adv. Anim. Vet. Sci. 2019, 7, 1015–1020. [Google Scholar] [CrossRef]
- Kanda, T.; Ishihara, S.; Oka, M.; Sako, K.; Sato, Y.; Maeta, N.; Tamura, K.; Furumoto, K.; Furukawa, T. Temporal effects of intramuscular administration of medetomidine hydrochloride or xylazine hydrochloride to healthy dogs on tear flow measured by use of a schirmer tear test I. Am. J. Vet. Res. 2016, 77, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.S.; Malmasi, A.; Bokaie, S. Effect of acepromazine or xylazine on tear production as measured by Schirmer tear test in normal cats. Vet. Ophthalmol. 2010, 13, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Selk Ghaffari, M.; Brooks, D.E.; Sabzevari, A.; Ghamsari, S.M.; Mansoor Lakooraj, H.; Shad, H. Effects of Intravenous Detomidine on Schirmer Tear Test Results in Clinically Normal Horses. J. Equine Vet. Sci. 2017, 55, 97–99. [Google Scholar] [CrossRef]
- Leonardi, F.; Costa, G.L.; Dubau, M.; Sabbioni, A.; Simonazzi, B.; Angelone, M. Effects of intravenous romifidine, detomidine, detomidine combined with butorphanol, and xylazine on tear production in horses. Equine Vet. Educ. 2020, 32, 53–57. [Google Scholar] [CrossRef]
- Kanda, T.; Mizoguchi, Y.; Furumoto, K.; Shimizu, Y.; Maeta, N.; Furukawa, T. Effect of Intramuscular Medetomidine Administration on Tear Flow in Rats. Vet. Sci. 2020, 7, 42. [Google Scholar] [CrossRef]
- Weisse, I.; Hoefke, W.; Greenberg, S.; Gaida, W.; Stötzer, H.; Kreuzer, H. Ophthalmological and pharmacological studies after administration of clonidine in rats. Arch. Toxicol. 1978, 41, 89–98. [Google Scholar] [CrossRef]
- Kanda, T.; Kajiyama, A.; Morimitsu, W.; Nishino, Y.; Oishi, Y.; Shimizu, Y.; Maeta, N.; Furumoto, K.; Itoh, Y.; Furukawa, T. Effect of medetomidine on tear flow measured by Schirmer tear test I in normal pigs. J. Vet. Med. Sci. 2019, 81, 538–540. [Google Scholar] [CrossRef]
- Kanda, T.; Shimizu, Y.; Hanazono, C.; Maki, S.; Maeta, N.; Itoi, T.; Furumoto, K.; Okamura, Y.; Itoh, Y.; Furukawa, T. Effect of intramuscular administration of medetomidine and xylazine on tear flow measured by the Schirmer tear test I in healthy cats. J. Feline Med. Surg. 2019, 21, 788–792. [Google Scholar] [CrossRef]
- Moore, C.P. Disease and surgery of the lacrimal secretory system. In Veterinary Ophthalmology; Gelatt, K.N., Ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1999; pp. 583–607. [Google Scholar]
- Meng, I.D.; Barton, S.T.; Mecum, N.E.; Kurose, M. Corneal Sensitivity Following Lacrimal Gland Excision in the Rat. Investig. Opthalmol. Vis. Sci. 2015, 56, 3347. [Google Scholar] [CrossRef]
- Peche, N.; Köstlin, R.; Reese, S.; Pieper, K. Postanaesthetic tear production and ocular irritation in cats. Tierarztl Prax. Ausg. K Kleintiere Heimtiere 2015, 43, 75–82. [Google Scholar]
- Dodam, J.R.; Branson, K.R.; Martin, D.D. Effects of intramuscular sedative and opioid combinations on tear production in dogs. Vet. Ophthalmol. 1998, 1, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, F.; Costa, G.L.; Stagnoli, A.; Zubin, E.; Boschi, P.; Sabbioni, A.; Simonazzi, B. The effect of intramuscular dexmedetomidine-butorphanol combination on tear production in dogs. Can. Vet. J. 2019, 60, 55–59. [Google Scholar] [PubMed]
- Virtanen, R. Pharmacological profiles of medetomidine and its antagonist, atipamezole. Acta Vet. Scand. Suppl. 1989, 85, 29–37. [Google Scholar] [PubMed]
- Nakamura, S.; Kimura, Y.; Mori, D.; Imada, T.; Izuta, Y.; Shibuya, M.; Sakaguchi, H.; Oonishi, E.; Okada, N.; Matsumoto, K.; et al. Restoration of Tear Secretion in a Murine Dry Eye Model by Oral Administration of Palmitoleic Acid. Nutrients 2017, 9, 364. [Google Scholar] [CrossRef]
- Hegarty, D.M.; David, L.L.; Aicher, S.A. Lacrimal Gland Denervation Alters Tear Protein Composition and Impairs Ipsilateral Eye Closures and Corneal Nociception. Investig. Opthalmol. Vis. Sci. 2018, 59, 5217–5224. [Google Scholar] [CrossRef]
- Vähä-Vahe, A.T. The clinical effectiveness of atipamezole as a medetomidine antagonist in the dog. J. Vet. Pharmacol. Ther. 1990, 13, 198–205. [Google Scholar] [CrossRef]
- Vainio, O.; Vähä-Vahe, T. Reversal of medetomidine sedation by atipamezole in dogs. J. Vet. Pharmacol. Ther. 1990, 13, 15–22. [Google Scholar] [CrossRef]
- Hu, C.; Flecknell, P.A.; Liles, J.H. Fentanyl and medetomidine anaesthesia in the rat and its reversal using atipamazole and either nalbuphine or butorphanol. Lab. Anim. 1992, 26, 15–22. [Google Scholar] [CrossRef]
- Jang, H.S.; Choi, H.S.; Lee, S.H.; Jang, K.H.; Lee, M.-G. Evaluation of the anaesthetic effects of medetomidine and ketamine in rats and their reversal with atipamezole. Vet. Anaesth. Analg. 2009, 36, 319–327. [Google Scholar] [CrossRef]
- Hedenqvist, P.; Roughan, J.V.; Flecknell, P.A. Sufentanil and medetomidine anaesthesia in the rat and its reversal with atipamezole and butorphanol. Lab. Anim. 2000, 34, 244–251. [Google Scholar] [CrossRef]
- Nishimura, R.; Kim, H.; Matsunaga, S.; Hayashi, K.; Sakai, N.; Tamura, H.; Takeuchi, A. Antagonistic Effects of Atipamezole and Flumazenil on Medetomidine-Midazolam Induced Sedation in Laboratory Pigs. J. Vet. Med. Sci. 1993, 55, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yonezawa, K.; Izumisawa, Y.; Kotani, T. Antagonistic Effects of Atipamezole on Medetomidine-Induced Sedation in Horses. J. Vet. Med. Sci. 1996, 58, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A.; Kauppila, T.; Tukeva, T. The effect of medetomidine, an alpha 2-adrenoceptor agonist, in various pain tests. Eur. J. Pharmacol. 1990, 179, 323–328. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Gotoh, M.; Makino, A.; Furumoto, K.; Shimizu, Y.; Itoi, T.; Maeta, N.; Furukawa, T. Effect of Different Doses of Atipamezole on Reversal of Medetomidine-Induced Tear-Flow Decrease in Rats. Vet. Sci. 2020, 7, 197. https://doi.org/10.3390/vetsci7040197
Kanda T, Gotoh M, Makino A, Furumoto K, Shimizu Y, Itoi T, Maeta N, Furukawa T. Effect of Different Doses of Atipamezole on Reversal of Medetomidine-Induced Tear-Flow Decrease in Rats. Veterinary Sciences. 2020; 7(4):197. https://doi.org/10.3390/vetsci7040197
Chicago/Turabian StyleKanda, Teppei, Manami Gotoh, Ayumi Makino, Kayo Furumoto, Yuki Shimizu, Takamasa Itoi, Noritaka Maeta, and Toshinori Furukawa. 2020. "Effect of Different Doses of Atipamezole on Reversal of Medetomidine-Induced Tear-Flow Decrease in Rats" Veterinary Sciences 7, no. 4: 197. https://doi.org/10.3390/vetsci7040197
APA StyleKanda, T., Gotoh, M., Makino, A., Furumoto, K., Shimizu, Y., Itoi, T., Maeta, N., & Furukawa, T. (2020). Effect of Different Doses of Atipamezole on Reversal of Medetomidine-Induced Tear-Flow Decrease in Rats. Veterinary Sciences, 7(4), 197. https://doi.org/10.3390/vetsci7040197




